Abstract
The assessment of sarcopenia is part of the nutritional assessment index and is essential in stroke management. This study aimed to identify and validate cutoff values of temporal muscle thickness (TMT) measured using computed tomography to identify sarcopenia after acute stroke. The participants were patients with stroke aged ≥65 years who were admitted to rehabilitation units. The recruited patients were randomly divided into the calculation and validation cohort. In the calculation cohort, TMT cutoff values for identifying sarcopenia were calculated using receiver operating characteristic analysis. The obtained values were validated in the validation cohort using sensitivity and specificity. The calculation cohort included 230 patients (125 men, mean age, 77.2 ± 7.2 years), whereas the validation cohort included 235 patients (125 men, mean age, 76.4 ± 6.95 years). The TMT cutoff values for identifying sarcopenia and low skeletal muscle index were the same: 3.83 mm for men and 2.78 mm for women. The TMT cutoff value for identifying sarcopenia showed a sensitivity and specificity of 0.642 and 0.750, respectively, for men, and 0.660 and 0.567, respectively, for women. We identified a valid cutoff value of temporal muscle thickness for identifying sarcopenia after acute stroke. TMT is easy to measure and may be useful for the early detection of sarcopenia.
1. Introduction
Stroke has a high global incidence. It can lead to various disabilities and a decreased ability to perform activities of daily living (ADL). Undernutrition and sarcopenia are negative factors for the recovery of physical function and ADL performance ability after acute stroke [1,2,3,4,5,6]. Stroke-related sarcopenia occurs due to immobility and decreased activity, as well as stroke-related muscle tissue changes such as inflammation, denervation, sympathetic activation, and shifts in muscle fiber type [7]. In addition, undernutrition and sarcopenia are associated, and the coexistence of undernutrition and sarcopenia is frequently observed in elderly patients [8]. In stroke, impaired consciousness, dysphagia, and functional impairment of the upper limbs can lead to poor nutritional intake [9,10], which can easily worsen nutritional status [3], and undernutrition can worsen sarcopenia [11]. An assessment of muscle mass is also important in the diagnostic criteria for malnutrition [12]. Therefore, it is essential to identify, prevent, and treat sarcopenia and undernutrition in patients with stroke. Sarcopenia is often diagnosed using criteria such as the Asian Working Group for Sarcopenia (AWGS) 2019 [13] and European Working Group on Sarcopenia in Older People 2 [14]. These criteria diagnose sarcopenia using indices of muscle strength, function, and muscle mass. In patients with stroke, it is often difficult to assess muscle strength and physical function due to paralysis and impaired consciousness. Dual-energy X-ray absorptiometry and bioelectrical impedance analysis (BIA), the gold standards for muscle mass assessment, are not yet widely used in clinical practice. Therefore, a sarcopenia assessment method that is easy to use clinically in patients with stroke is needed.
In recent years, temporal muscle thickness (TMT) has gained attention as a surrogate marker for identifying post-stroke function and prognosis [15]. TMT correlates with sarcopenia risk [16] and hand grip strength [17] in patients with stroke. A low TMT has been found to be associated with significantly decreased survival [18], and severe dysphagia [19] in patients with acute stroke. Katsuki et al. reported TMT cutoff values of 4.9 mm for women and 6.7 mm for men when identifying physical function at 3 months post-stroke in patients with subarachnoid hemorrhage under 75 years of age [20]. TMT has also been shown to be associated with nutritional status in older adults [21] and progression-free survival in patients with head and neck squamous cell carcinoma [18]. TMT can be measured using computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography. Since CT and MRI are routinely performed in patients with stroke, assessing sarcopenia based on TMT can enable early diagnosis and intervention and may contribute to improving the clinical outcomes of patients with acute stroke. However, no TMT cutoff values have been reported for identifying sarcopenia or skeletal muscle loss in patients after acute stroke. The purpose of this study was to calculate and validate the cutoff values of TMT for identifying sarcopenia in older Japanese patients with stroke.
2. Materials and Methods
2.1. Participants
This cross-sectional study was conducted at the Hamamatsu City Rehabilitation Hospital in Shizuoka, Japan. This hospital provides inpatient rehabilitation services and care covered by the Japanese national health insurance system [22]. The participants were patients aged ≥65 years who were admitted to rehabilitation units after acute stroke between June 2019 and June 2021. Patients were excluded if it was not possible to measure their body composition using BIA. This study was approved by the Ethics Committee of the Hamamatsu City Rehabilitation Hospital (ID:20-26). The requirement for informed consent was waved by the ethics committee because of the retrospective study design. Patients could withdraw from the study at any time using the opt-out feature on the study website.
2.2. Temporal Muscle Thickness
TMT was evaluated on CT images (window width: 100 mm and window level: 35 mm) obtained on the day of admission to the hospital using the method described by Nozoe et al. [16] TMT was measured at the level of the orbital roof, and the Sylvian fissure was used as a reference point to determine the anterior–posterior orientation. TMT was separately evaluated on the left and right sides in all patients by one registered dietician and one radiological technologist using Image J software (Ver. 1.52u) [23]. The TMT values of each side were summed and divided by two to obtain the mean TMT for each patient.
2.3. Sarcopenia Parameters
Sarcopenia was diagnosed according to the AWGS 2019 criteria [13]. Hand grip strength was measured using a Jamar dynamometer (MG-4800 digital grip strength meter; CHARDER Electronic, Taichung, Taiwan). The hand grip strength was measured separately for the left and right hands with the participant seated with their elbow at 90°, and the highest value from three measurements for each side was used for analysis. Muscle mass was calculated from the impedance measured using the bioimpedance analyzer (Inbody s10; InBody Japan, Tokyo, Japan). To minimize the influence of the measurement device, we used Yamada’s formula: [24]. The skeletal muscle index (SMI) was calculated as the ratio between the appendicular skeletal muscle mass and the height squared. Based on the cutoff mentioned in the AWGS 2019 criteria [13], women with an SMI < 5.7 kg/m2 and a hand grip strength < 18 kg and men with an SMI < 7.0 kg/m2 and a hand grip strength < 28 kg were diagnosed with sarcopenia. Gait speed and balance were not evaluated, as patients often have difficulties walking or standing after stroke. An SMI of <5.7 kg/m2 in men and <7.0 kg/m2 in women was considered a “low SMI” and a hand grip strength < 28 kg in men and <18 kg in women was considered a “low hand grip strength”.
2.4. Other Parameters
Stroke severity was assessed using the modified Rankin Scale [25]. The modified Rankin Scale score at stroke onset and time (in days) from stroke onset to rehabilitation hospital admission were determined from the records from the previous hospital. Nutritional status was assessed using the Mini Nutritional Assessment—Short Form, which is a tool for screening the nutritional status of older adults. Its scores range from 0 to 14, with scores of 11 or less indicating a poor nutritional status [26]. Comorbidities were assessed using the Charlson Comorbidity Index [27]. The ability to perform ADL was assessed by physical or occupational therapists using the Functional Independence Measure [28], which consists of motor (13 items) and cognitive (5 items) subscales. For each item, the patient’s independence level was graded on a scale from total assistance (1 point) to complete independence (7 points), and the total score ranged from 18 to 126 [28]. Each evaluation was performed within 3 days of admission. C-reactive protein values were obtained from blood samples taken at the time of admission.
2.5. Statistical Analysis
The recruited patients were randomly divided into two 1:1 cohorts: the calculation cohort, in which the cutoff value for identifying sarcopenia was calculated, and the validation cohort, in which the cutoff value obtained from analysis of the other cohort was validated. Descriptive statistics were used to describe patient characteristics in each cohort. Continuous and ordinal data are presented as mean ± standard deviation and median (25, 75 percentiles), respectively. Categorical data are expressed as frequencies and percentages. In the calculation cohort, TMT cutoff values for identifying low hand grip strength, low SMI, and sarcopenia were calculated using receiver operating characteristic analysis. In addition, the sensitivity, specificity, area under the curve (AUC), positive predictive value (PPV), and negative predictive value (NPV) for the obtained TMT cutoff values were calculated in the validation cohort.
3. Results
After excluding 20 patients whose muscle mass could not be measured using BIA, 465 patients were finally included. Of them, 230 (125 man, mean age, 77.2 ± 7.2 years) were included in the calculation cohort (Table 1). The mean TMT in this cohort was 3.98 ± 1.52 mm in men and 3.07 ± 1.20 mm in women. In the calculation cohort, the TMT cutoff values for identifying low SMI and sarcopenia were the same: 3.83 mm for men and 2.78 mm for women (Figure 1). The cutoff value for identifying low hand grip strength was 3.83 mm for men, which was the same as that for low SMI and sarcopenia, while it was 3.08 mm for women (Figure 1).

Table 1.
Characteristics of the calculation cohort.
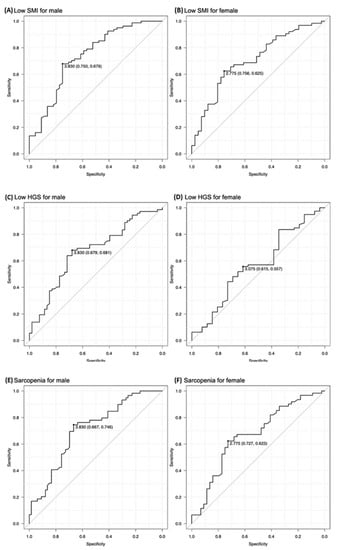
Figure 1.
The temporal muscle thickness (TMT) cutoff values for identifying low skeletal muscle index (SMI), low hand grip strength (HGS), and sarcopenia. The TMT cutoff values for identifying low SMI were 3.83 mm for men and 2.78 mm for women (A,B, respectively). The TMT cutoff values for identifying low HGS were 3.83 mm for men and 3.08 mm for women (C,D, respectively). The TMT cutoff values for identifying sarcopenia were 3.83 mm for men and 2.78 mm for women (E,F, respectively).
The validation cohort included 235 patients (125 men, mean age, 76.4 ± 6.95 years) (Table 2). The mean TMT for validation cohort was 4.09 ± 1.53 mm in men and 3.10 ± 1.20 mm in women. There was no statistically significant difference in characteristics between the two groups. The sensitivity and specificity of the cutoff values for identifying low SMI identified in the calculation cohort were 0.774 and 0.583, respectively, for men, and 0.700 and 0.517, respectively, for women. The AUC, PPV, and NPV, were 0.735, 0.557, and 0.778, respectively, for men, and 0.704, 0.547, and 0.674, respectively, for women. The sensitivity and specificity of the cutoff values for identifying sarcopenia identified in the calculation cohort were 0.642 and 0.750, respectively, for men, and 0.660 and 0.567, respectively, for women. The AUC, PPV, and NPV, were 0.726, 0.654, and 0.740, respectively, for men, and 0.681, 0.559, and 0.667, respectively, for women (Table 3).

Table 2.
Characteristics of the validation cohort.

Table 3.
Accuracy of low TMT for identifying low SMI and sarcopenia.
4. Discussion
In this study, we found a valid TMT cutoff value that predicts low SMI sarcopenia after acute stroke in older patients. We randomly divided older patients who had experienced acute stroke into two cohorts, calculated cutoff values for identifying low SMI and sarcopenia in one cohort, and validated the cutoff values in the other cohort. The TMT cutoff value for identifying low SMI and sarcopenia was 3.83 mm for men and 2.78 mm for women. The sensitivity, specificity, and AUC for identifying low SMI were 0.774, 0.583, and 0.735, respectively, for men, and 0.700, 0.517, and 0.704, respectively, for women. The TMT cutoff value for identifying sarcopenia showed a sensitivity, specificity, and AUC of 0.642, 0.750, and 0.726, respectively, for men, and 0.660, 0.567, and 0.681, respectively, for women.
The TMT cutoff values for identifying low SMI and sarcopenia identified in this study were found to be valid. Steindl et al. reported a correlation between TMT and hand grip strength measured using MRI in patients with neurological disease (Pearson correlation coefficient = 0.649; p < 0.001) [17]. Furthermore, Cho et al. found a weak correlation between TMT measured using MRI and appendicular skeletal mass (r = 0.379, p = 0.001) [29]. A correlation between pre-stroke sarcopenia risk, assessed using the strength, assistance in walking, rise from a chair, climb stairs, and falls questionnaire and TMT, has also been reported [16]. Thus, TMT may be useful in assessing sarcopenia as an indicator of muscle strength and mass. In this study, cutoff values of TMT for identifying sarcopenia were determined and validated in different cohorts. The sensitivity and specificity were 0.642 and 0.750, respectively, for men, and 0.660 and 0.567, respectively, for women. The AUC was 0.726 for men and 0.681 for women, with moderate accuracy, and we consider the cutoff values to be useful for screening for sarcopenia. In this study, TMT was calculated from CT images, but TMT can be measured using ultrasonography and MRI as well [21,30]. Ultrasonography has been used for nutritional status assessment, and the technology has been improved [31,32,33]. Ultrasonography also has potential uses for swallowing assessment [34]. Ultrasonography and CT are commonly used in clinical practice, and TMT measurement may be an alternative method of sarcopenia assessment in older patients with stroke.
Sex differences were observed in TMT in the present study. Sex differences in muscle mass are generally observed, with men having more muscle mass than women, and men and women have different cut-off values for muscle mass [13]. Yesil Cinkir et al. measured TMT using MRI in patients with newly diagnosed glioblastoma multiforme (median (range) age: 56 (18–79) years) and found that the median TMT was 4.7 mm (range: 2.8–6.6 mm) in women and 5.4 mm (range: 2.9–8.1) in men [35]. In addition, Katsuki et al. reported that, in patients with subarachnoid hemorrhage aged 75 years or younger (mean [range] age: 60.6 (32–74) years), the TMT cutoff values for identifying physical function at 3 months were 4.9 mm for women and 6.7 mm for men [20]. In our study, the cutoff values of TMT for sarcopenia were 2.78 mm for women and 3.83 mm for men. The mean TMT and cutoff values for identifying sarcopenia were lower in our study than in a previous study [20,35]. The patients in this study were older than 65 years of age (mean age 77.2 ± 7.2 years), whereas the patients in the previous report were much younger than those in this study, with a mean age of 56 or 60 years. TMT, and the thicknesses of other muscles, should be considered during the evaluation of age-related loss of muscle mass and sex differences in muscle mass.
The strength of this study is that, for the first time, we recognized TMT cutoff values for identifying low SMI and sarcopenia after acute stroke in older Japanese patients. In this study, cutoff values were calculated and validated using two different cohorts. Appropriate random sampling and reliable results were obtained. Sarcopenia affects the prognosis of patients with stroke [4,36,37]. Although it is difficult to measure motor and physical function in patients with stroke due to factors such as paralysis, head CT findings are easily obtainable in clinical practice, and the ability to use TMT for identifying sarcopenia may permit rapid intervention. Rehabilitation nutrition or nutritional management according to rehabilitation and rehabilitative practices that take nutritional status into account is necessary [38,39]. Although the accuracy and specificity of the TMT cutoff values identifying low SMI and sarcopenia shown in this study were not very high, the ability to assess the risk of sarcopenia from CT images, which are routinely obtained in patients with stroke, may be useful in clinical practice. Nevertheless, this study was conducted on Japanese patients, and the cutoff values of TMT may differ depending on race; therefore, further validation in non-Asian patients is needed. Although muscle mass is affected by age, age-adjusted cut-off values could not be calculated in this study due to the insufficient sample size. In addition, since sarcopenia is affected by various factors, such as hormonal changes and inflammation, it is necessary to consider the influence of these factors in the correlation between TMT and sarcopenia. However, the present study was conducted as a retrospective study with a limited sample size and information. Therefore, further validation is needed. Moreover, the validity of the cutoff values for identifying prognosis was not verified in our study. In a previous study, TMT was a predictor of severe dysphagia in patients with stroke [40], but an independent association with functional prognosis was not reported [16]. Future validation of the association of TMT with prognosis in patients with stroke is warranted.
5. Conclusions
In this study, we calculated and validated TMT cutoff values for identifying sarcopenia in older Japanese patients who had experienced acute stroke. TMT cutoff value for identifying sarcopenia showed a sensitivity, specificity, and AUC of 0.642, 0.750, and 0.726, respectively, for men, and 0.660, 0.567, and 0.681, respectively, for women. Assessing sarcopenia using TMT measured using CT may be clinically useful.
Author Contributions
Conceptualization, A.N., A.S. and K.M. (Keisuke Maeda); Methodology, A.N., A.S. and K.M. (Keisuke Maeda); Formal Analysis, A.N. and A.S.; Investigation, A.S.; Resources, A.S.; Data Curation, A.S.; Writing—Original Draft Preparation, A.N.; Writing—Review and Editing, A.S., K.M. (Keisuke Maeda), J.U., T.I., K.M. (Kenta Murotani), Y.I. and N.M.; Supervision, N.M.; Project Administration, K.M. (Keisuke Maeda); Funding Acquisition, K.M. (Keisuke Maeda) All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Japan Society for the Promotion of Science (Grant number: 21H03390 and JP22H04923 to Maeda) and the Research Funding of Longevity Sciences from the Ministry of Health, Labour and Welfare of Japan (Grant number: 22-4 to Maeda).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Hamamatsu City Rehabilitation Hospital (ID:20-26).
Informed Consent Statement
Patient consent was waived due to retrospective cohort design.
Data Availability Statement
The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nishiyama, A.; Wakabayashi, H.; Nishioka, S.; Nagano, A.; Momosaki, R. Energy Intake at Admission for Improving Activities of Daily Living and Nutritional Status among Convalescent Stroke Patients. Neurol. Med. Chir. 2019, 59, 313–320. [Google Scholar] [CrossRef]
- Nishioka, S.; Wakabayashi, H.; Nishioka, E.; Yoshida, T.; Mori, N.; Watanabe, R. Nutritional Improvement Correlates with Recovery of Activities of Daily Living among Malnourished Elderly Stroke Patients in the Convalescent Stage: A Cross-Sectional Study. J. Acad. Nutr. Diet. 2016, 116, 837–843. [Google Scholar] [CrossRef]
- Kokura, Y.; Wakabayashi, H.; Nishioka, S.; Maeda, K. Nutritional intake is associated with activities of daily living and complications in older inpatients with stroke. Geriatr. Gerontol. Int. 2018, 18, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition 2019, 61, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Tanoue, M. Prevalence of sarcopenia and its association with activities of daily living and dysphagia in convalescent rehabilitation ward inpatients. Clin. Nutr. 2018, 37, 2022–2028. [Google Scholar] [CrossRef]
- Nozoe, M.; Noguchi, M.; Kubo, H.; Kanai, M.; Shimada, S. Association between the coexistence of premorbid sarcopenia, frailty, and disability and functional outcome in older patients with acute stroke. Geriatr. Gerontol. Int. 2022, 22, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yue, T.; Liu, Y. New understanding of the pathogenesis and treatment of stroke-related sarcopenia. Biomed. Pharmacother. 2020, 131, 110721. [Google Scholar] [CrossRef]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Murotani, K.; Ohno, T.; Nomoto, A.; Nagami, S.; Nagano, A.; Sato, K.; Ueshima, J.; et al. Association between food texture levels consumed and the prevalence of malnutrition and sarcopenia in older patients after stroke. Eur. J. Clin. Nutr. 2022, 76, 1576–1582. [Google Scholar] [CrossRef]
- Nii, M.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Tanaka, A. Nutritional Improvement and Energy Intake Are Associated with Functional Recovery in Patients after Cerebrovascular Disorders. J. Stroke Cerebrovasc. Dis. 2016, 25, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kokura, Y.; Kato, M.; Taniguchi, Y.; Kimoto, K.; Okada, Y. Energy intake during the acute phase and changes in femoral muscle thickness in older hemiplegic inpatients with stroke. Nutrition 2020, 70, 110582. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Kakizawa, Y.; Nishikawa, A.; Yamamoto, Y.; Uchiyama, T.; Agata, M.; Wada, N.; Kawamura, S.; Koh, A. Temporal Muscle and Stroke-A Narrative Review on Current Meaning and Clinical Applications of Temporal Muscle Thickness, Area, and Volume. Nutrients 2022, 14, 687. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, M.; Kubo, H.; Kanai, M.; Yamamoto, M.; Okakita, M.; Suzuki, H.; Shimada, S.; Mase, K. Reliability and validity of measuring temporal muscle thickness as the evaluation of sarcopenia risk and the relationship with functional outcome in older patients with acute stroke. Clin. Neurol. Neurosurg. 2021, 201, 106444. [Google Scholar] [CrossRef]
- Steindl, A.; Leitner, J.; Schwarz, M.; Nenning, K.H.; Asenbaum, U.; Mayer, S.; Woitek, R.; Weber, M.; Schöpf, V.; Berghoff, A.S.; et al. Sarcopenia in Neurological Patients: Standard Values for Temporal Muscle Thickness and Muscle Strength Evaluation. J. Clin. Med. 2020, 9, 1272. [Google Scholar] [CrossRef]
- Li, Y.X.; Hou, J.; Liu, W.Y. Long-term prognostic significance of sarcopenia in acute ischemic stroke. Medicine 2022, 101, e30031. [Google Scholar] [CrossRef] [PubMed]
- Sporns, P.B.; Muhle, P.; Hanning, U.; Suntrup-Krueger, S.; Schwindt, W.; Eversmann, J.; Warnecke, T.; Wirth, R.; Zimmer, S.; Dziewas, R. Atrophy of Swallowing Muscles Is Associated With Severity of Dysphagia and Age in Patients With Acute Stroke. J. Am. Med. Dir. Assoc. 2017, 18, 635.e1–635.e7. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Kakizawa, Y.; Nishikawa, A.; Yamamoto, Y.; Uchiyama, T. Temporal muscle thickness and area are an independent prognostic factors in patients aged 75 or younger with aneurysmal subarachnoid hemorrhage treated by clipping. Surg. Neurol. Int. 2021, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Yoshida, M.; Sato, A.; Fujimoto, Y.; Minematsu, T.; Sugama, J.; Sanada, H. Temporal muscle thickness as a new indicator of nutritional status in older individuals. Geriatr. Gerontol. Int. 2019, 19, 135–140. [Google Scholar] [CrossRef]
- Miyai, I.; Sonoda, S.; Nagai, S.; Takayama, Y.; Inoue, Y.; Kakehi, A.; Kurihara, M.; Ishikawa, M. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil. Neural Repair 2011, 25, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.B.; Dobson, E.T.A.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ ecosystem: Open-source software for image visualization, processing, and analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef]
- Yamada, Y.; Yamada, M.; Yoshida, T.; Miyachi, M.; Arai, H. Validating muscle mass cutoffs of four international sarcopenia-working groups in Japanese people using DXA and BIA. J. Cachexia Sarcopenia Muscle 2021, 12, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Kidd, D.; Stewart, G.; Baldry, J.; Johnson, J.; Rossiter, D.; Petruckevitch, A.; Thompson, A.J. The Functional Independence Measure: A comparative validity and reliability study. Disabil. Rehabil. 1995, 17, 10–14. [Google Scholar] [CrossRef]
- Cho, J.; Park, M.; Moon, W.J.; Han, S.H.; Moon, Y. Sarcopenia in patients with dementia: Correlation of temporalis muscle thickness with appendicular muscle mass. Neurol. Sci. 2022, 43, 3089–3095. [Google Scholar] [CrossRef]
- Gomes, G.G.C.; Palinkas, M.; da Silva, G.P.; Gonçalves, C.R.; Lopes, R.F.T.; Verri, E.D.; Fabrin, S.C.V.; Fioco, E.M.; Siéssere, S.; Regalo, S.C.H. Bite Force, Thickness, and Thermographic Patterns of Masticatory Muscles Post-Hemorrhagic Stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106173. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Kim, S.A.; Hwang, I.; Kim, D.E. Utility of ultrasound as a promising diagnostic tool for stroke-related sarcopenia: A retrospective pilot study. Medicine 2022, 101, e30245. [Google Scholar] [CrossRef]
- Dhariwal, S.; Roy, A.; Taneja, S.; Bansal, A.; Gorsi, U.; Singh, S.; De, A.; Verma, N.; Premkumar, M.; Duseja, A.; et al. Assessment of Sarcopenia Using Muscle Ultrasound in Patients With Cirrhosis and Sarcopenic Obesity (AMUSE STUDY). J. Clin. Gastroenterol. 2022. [Google Scholar] [CrossRef]
- Nijholt, W.; Scafoglieri, A.; Jager-Wittenaar, H.; Hobbelen, J.S.M.; van der Schans, C.P. The reliability and validity of ultrasound to quantify muscles in older adults: A systematic review. J. Cachexia Sarcopenia Muscle 2017, 8, 702–712. [Google Scholar] [CrossRef]
- Miura, Y.; Tamai, N.; Kitamura, A.; Yoshida, M.; Takahashi, T.; Mugita, Y.; Tobita, I.; Arita, M.; Urai, T.; Dai, M.; et al. Diagnostic accuracy of ultrasound examination in detecting aspiration and pharyngeal residue in patients with dysphagia: A systematic review and meta-analysis. Jpn. J. Nurs. Sci. 2021, 18, e12396. [Google Scholar] [CrossRef]
- Yesil Cinkir, H.; Colakoglu Er, H. Is temporal muscle thickness a survival predictor in newly diagnosed glioblastoma multiforme? Asia Pac. J. Clin. Oncol. 2020, 16, e223–e227. [Google Scholar] [CrossRef]
- Kameyama, Y.; Ashizawa, R.; Honda, H.; Take, K.; Yoshizawa, K.; Yoshimoto, Y. Sarcopenia Affects Functional Independence Measure motor Scores in Elderly Patients with Stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106615. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.H.; Heo, J.; Baik, M.; Park, H.; Lee, H.S.; Nam, H.S.; Kim, Y.D. Impact of Sarcopenia on Functional Outcomes Among Patients With Mild Acute Ischemic Stroke and Transient Ischemic Attack: A Retrospective Study. Front. Neurol. 2022, 13, 841945. [Google Scholar] [CrossRef]
- Nagano, A.; Nishioka, S.; Wakabayashi, H. Rehabilitation Nutrition for Iatrogenic Sarcopenia and Sarcopenic Dysphagia. J. Nutr. Health Aging 2019, 23, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Aragane, H.; Suzuki, N.; Yoshimura, Y.; Fujiwara, D.; Mori, T.; Kanehisa, Y.; Iida, Y.; Higashi, K.; Yoshimura-Yokoi, Y.; et al. Clinical practice guidelines for rehabilitation nutrition in cerebrovascular disease, hip fracture, cancer, and acute illness: 2020 update. Clin. Nutr. ESPEN 2021, 43, 90–103. [Google Scholar] [CrossRef]
- Sakai, K.; Katayama, M.; Nakajima, J.; Inoue, S.; Koizumi, K.; Okada, S.; Suga, S.; Nomura, T.; Matsuura, N. Temporal muscle thickness is associated with the severity of dysphagia in patients with acute stroke. Arch. Gerontol. Geriatr. 2021, 96, 104439. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).