A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Effects of Multi-Strain Synbiotic in Patients with Functional Diarrhea and High Fecal Calprotectin Levels: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Estimation
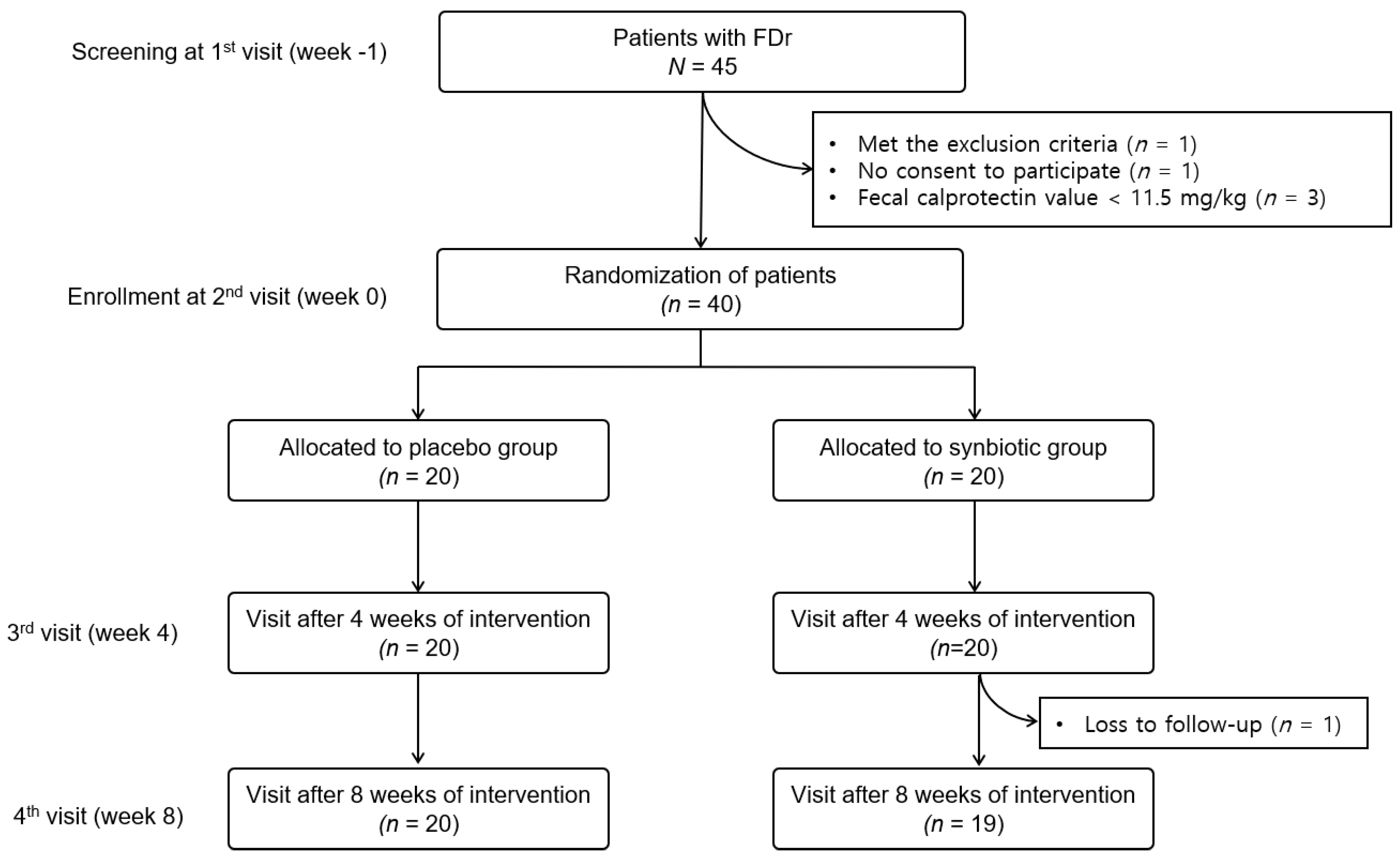
2.2. Study Design and Study Population
2.3. Synbiotic Preparation
2.4. Informed Consent and Ethical Approval
2.5. Measurements
2.5.1. Bowel Symptom Assessment Tools
2.5.2. Fecal Calprotectin and Microbiology Assays
2.6. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Intestinal Symptoms before and after Intervention
3.3. Between and Within-Group Fecal Calprotectin Levels
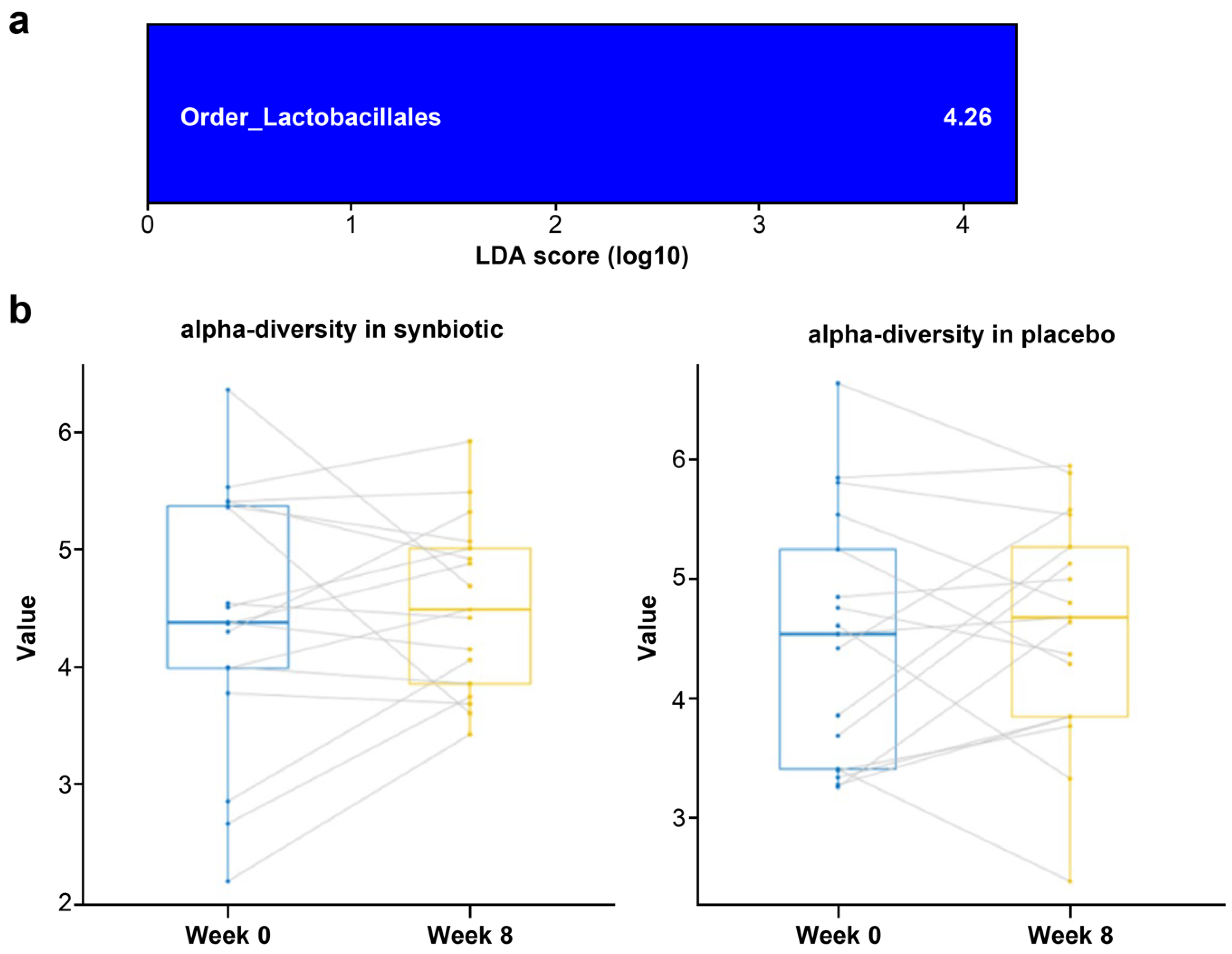
3.4. Fecal Microbiota Analysis
3.5. Safety of the Synbiotic and Placebo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273.e3. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Quigley, E.M.; Santos, J.; Vanner, S.; Vergnolle, N.; Zoetendal, E.G. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016, 150, 1305–1318.e8. [Google Scholar] [CrossRef] [PubMed]
- Carroll, I.M.; Ringel-Kulka, T.; Keku, T.O.; Chang, Y.H.; Packey, C.D.; Sartor, R.B.; Ringel, Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G799–G807. [Google Scholar] [CrossRef]
- Carroll, I.M.; Ringel-Kulka, T.; Siddle, J.P.; Ringel, Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 521-e248. [Google Scholar] [CrossRef]
- Liu, H.N.; Wu, H.; Chen, Y.Z.; Chen, Y.J.; Shen, X.Z.; Liu, T.T. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 331–337. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, C.R.; Kim, K.N. Changes in fecal calprotectin after rifaximin treatment in patients with nonconstipated irritable bowel syndrome. Am. J. Med. Sci. 2019, 357, 23–28. [Google Scholar] [CrossRef]
- Carrasco-Labra, A.; Lytvyn, L.; Falck-Ytter, Y.; Surawicz, C.M.; Chey, W.D. AGA technical review on the evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-D). Gastroenterology 2019, 157, 859–880. [Google Scholar] [CrossRef]
- Singh, P.; Lee, H.N.; Rangan, V.; Ballou, S.; Lembo, J.; Katon, J.; McMahon, C.; Friedlander, D.; Iturrino, J.; Nee, J.; et al. Similarities in clinical and psychosocial characteristics of functional diarrhea and irritable bowel syndrome with diarrhea. Clin. Gastroenterol. Hepatol. 2020, 18, 399–405.e1. [Google Scholar] [CrossRef]
- Shin, S.P.; Choi, Y.M.; Kim, W.H.; Hong, S.P.; Park, J.M.; Kim, J.; Kwon, O.; Lee, E.H.; Hahm, K.B. A double blind, placebo-controlled, randomized clinical trial that breast milk derived-Lactobacillus gasseri BNR17 mitigated diarrhea-dominant irritable bowel syndrome. J. Clin. Biochem. Nutr. 2018, 62, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, T.K. Ways of measuring drinking patterns and the difference they make: Experience with graduated frequencies. J. Subst. Abuse 2000, 12, 33–49. [Google Scholar] [CrossRef]
- Gunnarsson, J.; Simrén, M. Peripheral factors in the pathophysiology of irritable bowel syndrome. Dig. Liver Dis. 2009, 41, 788–793. [Google Scholar] [CrossRef]
- Ohman, L.; Simrén, M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig. Liver Dis. 2007, 39, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Ohman, L.; Simrén, M. Pathogenesis of IBS: Role of inflammation, immunity and neuroimmune interactions. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Parkes, G.C.; Brostoff, J.; Whelan, K.; Sanderson, J.D. Gastrointestinal microbiota in irritable bowel syndrome: Their role in its pathogenesis and treatment. Am. J. Gastroenterol. 2008, 103, 1557–1567. [Google Scholar] [CrossRef]
- Ducrotté, P.; Sawant, P.; Jayanthi, V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef]
- Xu, B.; Liang, S.; Zhao, J.; Li, X.; Guo, J.; Xin, B.; Li, B.; Huo, G.; Ma, W. Bifidobacterium animalis subsp. lactis XLTG11 improves antibiotic-related diarrhea by alleviating inflammation, enhancing intestinal barrier function and regulating intestinal flora. Food Funct. 2022, 13, 6404–6418. [Google Scholar] [CrossRef]
- Liang, D.; Longgui, N.; Guoqiang, X. Efficacy of different probiotic protocols in irritable bowel syndrome: A network meta-analysis. Medicine 2019, 98, e16068. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Turunen, K.; Anapliotis, P.; Zisi, D.; Spiliotis, V.; Kyriacou, A. Impact of a jelly containing short-chain fructo-oligosaccharides and Sideritis euboea extract on human faecal microbiota. Int. J. Food Microbiol. 2009, 135, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.; Hutkins, R.W. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl. Environ. Microbiol. 2000, 66, 2682–2684. [Google Scholar] [CrossRef]
- Paineau, D.; Payen, F.; Panserieu, S.; Coulombier, G.; Sobaszek, A.; Lartigau, I.; Brabet, M.; Galmiche, J.P.; Tripodi, D.; Sacher-Huvelin, S.; et al. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br. J. Nutr. 2008, 99, 311–318. [Google Scholar] [CrossRef]
- Jeurink, P.V.; van Esch, B.C.; Rijnierse, A.; Garssen, J.; Knippels, L.M. Mechanisms underlying immune effects of dietary oligosaccharides. Am. J. Clin. Nutr. 2013, 98, 572S–577S. [Google Scholar] [CrossRef]
- Xie, X.; He, Y.; Li, H.; Yu, D.; Na, L.; Sun, T.; Zhang, D.; Shi, X.; Xia, Y.; Jiang, T.; et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition 2019, 61, 132–142. [Google Scholar] [CrossRef]
- Medina, M.; Izquierdo, E.; Ennahar, S.; Sanz, Y. Differential immunomodulatory properties of Bifidobacterium logum strains: Relevance to probiotic selection and clinical applications. Clin. Exp. Immunol. 2007, 150, 531–538. [Google Scholar] [CrossRef]
- Chapman, C.M.; Gibson, G.R.; Rowland, I. In vitro evaluation of single- and multi-strain probiotics: Inter-species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe 2012, 18, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Jung, S.; Kim, N.; Ahn, H.; Yun, H.; Kim, K.N. A randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of Lactiplantibacillus plantarum CJLP243 in patients with functional diarrhea and high fecal calprotectin levels. Nutrients 2022, 14, 389. [Google Scholar] [CrossRef]
- Yang, B.; Yue, Y.; Chen, Y.; Ding, M.; Li, B.; Wang, L.; Wang, Q.; Stanton, C.; Ross, R.P.; Zhao, J.; et al. Lactobacillus plantarum CCFM1143 alleviates chronic diarrhea via inflammation regulation and gut microbiota modulation: A double-blind, randomized, placebo-controlled study. Front. Immunol. 2021, 12, 746585. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Kim, N.Y.; Kim, S.K.; Ra, C.H. Evaluation of gamma-aminobutyric acid (GABA) production by Lactobacillus plantarum using two-step fermentation. Bioprocess Biosyst. Eng. 2021, 44, 2099–2108. [Google Scholar] [CrossRef]
- Mazzoli, R.; Pessione, E. The neuro-endocrinological role of microbial glutamate and GABA signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Rong, C.; Wang, F.; Liu, X.; Sun, Y.; Zhang, H.T. GABAergic System in Stress: Implications of GABAergic Neuron Subpopulations and the Gut-Vagus-Brain Pathway. Neural Plast. 2020, 2020, e8858415. [Google Scholar] [CrossRef] [PubMed]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef]
- Albring, A.; Wendt, L.; Benson, S.; Witzke, O.; Kribben, A.; Engler, H.; Schedlowski, M. Placebo effects on the immune response in humans: The role of learning and expectation. PLoS ONE 2012, 7, e49477. [Google Scholar] [CrossRef][Green Version]
| Synbiotic (n = 19) | Placebo (n = 20) | p-Value | |
|---|---|---|---|
| Age (years) | 49.8 ± 2.1 | 46.3 ± 2.6 | 0.304 |
| Male, n (%) | 13 (68) | 14 (70) | 0.915 |
| BMI (kg/m2)° | 26.0 ± 1.9 | 26.1 ± 1.0 | 0.178 |
| Current smoker, n (%) | 2 (10.5) | 7 (35.0) | 0.127 |
| Ex-smoker, n (%) | 5 (26.3) | 4 (20.0) | 0.717 |
| Weekly alcohol intake | 5.5 ± 2.6 | 5.4 ± 1.7 | 0.967 |
| Fecal calprotectin (mg/kg) | 111.5 ± 118.5 | 217.5 ± 312.6 | |
| Log-transformed FC | 4.34 ± 0.19 | 4.71 ± 0.25 | 0.254 |
| Hypertension | 4 | 5 | 1.000 |
| Diabetes mellitus | 1 | 2 | 1.000 |
| Dyslipidemia | 7 | 9 | 0.605 |
| Synbiotic | Placebo | Estimate a | p-Value b | |
|---|---|---|---|---|
| Stool frequency (#/day) | ||||
| Week 0 | 1.3 ± 0.1 | 1.6 ± 0.2 | ||
| Week 4 | 1.6 ± 0.1 | 1.7 ± 0.1 | +0.2 | 0.148 |
| Week 8 | 1.6 ± 0.1 | 1.6 ± 0.9 | +0.2 | 0.05 * |
| Within-group comparison | 0.002 ** | 0.625 | ||
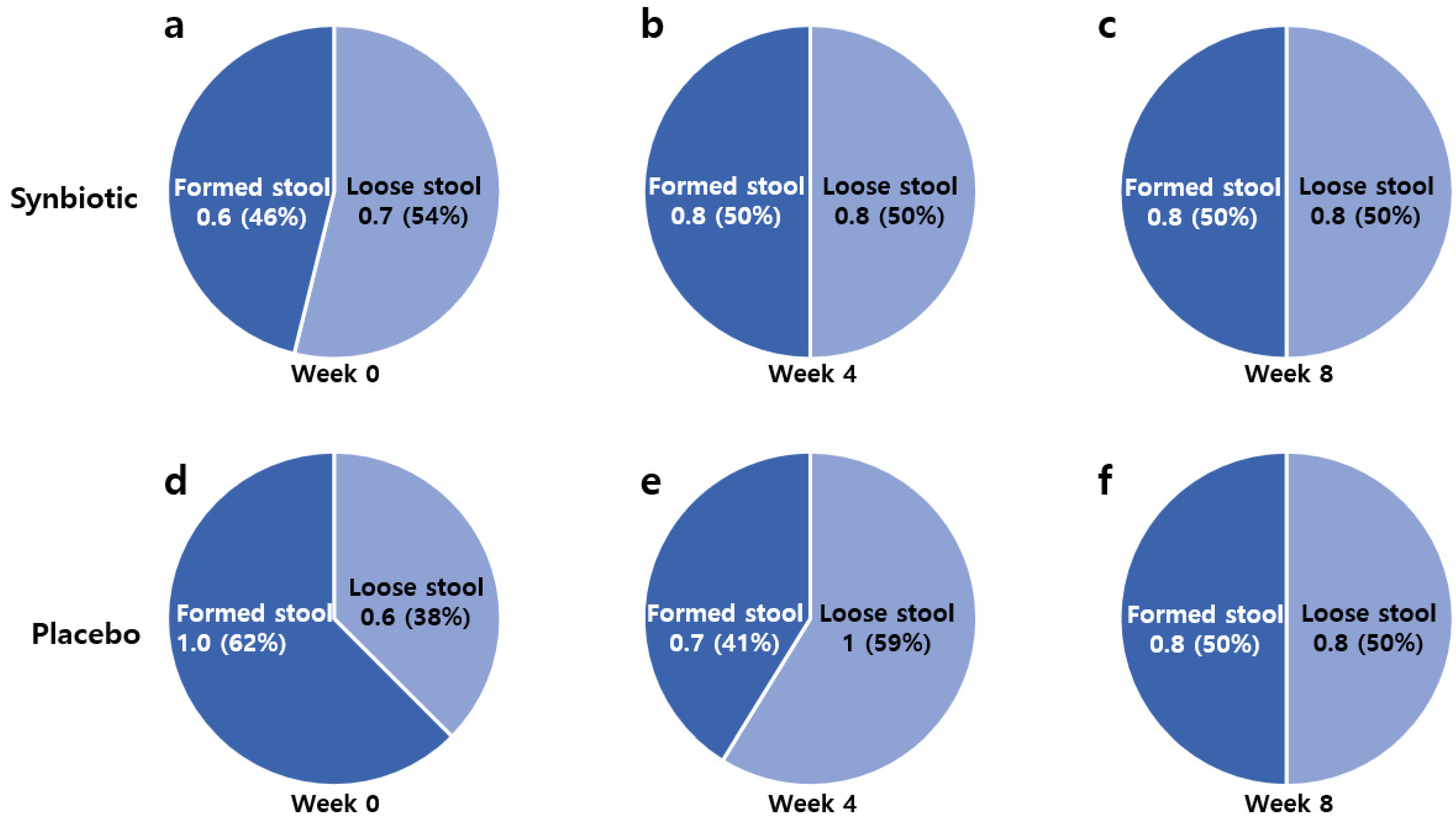
| Loose stool | ||||
| Week 0 | 0.7 ± 0.1 | 0.6 ± 0.1 | ||
| Week 4 | 0.8 ± 0.1 | 1.0 ± 0.1 | −0.3 | 0.084 * |
| Week 8 | 0.8 ± 0.2 | 0.8 ± 0.1 | −0.1 | 0.387 |
| Within-group comparison | 0.658 | 0.093 * | ||
| Formed stool | ||||
| Week 0 | 0.6 ± 0.1 | 1.0 ± 0.2 | ||
| Week 4 | 0.8 ± 0.1 | 0.7 ± 0.1 | +0.5 | 0.009 ** |
| Week 8 | 0.8 ± 0.2 | 0.8 ± 0.1 | +0.4 | 0.028 ** |
| Within-group comparison | 0.067 * | 0.205 | ||
| Bowel movement satisfaction | ||||
| Week 0 | 44.2 ± 4.9 | 45 ± 4.4 | ||
| Week 4 | 60.8 ± 5.0 | 46.5 ± 5.6 | +15.4 | 0.024 ** |
| Week 8 | 63.4 ± 5.3 | 49.4 ± 5.5 | +14.7 | 0.029 ** |
| Within-group comparison | <0.001 ** | 0.363 |
| Synbiotic | Placebo | Estimate a | p-Value b | |
|---|---|---|---|---|
| Week 0 | 4.34 ± 0.19 (111.5 ± 27.2) § | 4.71 ± 0.25 (217.5 ± 70.0) § | ||
| Week 8 | 3.51 ± 0.23 (56.7 ± 14.4) § | 3.91 ± 0.2 (73.6 ± 16.6) § | −0.06 | 0.889 |
| Within-group comparison | 0.006 | 0.008 |
| Synbiotic (n = 19) | Placebo (n = 20) | |||||
|---|---|---|---|---|---|---|
| Baseline | 8 Weeks | p-Value | Baseline | 8 Weeks | p-Value | |
| WBC (×103/μL) | 6.7 ± 0.6 | 6.4 ± 0.4 | 0.250 | 6.3 ± 0.4 | 6.3 ± 0.4 | 0.904 |
| Hb (g/dL) | 14.6 ± 0.3 | 14.6 ± 0.2 | 0.795 | 14.9 ± 0.3 | 14.3 ± 0.3 | 0.005 |
| BUN (mg/dL) | 13.5 ± 0.9 | 13.7 ± 0.9 | 0.841 | 8.8 ± 0.8 | 13.1 ± 0.9 | 0.754 |
| Creatinine (mg/dL) | 0.83 ± 0.03 | 0.84 ± 0.04 | 0.679 | 0.87 ± 0.03 | 0.84 ± 0.03 | 0.154 |
| ALT (IU/L) | 45.9 ± 9.1 | 45.4 ± 9.4 | 0.904 | 26.2 ± 3.2 | 29.6 ± 4.4 | 0.379 |
| AST (IU/L) | 35.1 ± 6.4 | 32.9 ± 5.5 | 0.506 | 22.7 ± 1.8 | 25.3 ± 2.5 | 0.431 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Kim, K.-M.; Youn, S.-M.; Kim, K.-N. A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Effects of Multi-Strain Synbiotic in Patients with Functional Diarrhea and High Fecal Calprotectin Levels: A Pilot Study. Nutrients 2022, 14, 5017. https://doi.org/10.3390/nu14235017
Jung S, Kim K-M, Youn S-M, Kim K-N. A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Effects of Multi-Strain Synbiotic in Patients with Functional Diarrhea and High Fecal Calprotectin Levels: A Pilot Study. Nutrients. 2022; 14(23):5017. https://doi.org/10.3390/nu14235017
Chicago/Turabian StyleJung, Susie, Kwang-Min Kim, Sung-Min Youn, and Kyu-Nam Kim. 2022. "A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Effects of Multi-Strain Synbiotic in Patients with Functional Diarrhea and High Fecal Calprotectin Levels: A Pilot Study" Nutrients 14, no. 23: 5017. https://doi.org/10.3390/nu14235017
APA StyleJung, S., Kim, K.-M., Youn, S.-M., & Kim, K.-N. (2022). A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Effects of Multi-Strain Synbiotic in Patients with Functional Diarrhea and High Fecal Calprotectin Levels: A Pilot Study. Nutrients, 14(23), 5017. https://doi.org/10.3390/nu14235017






