Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology
Abstract
1. Introduction
2. Functional Foods
3. Supplements and Nutraceuticals
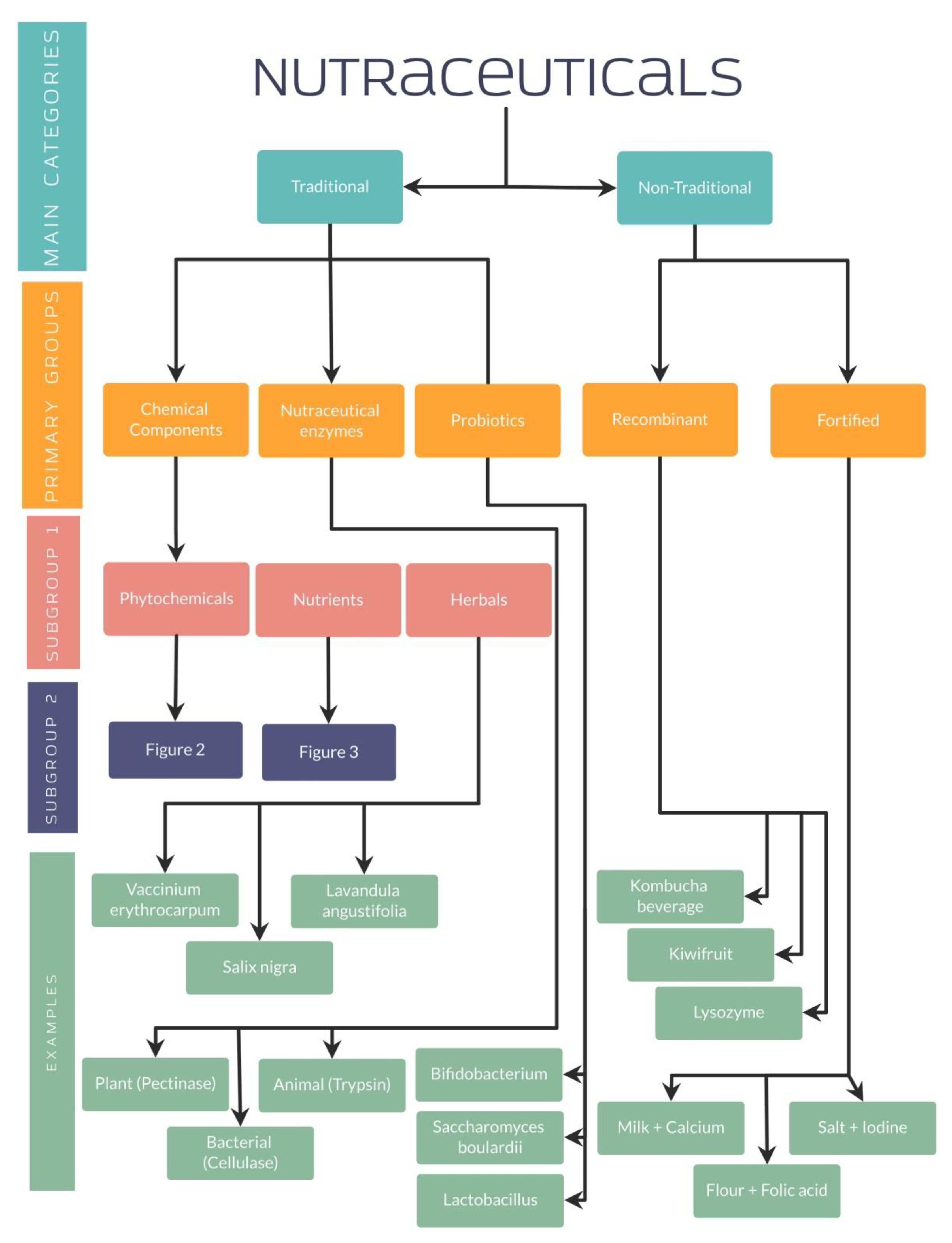
4. Classification of Nutraceuticals
4.1. Traditional and Non-Traditional Nutraceuticals
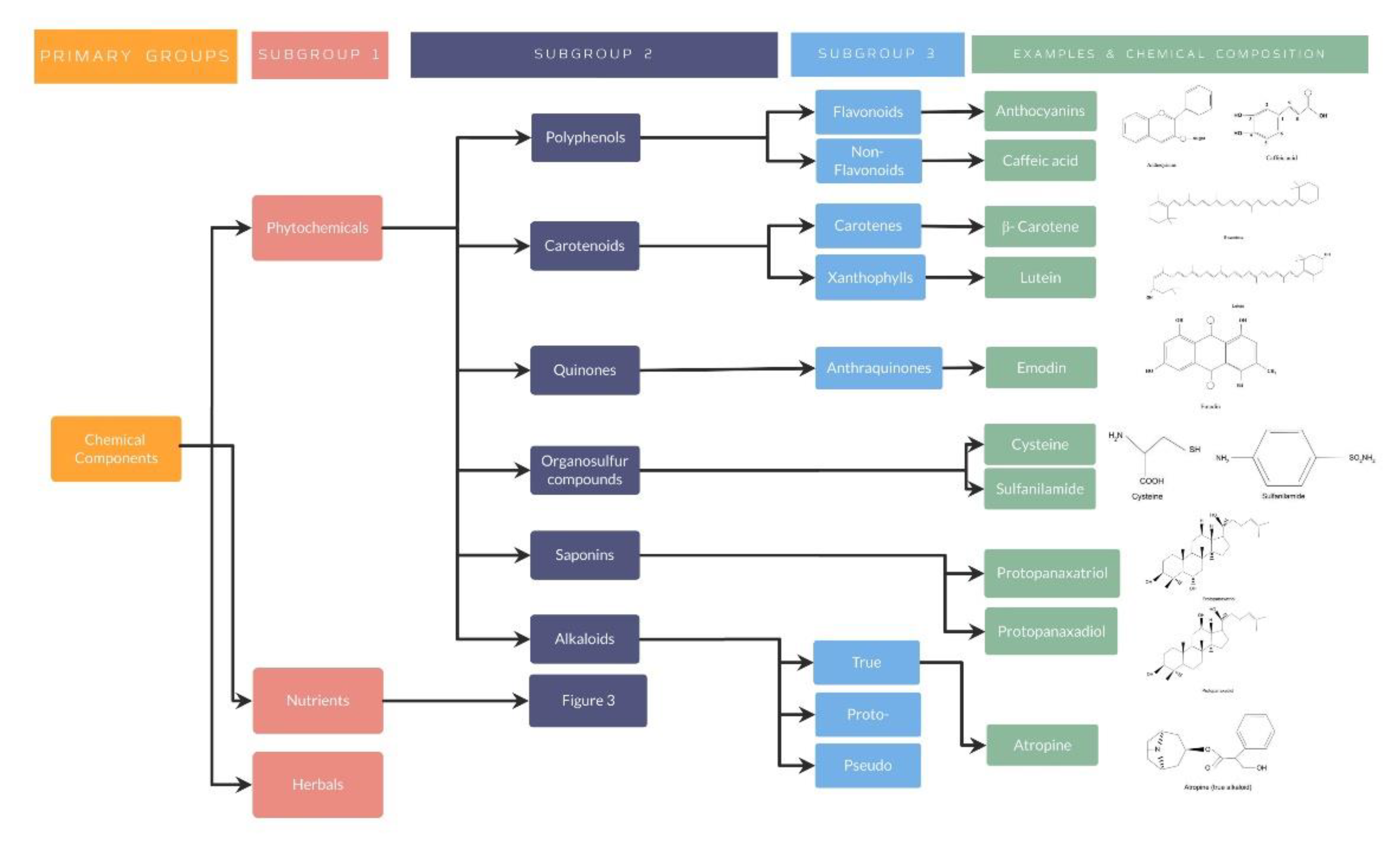
4.1.1. Traditional Nutraceuticals: Chemical Components
Phytochemical Nutraceuticals
- Phytochemical nutraceuticals: Polyphenols
- 2.
- Phytochemical nutraceuticals: Carotenoids
- 3.
- Phytochemical nutraceuticals: Quinones
- 4.
- Phytochemical nutraceuticals: Organosulfur compounds
- 5.
- Phytochemical nutraceuticals: Saponins
- 6.
- Phytochemical nutraceuticals: Alkaloids
Nutrients
- Nutrients: Peptides and Bioactive Peptides
- 2.
- Nutrients: Bioactive Carbohydrates
- 3.
- Nutrients: Fatty Acids
- 4.
- Nutrients: Vitamins
- 5.
- Nutrients: Minerals
Herbals
4.1.2. Traditional Nutraceuticals: Enzymes
4.1.3. Traditional Nutraceuticals: Probiotics
4.1.4. Non-Traditional Nutraceuticals: Recombinant Nutraceuticals
4.1.5. Non-Traditional Nutraceuticals: Fortified Nutraceuticals
5. Use of Nutraceuticals in Ophthalmology
5.1. Presbyopia
5.2. Cataract
5.3. Dry Eye Disease
5.4. Glaucoma
5.5. Age Macular Degeneration
5.6. Diabetic Retinopathy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kador, P.F. Topical applied nutraceutical antioxidant formulation reduces ocular oxidative stress. Funct. Foods Health Dis. 2017, 7, 68–87. [Google Scholar] [CrossRef][Green Version]
- Gatell-Tortajada, J. Oral supplementation with a nutraceutical formulation containing omega-3 fatty acids, vitamins, minerals, and antioxidants in a large series of patients with dry eye symptoms: Results of a prospective study. Clin. Interv. Aging 2016, 11, 571–578. [Google Scholar] [CrossRef][Green Version]
- Bucolo, C.; Musumeci, M.; Salomone, S.; Romano, G.L.; Leggio, G.M.; Gagliano, C.; Reibaldi, M.; Avitabile, T.; Uva, M.G.; Musumeci, S.; et al. Effects of topical fucosyl-lactose, a milk oligosaccharide, on dry eye model: An example of nutraceutical candidate. Front. Pharmacol. 2015, 6, 280. [Google Scholar] [CrossRef]
- Scuteri, D.; Rombolà, L.; Watanabe, C.; Sakurada, S.; Corasaniti, M.T.; Bagetta, G.; Tonin, P.; Russo, R.; Nucci, C.; Morrone, L.A. Impact of nutraceuticals on glaucoma: A systematic review. Prog. Brain Res. 2020, 257, 141–154. [Google Scholar] [CrossRef]
- López-Varela, S.; González-Gross, M.; Marcos, A. Functional foods and the immune system: A review. Eur. J. Clin. Nutr. 2002, 56, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Rastqar, A.; Keshvari, M. Functional Food and Cardiovascular Disease Prevention and Treatment: A Review. J. Am. Coll. Nutr. 2018, 37, 429–455. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. Nutraceuticals for older people: Facts, fictions and gaps in knowledge. Maturitas 2013, 75, 313–334. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–610. [Google Scholar] [CrossRef]
- Nagashima, H.; Sasaki, N.; Amano, S.; Nakamura, S.; Hayano, M.; Tsubota, K. Oral administration of resveratrol or lactic acid bacterium improves lens elasticity. Sci. Rep. 2021, 11, 2174. [Google Scholar] [CrossRef] [PubMed]
- Tsuneyoshi, Y.; Higuchi, A.; Negishi, K.; Tsubota, K. Suppression of presbyopia progression with pirenoxine eye drops: Experiments on rats and non-blinded, randomized clinical trial of efficacy. Sci. Rep. 2017, 7, 6819. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Hashizume, K.; Kishimoto, S.; Tezuka, Y.; Nishigori, H.; Yamamoto, N.; Kondo, Y.; Maruyama, N.; Ishigami, A.; Kurosaka, D. Effect of vitamin C depletion on UVR-B induced cataract in SMP30/GNL knockout mice. Exp. Eye Res. 2012, 94, 85–89. [Google Scholar] [CrossRef]
- Blondin, J.; Baragi, V.; Schwartz, E.; Sadowski, J.A.; Taylor, A. Delay of UV-induced eye lens protein damage in guinea pigs by dietary ascorbate. J. Free Radic. Biol. Med. 1986, 2, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, D.; Naziroglu, M.; Armagan, A.; Demirel, A.; Koroglu, B.K.; Colakoglu, N.; Kukner, A.; Sonmez, T.T. Dietary vitamin C and E modulates oxidative stress induced-kidney and lens injury in diabetic aged male rats through modulating glucose homeostasis and antioxidant systems. Cell Biochem. Funct. 2011, 29, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, D.N.; Pan, Z.; Lu, C.W.; Xue, C.Y.; Aass, I. Curcumin protects against hyperosmoticity-induced IL-1β elevation in human corneal epithelial cell via MAPK pathways. Exp. Eye Res. 2010, 90, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Lee, I.S.; Hyun, S.W.; Jo, K.; Lee, T.G.; Kim, J.S.; Kim, C.S. The Protective Effect of Polygonum cuspidatum (PCE) Aqueous Extract in a Dry Eye Model. Nutrients 2018, 10, 1550. [Google Scholar] [CrossRef]
- Chien, K.J.; Horng, C.T.; Huang, Y.S.; Hsieh, Y.H.; Wang, C.J.; Yang, J.S.; Lu, C.C.; Chen, F.A. Effects of Lycium barbarum (goji berry) on dry eye disease in rats. Mol. Med. Rep. 2018, 17, 809–818. [Google Scholar] [CrossRef]
- Li, L.; Jin, R.; Li, Y.; Yoon, H.S.; Yoon, H.J.; Yoon, K.C. Effects of eye drops containing a mixture of 3% diquafosol sodium and tocopherol acetate (vitamin E) on the ocular surface of murine dry eye. Cutan. Ocul. Toxicol. 2021, 40, 350–358. [Google Scholar] [CrossRef]
- Kamalden, T.A.; Ji, D.; Fawcett, R.J.; Osborne, N.N. Genistein blunts the negative effect of ischaemia to the retina caused by an elevation of intraocular pressure. Ophthalmic Res. 2011, 45, 65–72. [Google Scholar] [CrossRef]
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H.; et al. Topical Curcumin Nanocarriers are Neuroprotective in Eye Disease. Sci. Rep. 2018, 8, 11066. [Google Scholar] [CrossRef]
- Chichili, G.R.; Nohr, D.; Frank, J.; Flaccus, A.; Fraser, P.D.; Enfissi, E.M.A.; Biesalski, H.K. Protective effects of tomato extract with elevated Β-carotene levels on oxidative stress in ARPE-19 cells. Br. J. Nutr. 2006, 96, 643–649. [Google Scholar] [CrossRef]
- Bhatt, P.; Fnu, G.; Bhatia, D.; Shahid, A.; Sutariya, V. Nanodelivery of Resveratrol-Loaded PLGA Nanoparticles for Age-Related Macular Degeneration. AAPS PharmSciTech 2020 2020, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M.; Thandampallayam, M.; Putt, D.; Gierhart, D.L. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. 2014, 11, 8. [Google Scholar] [CrossRef]
- Singh, P.; Tripathi, M.K.; Yasir, M.; Khare, R.; Tripathi, M.K.; Shrivastava, R. Potential Inhibitors for SARS-CoV-2 and Functional Food Components as Nutritional Supplement for COVID-19: A Review. Plant Foods Hum. Nutr. 2020, 75, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Maki, K.C.; Dicklin, M.R.; Feinstein, S.B.; Witchger, M.S.; Bell, M.; McGuire, D.K.; Provost, J.C.; Liker, H.; Aviram, M. Effects of Consumption of Pomegranate Juice on Carotid Intima-Media Thickness in Men and Women at Moderate Risk for Coronary Heart Disease. Am. J. Cardiol. 2009, 104, 936–942. [Google Scholar] [CrossRef]
- Lionetti, V.; Tuana, B.S.; Casieri, V.; Parikh, M.; Pierce, G.N. Importance of functional food compounds in cardioprotection through action on the epigenome. Eur. Heart J. 2019, 40, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Al Alawi, R.; Alhamdani, M.S.S.; Hoheisel, J.D.; Baqi, Y. Antifibrotic and tumor microenvironment modulating effect of date palm fruit (Phoenix dactylifera L.) extracts in pancreatic cancer. Biomed. Pharmacother. 2020, 121, 109522. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Coleman, A.L.; Stone, K.L.; Kodjebacheva, G.; Yu, F.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.A.; Hochberg, M.C.; Topouzis, F.; Badala, F.; et al. Glaucoma Risk and the Consumption of Fruits and Vegetables Among Older Women in the Study of Osteoporotic Fractures. Am. J. Ophthalmol. 2008, 145, 1081–1089. [Google Scholar] [CrossRef]
- Al Owaifeer, A.M.; Al Taisan, A.A. The Role of Diet in Glaucoma: A Review of the Current Evidence. Ophthalmol. Ther. 2018, 7, 19–31. [Google Scholar] [CrossRef]
- Ameratunga, R.; Woon, S.T. Anaphylaxis to hyperallergenic functional foods. Allergy Asthma Clin. Immunol. 2010, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, R.; Crooks, C.; Simmons, G.; Woon, S.T. Health Risks and Adverse Reactions to Functional Foods. Crit. Rev. Food Sci. Nutr. 2015, 56, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.L.; Clarke, L.R. Salicylate intolerance: A masquerader of multiple adverse drug reactions. Case Rep. 2009, 2009, bcr0220091602. [Google Scholar] [CrossRef] [PubMed]
- Brazier, N.C.; Levine, M.A.H. Drug-herb interaction among commonly used conventional medicines: A compendium for health care professionals. Am. J. Ther. 2003, 10, 163–169. [Google Scholar] [CrossRef]
- Low Dog, T.; Markham, M.J. Dietary Supplements and Hemostasis. Consult. Hemost. Thromb. 2013, 561–566. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Rojas Jiménez, S.; Sebastián, J.; Valle, L.; Ocampo, A.U.; Correa Pérez, S.; Perilla Hernández, N.; Sebastián, J.; Cárdenas, M. Consumo de nutracéuticos, una alternativa en la prevención de las enfermedades crónicas no transmisibles. Biosalud 2015, 14, 91–103. [Google Scholar] [CrossRef]
- Nwosu, O.K.; Ubaoji, K.I. Nutraceuticals: History, Classification and Market Demand. In Functional Foods and Nutraceuticals; Springer: Cham, Switzerland, 2020; pp. 13–22. [Google Scholar]
- Alamgir, A.N.M. Vitamins, Nutraceuticals, Food Additives, Enzymes, Anesthetic Aids, and Cosmetics. In Progress in Drug Research; Springer: Cham, Switzerland, 2018; Volume 74, pp. 407–534. [Google Scholar]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation|Bentham Science. Mini Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Classification of Drugs, Nutraceuticals, Functional Food, and Cosmeceuticals; Proteins, Peptides, and Enzymes as Drugs. In Progress in Drug Research; Springer: Cham, Switzerland, 2017; Volume 73, pp. 125–175. [Google Scholar]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.-J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kgamma mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.P.; Liu, B.Y. Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur. J. Med. Chem. 2016, 121, 47–57. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, B.-f.; Hu, Q.; Liu, X.-p.; Chen, J. Syringic acid mitigates myocardial ischemia reperfusion injury by activating the PI3K/Akt/GSK-3β signaling pathway. Biochem. Biophys. Res. Commun. 2020, 531, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Islam, M.S. Caffeic Acid Protects against Iron-Induced Cardiotoxicity by Suppressing Angiotensin-Converting Enzyme Activity and Modulating Lipid Spectrum, Gluconeogenesis and Nucleotide Hydrolyzing Enzyme Activities. Biol. Trace Elem. Res. 2021, 199, 1052–1061. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, D.; Han, H.; Cao, Y.; Zhu, H.; Cui, G. Caffeic acid phenethyl ester protects against doxorubicin-induced cardiotoxicity and increases chemotherapeutic efficacy by regulating the unfolded protein response. Food Chem. Toxicol. 2022, 159, 112770. [Google Scholar] [CrossRef] [PubMed]
- Neto-Neves, E.M.; da Silva Maia Bezerra Filho, C.; Dejani, N.N.; de Sousa, D.P. Ferulic Acid and Cardiovascular Health: Therapeutic and Preventive Potential. Mini Rev. Med. Chem. 2021, 21, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rui, Y.-x.; Guo, S.-d.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, Q.; Zhang, L.; Qiao, X.; Zhang, Y.; Tang, F.; Li, Z. Effect of CAPE-pNO2 against type 2 diabetes mellitus via the AMPK/GLUT4/ GSK3β/PPARα pathway in HFD/STZ-induced diabetic mice. Eur. J. Pharmacol. 2019, 853, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Wang, P.; Ye, X.-l.; Liu, R.; Chen, H.-l.; Liang, X.; Li, W.-l.; Zhang, X.-d.; Qin, X.-j.; Bai, H.; Zhang, W.; et al. Mechanism of acute lung injury due to phosgene exposition and its protection by cafeic acid phenethyl ester in the rat. Exp. Toxicol. Pathol. 2013, 65, 311–318. [Google Scholar] [CrossRef]
- Mu, M.; Zuo, S.; Wu, R.M.; Deng, K.S.; Lu, S.; Zhu, J.J.; Zou, G.L.; Yang, J.; Cheng, M.L.; Zhao, X.K. Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF- β/Smad signaling pathway. Drug Des. Dev. Ther. 2018, 12, 4107–4115. [Google Scholar] [CrossRef]
- Ellison, S.L. Carotenoids: Physiology. Encycl. Food Health 2016, 670–675. [Google Scholar] [CrossRef]
- Papas, A.M. Vitamin E TPGS and its applications in nutraceuticals. In Nutraceuticals, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 991–1010. [Google Scholar]
- Harrison, E.H.; Curley, R.W. Carotenoids and Retinoids: Nomenclature, Chemistry, and Analysis. Subcell. Biochem. 2016, 81, 1–19. [Google Scholar] [CrossRef]
- Toti, E.; Chen, C.-Y.O.; Palmery, M.; Villaño Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxidative Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef]
- Fazal, Y.; Fatima, S.N.; Shahid, S.M.; Mahboob, T. Nephroprotective effects of b-carotene on ACE gene expression, oxidative stress and antioxidant status in thioacetamide induced renal toxicity in rats. Pak. J. Pharm. Sci. 2016, 29, 1139–1144. [Google Scholar] [PubMed]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein inhibits breast cancer cell growth by suppressing antioxidant and cell survival signals and induces apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.-C.; Wu, Y.-C.; Chen, Z.-W.; Yang, W.-C. Naturally Occurring Anthraquinones: Chemistry and Therapeutic Potential in Autoimmune Diabetes. Evid.-Based Complement. Altern. Med. 2015, 2015, 357357. [Google Scholar] [CrossRef] [PubMed]
- Malik, E.M.; Müller, C.E. Anthraquinones As Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.G. Health functions and structure–activity relationships of natural anthraquinones from plants. Food Funct. 2018, 9, 6063–6080. [Google Scholar] [CrossRef] [PubMed]
- Abderrahmane, B.; Noureddine, C.; Meriem, D.; Haythem, A.S.; Arrar, L.; Mohammad, S.M. Free radical scanvenging and antioxidant effects of some anthraquinone derivatives. Med. Chem. 2011, 7, 639–644. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Belinskaia, D.A.; Ukolov, A.I.; Jenkins, R.O.; Avdonin, P.V. Organosulfur compounds as nutraceuticals. In Nutraceuticals, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 911–924. [Google Scholar]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur Compounds: A Review of Their Anti-inflammatory Effects in Human Health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Chukwuebuka, E.; Genevieve, T. Functional Foods and Nutraceuticals; Springer: Cham, Switzerland, 2020; Volume 1, XII, p. 642. [Google Scholar] [CrossRef]
- Petropoulos, S.; Di Gioia, F.; Ntatsi, G. Vegetable Organosulfur Compounds and their Health Promoting Effects. Curr. Pharm. Des. 2017, 23, 2850–2875. [Google Scholar] [CrossRef] [PubMed]
- Khubber, S.; Hashemifesharaki, R.; Mohammadi, M.; Gharibzahedi, S.M.T. Garlic (Allium sativum L.): A potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr. J. 2020, 19, 124. [Google Scholar] [CrossRef]
- Kim, I.H.; Choi, J.W.; Lee, M.K.; Kwon, C.J.; Nam, T.J. Anti-obesity effects of pectinase and cellulase enzyme-treated Ecklonia cava extract in high-fat diet-fed C57BL/6N mice. Int. J. Mol. Med. 2018, 41, 924–934. [Google Scholar] [CrossRef]
- Moriarty, R.; Naithani, R.; Surve, B. Organosulfur compounds in cancer chemoprevention. Mini Rev. Med. Chem. 2007, 7, 827–838. [Google Scholar] [CrossRef]
- Percival, S.S. Aged Garlic Extract Modifies Human Immunity. J. Nutr. 2016, 146, 433S–436S. [Google Scholar] [CrossRef] [PubMed]
- Nantz, M.P.; Rowe, C.A.; Muller, C.E.; Creasy, R.A.; Stanilka, J.M.; Percival, S.S. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind, placebo-controlled nutrition intervention. Clin. Nutr. 2012, 31, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Byran, G.; Jupudi, S.; Vadivelan, R. Activity of phytochemical constituents of black pepper, ginger, and garlic against coronavirus (COVID-19): An in silico approach. Int. J. Health Allied Sci. 2022, 9, 43–50. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Kumar, A.; Srivastava, A.; Jha, N.K. Screening of potent inhibitors against 2019 novel coronavirus (COVID-19) from alliumsativum and allium cepa: An in silico approach. Biointerface Res. Appl. Chem. 2020, 11, 7981–7993. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Shi, J.; Arunasalam, K.; Yeung, D.; Kakuda, Y.; Mittal, G.; Jiang, Y. Saponins from Edible Legumes: Chemistry, Processing, and Health Benefits. J. Med. Food 2004, 7, 67–78. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Choi, Y.H.; Cha, M.R.; Yang, H.J.; Park, S. Platyconic acid, a saponin from Platycodi radix, improves glucose homeostasis by enhancing insulin sensitivity in vitro and in vivo. Eur. J. Nutr. 2012, 51, 529–540. [Google Scholar] [CrossRef]
- Casciaro, B.; Mangiardi, L.; Cappiello, F.; Romeo, I.; Loffredo, M.R.; Iazzetti, A.; Calcaterra, A.; Goggiamani, A.; Ghirga, F.; Mangoni, M.L.; et al. Naturally-Occurring Alkaloids of Plant Origin as Potential Antimicrobials against Antibiotic-Resistant Infections. Molecules 2020, 25, 3619. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Kim, K.H.; Noh, H.J.; Choi, S.U.; Lee, K.R. Isohericenone, a new cytotoxic isoindolinone alkaloid from Hericium erinaceum. J. Antibiot. 2012, 65, 575–577. [Google Scholar] [CrossRef]
- Arpha, K.; Phosri, C.; Suwannasai, N.; Mongkolthanaruk, W.; Sodngam, S. Astraodoric acids A-D: New lanostane triterpenes from edible mushroom astraeus odoratus and their anti-mycobacterium tuberculosis H37Ra and cytotoxic activity. J. Agric. Food Chem. 2012, 60, 9834–9841. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef]
- Griffith, G.L.; Kasus-Jacobi, A.; Pereira, H.A. Bioactive Antimicrobial Peptides as Therapeutics for Corneal Wounds and Infections. Adv. Wound Care 2017, 6, 175–190. [Google Scholar] [CrossRef]
- Griffith, G.L.; Kasus-Jacobi, A.; Lerner, M.R.; Anne Pereira, H. Corneal Wound Healing, a Newly Identified Function of CAP37, Is Mediated by Protein Kinase C Delta (PKCδ). Investig. Ophthalmol. Vis. Sci. 2014, 55, 4886–4895. [Google Scholar] [CrossRef][Green Version]
- Lee, T.D.; Gonzalez, M.L.; Kumar, P.; Chary-Reddy, S.; Grammas, P.; Pereira, H.A. CAP37, a novel inflammatory mediator: Its expression in endothelial cells and localization to atherosclerotic lesions. Am. J. Pathol. 2002, 160, 841–848. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kawaguchi, M.; Kitamura, K.; Narumiya, S.; Kawamura, M.; Tengan, I.; Nishimoto, S.; Hanamure, Y.; Majima, Y.; Tsubura, S.; et al. An Exploratory Study on the Anti-inflammatory Effects of Fucoidan in Relation to Quality of Life in Advanced Cancer Patients. Integr. Cancer Ther. 2018, 17, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The lignicolous fungus Trametes versicolor (L.) Lloyd (1920): A promising natural source of antiradical and AChE inhibitory agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Z.; Cui, R.; Chu, H. Polysaccharopeptide from Trametes versicolor blocks inflammatory osteoarthritis pain-morphine tolerance effects via activating cannabinoid type 2 receptor. Int. J. Biol. Macromol. 2019, 126, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Rahman, M.; Zaman, S.; Jahangir, T.A.; Razu, M.H. Omega-3 Polyunsaturated Fatty Acids from Algae. Recent Adv. Microalgal Biotechnol. 2015, 43-5. [Google Scholar]
- Zhang, A.C.; Singh, S.; Craig, J.P.; Downie, L.E. Omega-3 fatty acids and eye health: Opinions and self-reported practice behaviors of optometrists in Australia and New Zealand. Nutrients 2020, 12, 1179. [Google Scholar] [CrossRef]
- Pellegrini, M.; Senni, C.; Bernabei, F.; Cicero, A.F.G.; Vagge, A.; Maestri, A.; Scorcia, V.; Giannaccare, G. The Role of Nutrition and Nutritional Supplements in Ocular Surface Diseases. Nutrients 2020, 12, 952. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Ng, S.M.; Lindsley, K.B.; Akpek, E.K. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst. Rev. 2019, 12, CD011016. [Google Scholar] [CrossRef]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Adv. Dermatol. Allergol. Postępy Dermatol. I Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S. Chapter 5—Vitamin A and provitamin A carotenoids. In Present Knowledge in Nutrition, 11th ed.; Marriott, B.P., Birt, D.F., Stallings, V.A., Yates, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 73–91. [Google Scholar] [CrossRef]
- Information, N.C.F.B. PubChem Compound Summary for CID 638015, Retinal. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Retinal (accessed on 28 April 2020).
- Proinsias, K.; Giedyk, M.; Gryko, D. Vitamin B12: Chemical modifications. Chemical Society Reviews 2013, 42, 6605–6619. [Google Scholar] [CrossRef] [PubMed]
- Romain, M.; Sviri, S.; Linton, D.M.; Stav, I.; Van Heerden, P.V. The role of Vitamin B12 in the critically ill—A review. Anaesth. Intensive Care 2016, 44, 447–452. [Google Scholar] [CrossRef]
- Alonso, E.R.; León, I.; Alonso, J.L. The role of the intramolecular interactions in the structural behavior of biomolecules: Insights from rotational spectroscopy. In Intra- and Intermolecular Interactions between Non-Covalently Bonded Species; Elsevier: Amsterdam, Netherlands, 2021; pp. 93–141. [Google Scholar] [CrossRef]
- Macan, A.M.; Kraljević, T.G.; Raić-Malić, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef] [PubMed]
- Peponis, V.; Papathanasiou, M.; Kapranou, A.; Magkou, C.; Tyligada, A.; Melidonis, A.; Droso, T.; Sitaras, N.M. Protective role of oral antioxidant supplementation in ocular surface of diabetic patients. Br. J. Ophthalmol. 2002, 86, 1369–1373. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Okamura, W.H.; Midland, M.M.; Hammond, M.W.; Abd Rahman, N.; Dormanen, M.C.; Nemere, I.; Norman, A.W. Chemistry and conformation of vitamin D molecules. J. Steroid Biochem. Mol. Biol. 1995, 53, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R. Selenium: Chemistry, Analysis, Function and Effects; Royal Society of Chemistry: London, UK, 2015; p. 642. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Barak, P.; Helmke, P.A. The Chemistry of Zinc. In Zinc in Soils and Plants; Springer: Dordrecht, The Netherlands, 1993; pp. 1–13. [Google Scholar] [CrossRef]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef]
- Grahn, B.H.; Paterson, P.G.; Gottschall-Pass, K.T.; Zhang, Z. Zinc and the Eye. J. Am. Coll. Nutr. 2001, 20, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhães, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Front. Pharmacol. 2020, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Pokkalath, A.S.; Sawant, A.; Sawarkar, S.P. Herbal medicine for ocular diseases: An age old therapy and its future perspective. J. Drug Deliv. Sci. Technol. 2022, 68, 102979. [Google Scholar] [CrossRef]
- De Almeida Alvarenga, L.; Borges, N.A.; Moreira, L.D.S.G.; Resende Teixeira, K.T.; Carraro-Eduardo, J.C.; Dai, L.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Cranberries—potential benefits in patients with chronic kidney disease. Food Funct. 2019, 10, 3103–3112. [Google Scholar] [CrossRef]
- Gbinigie, O.A.; Spencer, E.A.; Heneghan, C.J.; Lee, J.J.; Butler, C.C. Cranberry Extract for Symptoms of Acute, Uncomplicated Urinary Tract Infection: A Systematic Review. Antibiotics 2020, 10, 12. [Google Scholar] [CrossRef]
- Krueger, C.G.; Reed, J.D.; Feliciano, R.P.; Howell, A.B. Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Anal. Bioanal. Chem. 2013, 405, 4385–4395. [Google Scholar] [CrossRef]
- Jepson, R.G.; Williams, G.; Craig, J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, H.; Gu, L. American cranberries and health benefits—An evolving story of 25 years. J. Sci. Food Agric. 2020, 100, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Cranberries: Ripe for more cancer research? J. Sci. Food Agric. 2011, 91, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sahu, D.; Das, H.R.; Sharma, D. Amelioration of collagen-induced arthritis by Salix nigra bark extract via suppression of pro-inflammatory cytokines and oxidative stress. Food Chem. Toxicol. 2011, 49, 3395–3406. [Google Scholar] [CrossRef]
- da Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; da Silva Melo, D.A.; Donadio, M.V.F.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.B.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Ciências 2015, 87, 1397–1408. [Google Scholar] [CrossRef]
- Seo, E.; Shin, Y.K.; Hsieh, Y.S.; Lee, J.M.; Seol, G.H. Linalyl acetate as a potential preventive agent against muscle wasting in rheumatoid arthritis rats chronically exposed to nicotine. J. Pharmacol. Sci. 2021, 147, 27–32. [Google Scholar] [CrossRef]
- Aboutaleb, N.; Jamali, H.; Abolhasani, M.; Pazoki Toroudi, H. Lavender oil (Lavandula angustifolia) attenuates renal ischemia/reperfusion injury in rats through suppression of inflammation, oxidative stress and apoptosis. Biomed. Pharmacother. 2019, 110, 9–19. [Google Scholar] [CrossRef]
- Donelli, D.; Antonelli, M.; Bellinazzi, C.; Gensini, G.F.; Firenzuoli, F. Effects of lavender on anxiety: A systematic review and meta-analysis. Phytomedicine 2019, 65, 153099. [Google Scholar] [CrossRef]
- Srivastava, P.; Tiwari, A. A New Insight of Herbal Promises Against Ocular Disorders: An Occuloinformatics Approach. Curr. Top. Med. Chem. 2016, 16, 634–654. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Ramírez-Suarez, J.C.; Yada, R.Y. Plant proteases for bioactive peptides release: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2147–2163. [Google Scholar] [CrossRef]
- Cho, H.D.; Kim, J.H.; Won, Y.S.; Moon, K.D.; Seo, K.I. Inhibitory Effects of Pectinase-Treated Prunus Mume Fruit Concentrate on Colorectal Cancer Proliferation and Angiogenesis of Endothelial Cells. J. Food Sci. 2019, 84, 3284–3295. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Z.; Guo, M.M.; Hua, Y.F.; Cao, D.; Zhang, C.M. Enzymatic preparation of immunomodulating hydrolysates from soy proteins. Bioresour. Technol. 2008, 99, 8873–8879. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.L.; Zamith-Miranda, D.; Martins, F.S.; Bozza, F.A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E.T.A.; Douradinha, B. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: Is there room for improvement? Appl. Microbiol. Biotechnol. 2015, 99, 6563–6570. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Timson, D.J.; Annapure, U.S. Antioxidant properties and global metabolite screening of the probiotic yeast Saccharomyces cerevisiae var. boulardii. J. Sci. Food Agric. 2017, 97, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Smecuol, E.; Hwang, H.J.; Sugai, E.; Corso, L.; Cherñavsky, A.C.; Bellavite, F.P.; González, A.; Vodánovich, F.; Moreno, M.L.; Vázquez, H.; et al. Exploratory, Randomized, Double-blind, Placebo-controlled Study on the Effects of Bifidobacterium infantis Natren Life Start Strain Super Strain in Active Celiac Disease. J. Clin. Gastroenterol. 2013, 47, 139–147. [Google Scholar] [CrossRef]
- Olivares, M.; Castillejo, G.; Varea, V.; Sanz, Y. Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. Br. J. Nutr. 2014, 112, 30–40. [Google Scholar] [CrossRef]
- Quagliariello, A.; Aloisio, I.; Bozzi Cionci, N.; Luiselli, D.; D’Auria, G.; Martinez-Priego, L.; Pérez-Villarroya, D.; Langerholc, T.; Primec, M.; Mičetić-Turk, D.; et al. Effect of Bifidobacterium breve on the Intestinal Microbiota of Coeliac Children on a Gluten Free Diet: A Pilot Study. Nutrients 2016, 8, 660. [Google Scholar] [CrossRef]
- Caio, G.; Riegler, G.; Patt Urelli, M.; Facchiano, A.; De Magistris, L.; Sapone, A. Pathophysiology of non-celiac gluten sensitivity: Where are we now? Minerva Gastroenterol. E Dietol. 2017, 63, 16–21. [Google Scholar] [CrossRef]
- D’Arienzo, R.; Stefanile, R.; Maurano, F.; Mazzarella, G.; Ricca, E.; Troncone, R.; Auricchio, S.; Rossi, M. Immunomodulatory Effects of Lactobacillus casei Administration in a Mouse Model of Gliadin-Sensitive Enteropathy. Scand. J. Immunol. 2011, 74, 335–341. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef]
- Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Čanadanović-Brunet, J.M. Influence of starter cultures on the antioxidant activity of kombucha beverage. Food Chem. 2011, 127, 1727–1731. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Tang, B.; Liu, Y.; Guo, C.; Yang, P.; Yu, T.; Li, R.; Zhao, J.; Zhang, L.; et al. Characterization of Bioactive Recombinant Human Lysozyme Expressed in Milk of Cloned Transgenic Cattle. PLoS ONE 2011, 6, e17593. [Google Scholar] [CrossRef]
- Cormick, G.; Betran, A.P.; Romero, I.B.; Cormick, M.S.; Belizán, J.M.; Bardach, A.; Ciapponi, A. Effect of Calcium Fortified Foods on Health Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Nutrition and bone health. Oral Dis. 2017, 23, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Tablante, E.C.; Pachón, H.; Guetterman, H.M.; Finkelstein, J.L. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database Syst. Rev. 2019, 2019, 31257574. [Google Scholar] [CrossRef]
- Assessment, S.C.o.H.T. Benefits and Risks of Fortifying Flour with Folic Acid to Reduce the Risk of Neural Tube Defects: A Systematic Review; Swedish Council on Technology Assessment in Health Care (SBU): Stockholm, Swedish, 2007. [Google Scholar] [PubMed]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2018, 78, 34–44. [Google Scholar] [CrossRef]
- Joussen, A.M.; Rohrschneider, K.; Reichling, J.; Kirchhof, B.; Kruse, F.E. Treatment of Corneal Neovascularization with Dietary Isoflavonoids and Flavonoids. Exp. Eye Res. 2000, 71, 483–487. [Google Scholar] [CrossRef]
- Wang, B.Z.; Zou, Y.; Li, H.; Yan, H.; Pan, J.S.; Yuan, Z.L. Genistein Inhibited Retinal Neovascularization and Expression of Vascular Endothelial Growth Factor and Hypoxia Inducible Factor 1α in a Mouse Model of Oxygen-Induced Retinopathy. J. Ocul. Pharmacol. Ther. 2005, 21, 107–113. [Google Scholar] [CrossRef]
- Huang, R.; Shi, F.; Lei, T.; Song, Y.; Hughes, C.L.; Liu, G. Effect of the Isoflavone Genistein Against Galactose-Induced Cataracts in Rats. Exp. Biol. Med. 2007, 232, 118–125. [Google Scholar] [CrossRef]
- Yilmaz, A.; Yildirim, Ö.; Tamer, L.; Öz, Ö.; Cinel, L.; Vatansever, H.; Değirmenci, U.; Kanik, A.; Atik, U. Effects of Caffeic Acid Phenethyl Ester on Endotoxin-Induced Uveitis in Rats. Curr. Eye Res. 2005, 30, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Pittalà, V.; Salerno, L.; Romeo, G.; Siracusa, M.A.; Modica, M.N.; Romano, G.L.; Salomone, S.; Drago, F.; Bucolo, C. Effects of novel hybrids of caffeic acid phenethyl ester and NSAIDs on experimental ocular inflammation. Eur. J. Pharmacol. 2015, 752, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Şahin, A.; Kürşat Cingü, A.; Kaya, S.; Türkcü, G.; Arı, Ş.; Evliyaoğlu, O.; Çınar, Y.; Türkcü, F.M.; Yüksel, H.; Murat, M.; et al. The protective effects of caffeic acid phenethyl ester in isoniazid and ethambutol-induced ocular toxicity of rats. Cutan. Ocul. Toxicol. 2013, 32, 228–233. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Wen, D.; Ren, C.; Chen, S.; Zhang, Z.; Hu, L.; Yu, Z.; Tombran-Tink, J.; Zhang, X.; et al. Neuroprotection of retinal cells by Caffeic Acid Phenylethyl Ester (CAPE) is mediated by mitochondrial uncoupling protein UCP2. Neurochem. Int. 2021, 151, 105214. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.A.; Karpecki, P.M.; Dorca, A.; Chiva-Razavi, S.; Floyd, H.; Barnes, E.; Wuttke, M.; Donnenfeld, E. Presbyopia—A Review of Current Treatment Options and Emerging Therapies. Clin. Ophthalmol. 2021, 15, 2167–2178. [Google Scholar] [CrossRef]
- Sharma, G.; Chiva-Razavi, S.; Viriato, D.; Naujoks, C.; Patalano, F.; Bentley, S.; Findley, A.; Johnson, C.; Arbuckle, R.; Wolffsohn, J. Patient-reported outcome measures in presbyopia: A literature review. BMJ Open Ophthalmol 2020, 5, e000453. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Shimizu, Y.; Takahashi, S.; Matsuoka, S.; Yui, K. Effect of Multiple Dietary Supplement Containing Lutein, Astaxanthin, Cyanidin-3-Glucoside, and DHA on Accommodative Ability. Curr. Med. Chem. 2014, 14, 114–125. [Google Scholar] [CrossRef]
- Horng, C.-T.; Ma, J.-W.; Shieh, P.-C. Improvement of Presbyopia Using a Mixture of Traditional Chinese Herbal Medicines, Including Cassiae Semen, Wolfberry, and Dendrobium huoshanense. Evid. Based Complement. Altern. Med. 2021, 2021, 9902211. [Google Scholar] [CrossRef]
- Grzybowski, A.; Markeviciute, A.; Zemaitiene, R. A Review of Pharmacological Presbyopia Treatment. Asia Pac. J. Ophthalmol. 2020, 9, 226–233. [Google Scholar] [CrossRef]
- Kaur, A.; Gupta, V.; Christopher, A.F.; Malik, M.A.; Bansal, P. Nutraceuticals in prevention of cataract—An evidence based approach. Saudi J. Ophthalmol. 2017, 31, 30–37. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Donaldson, C.I.; Lim, J.C.; Donaldson, P.J. Nutritional Strategies to Prevent Lens Cataract: Current Status and Future Strategies. Nutrients 2019, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Afshari, N.A. The global state of cataract blindness. Curr. Opin. Ophthalmol. 2017, 28, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. Prog. Mol. Biol. Transl. Sci. 2015, 134, 169–201. [Google Scholar] [CrossRef]
- Wei, L.; Liang, G.; Cai, C.; Lv, J. Association of vitamin C with the risk of age-related cataract: A meta-analysis. Acta Ophthalmol. 2016, 94, e170–e176. [Google Scholar] [CrossRef] [PubMed]
- Mares-Perlman, J.A.; Lyle, B.J.; Klein, R.; Fisher, A.I.; Brady, W.E.; VandenLangenberg, G.M.; Trabulsi, J.N.; Palta, M. Vitamin supplement use and incident cataracts in a population-based study. Arch. Ophthalmol. 2000, 118, 1556–1563. [Google Scholar] [CrossRef]
- Robertson, J.M.; Donner, A.P.; Trevithick, J.R. A possible role for vitamins C and E in cataract prevention. Am. J. Clin. Nutr. 1991, 53, 346S–351S. [Google Scholar] [CrossRef]
- Vitale, S.; West, S.; Hallfrisch, J.; Alston, C.; Wang, F.; Moorman, C.; Muller, D.; Singh, V.; Taylor, H.R. Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiology 1993, 4, 195–203. [Google Scholar] [CrossRef]
- Weikel, K.A.; Garber, C.; Baburins, A.; Taylor, A. Nutritional modulation of cataract. Nutr. Rev. 2014, 72, 30–47. [Google Scholar] [CrossRef]
- Jacques, P.F.; Chylack, L.T., Jr.; Hankinson, S.E.; Khu, P.M.; Rogers, G.; Friend, J.; Tung, W.; Wolfe, J.K.; Padhye, N.; Willett, W.C.; et al. Long-term nutrient intake and early age-related nuclear lens opacities. Arch. Ophthalmol. 2001, 119, 1009–1019. [Google Scholar] [CrossRef]
- Leske, M.C.; Chylack, L.T., Jr.; Wu, S.Y. The Lens Opacities Case-Control Study. Risk factors for cataract. Arch. Ophthalmol. 1991, 109, 244–251. [Google Scholar] [CrossRef]
- Valero, M.P.; Fletcher, A.E.; De Stavola, B.L.; Vioque, J.; Alepuz, V.C. Vitamin C is associated with reduced risk of cataract in a Mediterranean population. J. Nutr. 2002, 132, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.D.; Vashist, P.; Gupta, S.K.; Young, I.S.; Maraini, G.; Camparini, M.; Jayanthi, R.; John, N.; Fitzpatrick, K.E.; Chakravarthy, U.; et al. Inverse association of vitamin C with cataract in older people in India. Ophthalmology 2011, 118, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.; Knudtson, M.D.; Lee, K.E.; Reinke, J.O.; Danforth, L.G.; Wealti, A.M.; Moore, E.; Klein, R. Supplements and age-related eye conditions the beaver dam eye study. Ophthalmology 2008, 115, 1203–1208. [Google Scholar] [CrossRef]
- Dherani, M.; Murthy, G.V.; Gupta, S.K.; Young, I.S.; Maraini, G.; Camparini, M.; Price, G.M.; John, N.; Chakravarthy, U.; Fletcher, A.E. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian population. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3328–3335. [Google Scholar] [CrossRef] [PubMed]
- Berendschot, T.T.; Broekmans, W.M.; Klopping-Ketelaars, I.A.; Kardinaal, A.F.; Van Poppel, G.; Van Norren, D. Lens aging in relation to nutritional determinants and possible risk factors for age-related cataract. Arch. Ophthalmol. 2002, 120, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, E.; Ortega, R.M.; Lopez-Sobaler, A.M.; Aparicio, A.; Bermejo, L.M.; Marin-Arias, L.I. The relationship between antioxidant nutrient intake and cataracts in older people. Int. J. Vitam. Nutr. Res. 2006, 76, 359–366. [Google Scholar] [CrossRef]
- Karppi, J.; Laukkanen, J.A.; Kurl, S. Plasma lutein and zeaxanthin and the risk of age-related nuclear cataract among the elderly Finnish population. Br. J. Nutr. 2012, 108, 148–154. [Google Scholar] [CrossRef]
- Christen, W.G.; Liu, S.; Glynn, R.J.; Gaziano, J.M.; Buring, J.E. Dietary carotenoids, vitamins C and E, and risk of cataract in women: A prospective study. Arch. Ophthalmol. 2008, 126, 102–109. [Google Scholar] [CrossRef]
- Moeller, S.M.; Voland, R.; Tinker, L.; Blodi, B.A.; Klein, M.L.; Gehrs, K.M.; Johnson, E.J.; Snodderly, D.M.; Wallace, R.B.; Chappell, R.J.; et al. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an Ancillary Study of the Women’s Health Initiative. Arch. Ophthalmol. 2008, 126, 354–364. [Google Scholar] [CrossRef]
- Chylack, L.T., Jr.; Brown, N.P.; Bron, A.; Hurst, M.; Kopcke, W.; Thien, U.; Schalch, W. The Roche European American Cataract Trial (REACT): A randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol. 2002, 9, 49–80. [Google Scholar] [CrossRef]
- Rouen, P.A.; White, M.L. Dry eye disease: Prevalence, assessment, and management. Home Healthc. Now 2018, 36, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zemanová, M. Dry eye disease. A review. In Ceska a Slovenska Oftalmologie: Casopis Ceske Oftalmologicke Spolecnosti a Slovenske Oftalmologicke Spolecnosti; Czech and Slovak Ophtalmology: Brno, Czech Republic, 2021; Volume 77, pp. 107–119. [Google Scholar] [CrossRef]
- Messmer, E.M. The Pathophysiology, Diagnosis, and Treatment of Dry Eye Disease. Dtsch. Ärzteblatt Int. 2015, 112, 71. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Chhadva, P.; Goldhardt, R.; Galor, A. Meibomian Gland Disease: The Role of Gland Dysfunction in Dry Eye Disease. Ophthalmology 2017, 124, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K. The Ocular Surface: The Challenge to Enable and Protect Vision: The Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4391–4398. [Google Scholar] [CrossRef]
- Navarro-Partida, J.; Rodrigo Castro-Castaneda, C.; Santa Cruz-Pavlovich, F.J.; Abraham Aceves-Franco, L.; Ori Guy, T.; Santos, A.; Cruz-Pavlovich, S.; Lipid-Based Nanocarriers, A.; Lopes, C.M.; Lucio, M. Lipid-Based Nanocarriers as Topical Drug Delivery Systems for Intraocular Diseases. Pharmaceutics 2021, 13, 678. [Google Scholar] [CrossRef]
- Stern, M.E.; Gao, J.; Siemasko, K.F.; Beuerman, R.W.; Pflugfelder, S.C. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res. 2004, 78, 409–416. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of Hyperosmolarity in the Pathogenesis and Management of Dry Eye Disease: Proceedings of the OCEAN Group Meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Chatterjee, S.; Agrawal, D.; Chaturvedi, P. Ocular Surface Disease Index©and the five-item dry eye questionnaire: A comparison in Indian patients with dry eye disease. Indian J. Ophthalmol. 2021, 69, 2396–2400. [Google Scholar] [CrossRef] [PubMed]
- Dibajnia, P.; Mohammadinia, M.; Moghadasin, M.; Amiri, M.A. Tear Film Break-up Time in Bipolar Disorder. Iran. J. Psychiatry 2012, 7, 191. [Google Scholar] [PubMed]
- Brott, N.R.; Ronquillo, Y. Schirmer Test. In Encyclopedia of Ophthalmology; StatPearls Publishing: Berlin, Germany, 2021; pp. 1–2. [Google Scholar] [CrossRef]
- Chi, S.C.; Tuan, H.I.; Kang, Y.N. Effects of Polyunsaturated Fatty Acids on Nonspecific Typical Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2019, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Bernabei, F.; Roda, M.; Taroni, L.; Versura, P.; Campos, E.C. Efficacy of Omega-3 Fatty Acid Supplementation for Treatment of Dry Eye Disease: A Meta-Analysis of Randomized Clinical Trials. Cornea 2019, 38, 565–573. [Google Scholar] [CrossRef]
- Bhargava, R.; Chandra, M.; Bansal, U.; Singh, D.; Ranjan, S.; Sharma, S. A Randomized Controlled Trial of Omega 3 Fatty Acids in Rosacea Patients with Dry Eye Symptoms. Curr. Eye Res. 2016, 41, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Kumar, P. Oral omega-3 fatty acid treatment for dry eye in contact lens wearers. Cornea 2015, 34, 413–420. [Google Scholar] [CrossRef]
- Bhargava, R.; Kumar, P.; Arora, Y. Short-Term Omega 3 Fatty Acids Treatment for Dry Eye in Young and Middle-Aged Visual Display Terminal Users. Eye Contact Lens Sci. Clin. Pract. 2016, 42, 231–236. [Google Scholar] [CrossRef]
- Bhargava, R.; Kumar, P.; Kumar, M.; Mehra, N.; Mishra, A. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int. J. Ophthalmol. 2013, 6, 811–816. [Google Scholar] [CrossRef]
- Bhargava, R.; Kumar, P.; Phogat, H.; Kaur, A.; Kumar, M. Oral omega-3 fatty acids treatment in computer vision syndrome related dry eye. Contact Lens Anterior Eye 2015, 38, 206–210. [Google Scholar] [CrossRef]
- Epitropoulos, A.T.; Donnenfeld, E.D.; Shah, Z.A.; Holland, E.J.; Gross, M.; Faulkner, W.J.; Matossian, C.; Lane, S.S.; Toyos, M.; Bucci, F.A.; et al. Effect of oral re-esterified Omega-3 nutritional supplementation on dry eyes. Cornea 2016, 35, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- The Dry Eye Assessment and Management Study Research Group. n−3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N. Engl. J. Med. 2018, 378, 1681–1690. [Google Scholar] [CrossRef]
- Kawakita, T.; Kawabata, F.; Tsuji, T.; Kawashima, M.; Shimmura, S.; Tsubota, K. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: Randomized controlled trial. Biomed. Res. 2013, 34, 215–220. [Google Scholar] [CrossRef]
- Deinema, L.A.; Vingrys, A.J.; Wong, C.Y.; Jackson, D.C.; Chinnery, H.R.; Downie, L.E. A Randomized, Double-Masked, Placebo-Controlled Clinical Trial of Two Forms of Omega-3 Supplements for Treating Dry Eye Disease. Ophthalmology 2017, 124, 43–52. [Google Scholar] [CrossRef]
- Chinnery, H.R.; Naranjo Golborne, C.; Downie, L.E. Omega-3 supplementation is neuroprotective to corneal nerves in dry eye disease: A pilot study. Ophthalmic Physiol. Opt. 2017, 37, 473–481. [Google Scholar] [CrossRef]
- Rand, A.L.; Asbell, P.A. Nutritional supplements for dry eye syndrome. Curr. Opin. Ophthalmol. 2011, 22, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.A.; El-Hiti, G.A.; Al-Baloud, A.A.; Alfarhan, M.I.; Al-Shahrani, A.; Albakri, A.A.; Alqahtani, S.; Masmali, A.M. Effects of short-term oral vitamin A supplementation on the ocular tear film in patients with dry eye. Clin. Ophthalmol. 2019, 13, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.D.; Singh, R.; McClellan, A.J.; Weikert, M.P.; Scoper, S.V.; Joly, T.J.; Whitley, W.O.; Kakkar, E.; Pflugfelder, S.C. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca: A Randomized Double-Blind Clinical Trial. Cornea 2013, 32, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.I.; Suzuki, N.; Yamamoto, K.; Iio, S.I.; Yamada, T. Effects of MaquiBright((R)) on improving eye dryness and fatigue in humans: A randomized, double-blind, placebo-controlled trial. J. Tradit. Complement. Med. 2019, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Hyon, J.Y.; Han, S.B. Dry Eye Disease and Vitamins: A Narrative Literature Review. Appl. Sci. 2022, 12, 4567. [Google Scholar] [CrossRef]
- Kador, P.F. Antioxidant Eye Drops. US 9,173,915 B1, 3 November 2015. [Google Scholar]
- Fogagnolo, P.; Quisisana, C.; Caretti, A.; Marchina, D.; Dei Cas, M.; Melardi, E.; Rossetti, L. Efficacy and Safety of VisuEvo((R)) and Cationorm((R)) for the Treatment of Evaporative and Non-Evaporative Dry Eye Disease: A Multicenter, Double-Blind, Cross-Over, Randomized Clinical Trial. Clin. Ophthalmol. 2020, 14, 1651–1663. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Vetrugno, M.; Uva, M.G.; Russo, V.; Iester, M.; Ciancaglini, M.; Brusini, P.; Centofanti, M.; Rossetti, L.M. Oral Administration of Forskolin and Rutin Contributes to Intraocular Pressure Control in Primary Open Angle Glaucoma Patients Under Maximum Tolerated Medical Therapy. J. Ocular Pharmacol. Ther. 2012, 28, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Mutolo, M.G.; Albanese, G.; Rusciano, D.; Pescosolido, N. Oral Administration of Forskolin, Homotaurine, Carnosine, and Folic Acid in Patients with Primary Open Angle Glaucoma: Changes in Intraocular Pressure, Pattern Electroretinogram Amplitude, and Foveal Sensitivity. J. Ocular Pharmacol. Ther. 2016, 32, 178–183. [Google Scholar] [CrossRef]
- Romeo Villadóniga, S.; Rodríguez García, E.; Sagastagoia Epelde, O.; Álvarez Díaz, M.D.; Domingo Pedrol, J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018, 2018, 8259371. [Google Scholar] [CrossRef] [PubMed]
- Galbis-Estrada, C.; Pinazo-Durán, M.D.; Cantú-Dibildox, J.; Marco-Ramírez, C.; Díaz-Llópis, M.; Benítez-del-Castillo, J. Patients undergoing long-term treatment with antihypertensive eye drops responded positively with respect to their ocular surface disorder to oral supplementation with antioxidants and essential fatty acids. Clin. Interv. Aging 2013, 8, 711–719. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonyadi, M.H.J.; Yazdani, S.; Saadat, S. The ocular hypotensive effect of saffron extract in primary open angle glaucoma: A pilot study. BMC Complement. Altern. Med. 2014, 14, 339. [Google Scholar] [CrossRef]
- Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update. Nutrients 2022, 14, 534. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, H.; Ohguro, I.; Katai, M.; Tanaka, S. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Ophthalmologica 2012, 228, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Centofanti, M.; Ziccardi, L.; Tanga, L.; Michelessi, M.; Roberti, G.; Manni, G. Treatment with citicoline eye drops enhances retinal function and neural conduction along the visual pathways in open angle glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Centofanti, M.; Gandolfi, S.; Marangoni, D.; Rossetti, L.; Tanga, L.; Tardini, M.; Traina, S.; Ungaro, N.; Vetrugno, M.; et al. Effects of coenzyme Q10 in conjunction with vitamin e on retinal-evoked and cortical-evoked responses in patients with open-angle glaucoma. J. Glaucoma 2014, 23, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Kassoff, A.; Kassoff, J.; Buehler, J.; Eglow, M.; Kaufman, F.; Mehu, M.; Kieval, S.; Mairs, M.; Graig, B.; Quattrocchi, A.; et al. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.P.; Ferris, F.L.; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression: AREDS2 Report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Chakravarthy, U.; Nolan, J.M.; Muldrew, K.A.; Woodside, J.V.; Denny, F.; Stevenson, M.R. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology 2013, 120, 600–606. [Google Scholar] [CrossRef]
- Berrow, E.J.; Bartlett, H.E.; Eperjesi, F.; Gibson, J.M. The effects of a lutein-based supplement on objective and subjective measures of retinal and visual function in eyes with age-related maculopathy—A randomised controlled trial. Br. J. Nutr. 2013, 109, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhöfer, G.; Schmidt-Erfurth, U.; et al. Effects of Lutein Supplementation on Macular Pigment Optical Density and Visual Acuity in Patients with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar] [CrossRef]
- Bovier, E.R.; Hammond, B.R. A randomized placebo-controlled study on the effects of lutein and zeaxanthin on visual processing speed in young healthy subjects. Arch. Biochem. Biophys. 2015, 572, 54–57. [Google Scholar] [CrossRef] [PubMed]
- García-Layana, A.; Recalde, S.; Alamán, A.S.; Robredo, P.F. Effects of Lutein and Docosahexaenoic Acid Supplementation on Macular Pigment Optical Density in a Randomized Controlled Trial. Nutrients 2013, 5, 543–551. [Google Scholar] [CrossRef]
- Allegrini, D.; Raimondi, R.; Angi, M.; Ricciardelli, G.; Montericcio, A.; Borgia, A.; Romano, M.R. Curcuma-Based Nutritional Supplement in Patients with Neovascular Age-Related Macular Degeneration. J. Med. Food 2021, 24, 1191–1196. [Google Scholar] [CrossRef]
- Matos, A.L.; Bruno, D.F.; Ambrósio, A.F.; Santos, P.F. The Benefits of Flavonoids in Diabetic Retinopathy. Nutrients 2020, 12, 3169. [Google Scholar] [CrossRef]
- Moschos, M.M.; Dettoraki, M.; Tsatsos, M.; Kitsos, G.; Kalogeropoulos, C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis. 2017, 4, 23. [Google Scholar] [CrossRef]
- Zhang, P.C.; Wu, C.R.; Wang, Z.L.; Wang, L.Y.; Han, Y.; Sun, S.L.; Li, Q.S.; Ma, L. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac. J. Clin. Nutr. 2017, 26, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.J.; Hu, Y.N.; Lin, S.; Ma, W.J.; Li, X.R. Application of Lutein and Zeaxanthin in nonproliferative diabetic retinopathy. Int. J. Ophthalmol. 2011, 4, 303. [Google Scholar] [CrossRef] [PubMed]
- Domanico, D.; Fragiotta, S.; Cutini, A.; Carnevale, C.; Zompatori, L.; Vingolo, E.M. Circulating levels of reactive oxygen species in patients with nonproliferative diabetic retinopathy and the influence of antioxidant supplementation: 6-month follow-up. Indian J. Ophthalmol. 2015, 63, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Korenfeld, M.S.; Robertson, S.M.; Stein, J.M.; Evans, D.G.; Rauchman, S.H.; Sall, K.N.; Venkataraman, S.; Chen, B.L.; Wuttke, M.; Burns, W. Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: A safety and preliminary efficacy trial. Eye 2021, 35, 3292–3301. [Google Scholar] [CrossRef] [PubMed]
- Kangari, H.; Eftekhari, M.H.; Sardari, S.; Hashemi, H.; Salamzadeh, J.; Ghassemi-Broumand, M.; Khabazkhoob, M. Short-term Consumption of Oral Omega-3 and Dry Eye Syndrome. Ophthalmology 2013, 120, 2191–2196. [Google Scholar] [CrossRef]
- Teoh, S.L.; Ngorsuraches, S.; Lai, N.M.; Chaiyakunapruk, N. Consumer Preferences and Willingness to Pay for Nutraceuticals: A Discrete Choice Experiment. Value Health Reg. Issues 2021, 24, 167–172. [Google Scholar] [CrossRef]

| Ocular Condition | Nutraceutical | Type of Study | Description | Outcomes | References |
|---|---|---|---|---|---|
| Presbyopia | Combination of DHA, Astaxanthin, Lutein, Cyanidin-3- Glucoside | Clinical Trial | Nutraceutical combination administered orally as two gel capsules/day (Daily dose: 50 mg DHA, 4 mg Astaxanthin, 10 mg Lutein, 2.3 mg Cyanidin-3- Glucoside). | Enhanced near point accommodation and a decrease in eye strain and blurred vision. | [169] |
| Ophthalmic Solution: EV06 (lipoic acid choline ester) | Clinical trial | Nutraceutical formulation administered topically one drop in one eye twice/day. (Each with 1.5% lipoic acid choline ester) | Significant amelioration in distance-corrected near vision acuity (DCNVA). | [249] | |
| Pirenoxine | Clinical trial | Nutraceutical compound administered topically one drop in each eye four/day. (Each with 0.005% pirenoxine) | Maintenance of the accommodative amplitude and aid in the prevention of presbyopia progression. | [10] | |
| Cataract | Vitamin C, β-carotene, and vitamin E | Clinical trial | Nutraceutical formulation administered orally, three capsules/day (Each with vitamin C 200 mg, β-carotene 6 mg, and vitamin E 200 mg). | Slight impact in cataract progression measured by an increase percentage pixel opaque. | [192] |
| DED | Omega 3 | Clinical Trial | Omega 3 fatty acids administered orally, two capsules/day (each with 120 mg DHA, 180 mg of EPA). | Improvement in dry eye symptoms, Nelson grade and TBUT. | [208] |
| Omega 3 | Clinical Trial | Omega 3 fatty acids administered orally, 8 capsules/day (each with 120 mg DHA, 180 mg of EPA). | Improvement in dry eye symptoms, Nelson grade and TBUT. No changes in Schirmer test values. | [209] | |
| Omega 3 | Clinical Trial | Omega 3 fatty acids administered orally, 4 capsules/day (each with 120 mg DHA, 180 mg of EPA). | Improvement in dry eye symptoms, lens wear comfort, increased TBUT and Nelson scores, improvement in epithelial cell morphology. | [211] | |
| Omega 3 | Clinical Trial | Omega 3 fatty acids administered orally, 4 capsules/day (each with 120 mg DHA, 180 mg of EPA). | Improvement in Meibomian Gland Score, TBUT, Schirmer scores and dry eye symptoms. | [207] | |
| Omega 3 | Clinical Trial | Omega 3 fatty acids administered orally, 2 capsules/day (each with 175 mg DHA, 325 mg EPA). | Improvement in dry eye symptoms, TBUT and Schirmer test values. | [210] | |
| Omega 3 | Clinical Trial | Omega 3 fatty acids administered orally, 4 gel capsules/day (Daily dose: 1680 mg EPA and 560 mg DHA). | Significant improvement in tear osmolarity, OSDI, TBUT and a decrease in Metalloproteinase 9 levels. | [212] | |
| Omega 3 | Clinical Trial | Omega 3 fatty acid administered orally, 5 gelatin capsules/day (each with 400 mg EPA, 200 mg DHA). | No significant differences were observed in DED patients. | [213] | |
| Omega 3 | Clinical Trial | Omega 3 fatty acid administered orally, 15 capsules/day (Daily dose: 1245 mg EPA, 540 mg DHA). | Improvement in dry eye symptoms, TBUT, and rose bengal staining score. | [214] | |
| Omega 3 | Clinical Trial | Omega 3 fatty acid administered orally, 5 capsules/day (Daily dose: 945 mg EPA, 510 mg DHA). | Improvement in tear osmolarity, tear stability, OSDI score, TBUT, ocular bulbar redness. Decrease in IL-17A. | [215] | |
| Omega 3 | Clinical Trial | Omega 3 fatty acid administered orally, 2 capsules/day (each with 180 mg EPA, 120 mg DHA). | Improvement in TBUT, OSDI, Schirmer’s scores. | [250] | |
| Omega 3 | Clinical Trial | Omega 3 fatty acids administered orally, 5 capsules/day (Daily dose: 1000 mg EPA, 500 mg DHA). | Reduction is OSDI, higher corneal total nerve branch density and length on the main fiber, improving tear osmolarity. | [216] | |
| Vitamin A | Clinical Trial | Vitamin A (Daily dose 1500 mg or 5000 IU) administered orally as a tablet. | Improvement in tear osmolarity and reduction in tear ferning. | [218] | |
| Brudysec® (Minerals, vitamins, omega-3, and antioxidants) | Clinical Series | Nutraceutical formulation administered orally, 3 capsules/day. Each containing 500 mg w-3 fatty acids: 30 mg DPA, 42.5 mg EPA, 350 mg DHA; 4 mg Vitamin E, 133.3 µg Vitamin A, 26.7 mg Vitamin C, 9.17 µg Selenium, 0.33 mg Magnesium, 1.6 mg Zinc, 0.16 mg Copper; 10.8 mg Tyrosine, 5.83 mg Cysteine, 2 mg Glutathione. | Increase in TBUT and Schirmer test scores, and improvement in symptoms. | [2] | |
| HydroEye®: Polyunsaturated fatty acids, GLA, vitamins and minerals | Clinical Trial | Nutraceutical formulation administered orally as 4 softgels/day each containing: -Omega 3 Fatty acids: 196 mg ALA, 126 mg EPA, 99 mg DHA, -Omega 6 fatty acids: 710 mg LA, <30 mg ARA, 240 mg GLA. -Vitamins: 2180 IU vitamin A, 12.8 mg vitamin B6, 262 mg vitamin C, 13.7 mg vitamin E. 8 mg -Magnesium 40 mg | Improvement in OSDI, surface asymmetry index, inhibited dendritic cell maturation. No effect in tear production, TBUT. | [219] | |
| MaquiBright®: Anthocyanins and delphinidins | Clinical trial | Nutraceutical formulation administered orally as 1 capsule/day each containing: 120 mg of Dextrin, 4 mg delphinidin-3,5-O-diglucoside, 21 mg anthocyanins, and 15 mg delphinidins | Eye dryness relief, improvement in Schirmer test and eye fatigue alleviation. | [220] | |
| Topical nutraceutical: Colostrum (2-fucosyl-lactose) | Animal model: rabbit | 2-fucosyl-lactose was administered topically at 3 different doses (0.01%, 0.1% and 1%). | TBUT, Schirmer test and tear osmolarity were improved. | [3] | |
| Optixcare EH: Topical nutraceutical | Animal model: rat | Nutraceutical formulation administered topically, 2 drops/day. Each drop containing EGCG (4%), resveratrol (4%), astaxanthin (4%), and ethyl pyruvate (4%). | Increased maintenance of tear flow. | [1] | |
| VisuEvo®: vitamin A, omega-3, and vitamin D3. | Clinical trial | Nutraceutical formulation administered topically, one drop/three times a day. Each drop with vitamin A, omega-3 (DHA and EPA), and vitamin D3 | Improved ocular surface inflammation, restoration of tear film stability and composition. | [223] | |
| Glaucoma | Kronek®: Forskolin, Rutin, Vitamin B1 and B2 | Clinical trial | Nutraceutical formulation administered orally, 2 tablets/day (each with 0.7 mg vitamin B1, 0.8 vitamin B2, 15 mg Forskolin, 200 mg Rutin). | Reduction in intraocular pressure. | [226] |
| Gangliolife®: Carnosine, Forskolin, folic acid, homotaurine, vitamins and magnesium | Clinical Trial | Combined nutraceutical administered orally, 2 tablets/day (each with 50 mg Carnosine, 15 mg Forskolin, 0.2 mg Folic acid, 100 mg, Homotaurine, 1.4 mg vitamin B6, 1.4 mg vitamin B2, 1.1 mg vitamin B1, 150 mg Magnesium). | Increase in foveal sensitivity, better PERG and a reduction in IOP. | [227] | |
| BrudyPio: Vitamins, minerals, fatty acids, and antioxidants | Clinical Trial | Nutraceutical formulation, administered orally, 3 capsules/day. Each containing: 30 mg DPA, 42.5 mg EPA, 350 mg DHA; 4 mg Vitamin E, 133.3 µg Vitamin A, 26.7 mg Vitamin C, 0.36 mg Vitamin B1, 0.46 mg Vitamin B2, 5.33 mg Vitamin B3, 0.46 mg Vitamin B6, 66.7 µg Vitamin B9, 0.83 µg Vitamin B12, 18.3 µg Selenium, 0.66 mg Manganese, 3.33 mg Zinc, 0.33 mg Copper; 0.33 mg Zeaxanthin, 3.33 mg Lutein, 2 mg Glutathione, 2 mg Coenzyme Q10, 2 mg Lycopene, 67 µg Oleuropein, 5 mg Anthocyanins. | Decrease in IOP and proinflammatory cytokines. | [228] | |
| Saffron (Crocus Sativus) | Clinical Trial | Saffron administered orally, 1 capsule/day (30 mg). | Reduction of IOP after three weeks of administration. | [230] | |
| Black currant anthocyanins (BCACs) | Clinical Trial | BCACs administered orally, 2 capsules/day (50 mg/day) | Enhancement of the blood flow to the ONH and delay in the visual field damage progression. | [232] | |
| OMK1®: HA, citicoline and benzalkonium chloride. | Clinical trial | OMK1 administered topically, 3 drops/day (each containing 0.02 g HA, 0.2 g citicoline, 0.001 g benzalkonium chloride). | Significant increase in PERG and shortened VEP. | [233] | |
| Coqun®: Coenzyme Q10 and vitamin E | Clinical trial | Nutraceutical formulation administered topically, 2 drops/day (each with 100 mg Coenzyme Q10 with 500 mg vitamin E). | Beneficial effect on the retinal function (PERG improvement) and enhancement of visual cortical responses (VEP improvement). | [234] | |
| AMD | AREDS: Beta-carotene, Vitamin C and E, and Zinc | Clinical Trial | Nutraceutical formulation administered orally, 4 tablets/day (each with 15 mg Beta-carotene, 500 mg Vitamin C, 400 IU Vitamin E, 80 mg Zinc). | Decrease progression to advanced disease. | [235] |
| AREDS 2: Lutein/zeaxanthin, Vitamin C and E, and Zinc | Clinical Trial | Nutraceutical formulation administered orally, 4 pills/day (each with 10 mg/2 mg Lutein/zeaxanthin, 500 mg Vitamin C, 400 IU Vitamin E, 25 mg Zinc). | Decrease progression to advanced disease, mainly neovascular AMD. | [236] | |
| Ocuvite: lutein, vitamin E, zeaxanthin, copper, vitamin C, and zinc oxide | Clinical Trial | Nutraceutical formulation administered orally, 1 pill/twice daily (Each with lutein 12 mg, vitamin E 15 mg, zeaxanthin 0.6 mg, copper 0.4 mg, vitamin C 150 mg, zinc oxide 20 mg) | Significant difference in the best-corrected visual acuity (BCVA). | [237] | |
| Ocuvite Duo: vitamin E, vitamin C, cupric oxide, lutein, zinc oxide, EPA, zeaxanthin, and DHA | Clinical Trial | Nutraceutical formulation administered orally, 1 pill/day (Each with vitamin E 15 mg, vitamin C 150 mg, cupric oxide 400 μg, lutein 12 mg, zinc oxide 20 mg, EPA 240 mg, zeaxanthin 0.6 mg, and DHA 840 mg) | Significant increase in multifocal electroretinography (mfERG) latency. | [238] | |
| Lutamax DUO: Lutein (L) | Clinical Trial | Nutraceutical formulation administered orally, 1 tablet/day (20 mg L for 3 months, then 10 mg L for another 3 months). | Increased MPOD, no effect on macular function or visual activity. | [240] | |
| Lutein (L), zeaxanthin (Z) | Clinical Trial | Nutraceutical formulation administered orally, 1 pill/day (Each with 8 mg L, 20 mg Z, 190 mg mixed fatty acids; or pure 20 mg Z). | Improvement in the temporal contrast sensitivity function (tCSF) and MPOD. Increased the visual processing speed. | [241] | |
| Lutein (L), Zeaxanthin (Z), DHA | Clinical Trial | Nutraceutical formulation administered orally, 2 tablets/day (Daily dose: 0.6 mg Z, 12 mg L, 280 mg DHA). | Improvement in MPOD. | [242] | |
| Curcumin | Clinical Trial | Curcumin administered orally/day. | Improve visual acuity and decrease the total number of intravitreal anti-VEGF injections. | [243] | |
| Diabetic retinopathy (non-proliferative) | Carotenoids | Clinical trial | Nutraceutical formulation administered orally, 1 capsule/day (Daily dose: Lutein 10 mg, meso-zeaxanthin 10 mg, zeaxanthin 2 mg). | Increase in foveal thickness and improvement in mfERG. | [245] |
| Lutein | Clinical trial | Lutein (10 mg/day) administered orally, 1 capsule/day. | Improvement in contrast sensitivity and visual acuity. | [246] | |
| Lutein and Zeaxanthin | Clinical trial | Lutein (6 mg) and zeaxanthin (0.5 mg) administered orally/day. | Improvement in visual acuity and decreased foveal thickness. | [247] | |
| Diaberet®: vitamin E, pycnogenol, and coenzyme Q10 | Clinical trial | Nutraceutical formulation administered orally 1 tablet/day. (Daily dose: vitamin E 30 mg, pycnogenol 50 mg, and coenzyme Q10 20 mg). | Improvement in central macular thickness. | [248] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Castaneda, C.R.; Altamirano-Lamarque, F.; Ortega-Macías, A.G.; Santa Cruz-Pavlovich, F.J.; Gonzalez-De la Rosa, A.; Armendariz-Borunda, J.; Santos, A.; Navarro-Partida, J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients 2022, 14, 5014. https://doi.org/10.3390/nu14235014
Castro-Castaneda CR, Altamirano-Lamarque F, Ortega-Macías AG, Santa Cruz-Pavlovich FJ, Gonzalez-De la Rosa A, Armendariz-Borunda J, Santos A, Navarro-Partida J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients. 2022; 14(23):5014. https://doi.org/10.3390/nu14235014
Chicago/Turabian StyleCastro-Castaneda, Carlos Rodrigo, Francisco Altamirano-Lamarque, Alan Gabriel Ortega-Macías, Francisco J. Santa Cruz-Pavlovich, Alejandro Gonzalez-De la Rosa, Juan Armendariz-Borunda, Arturo Santos, and Jose Navarro-Partida. 2022. "Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology" Nutrients 14, no. 23: 5014. https://doi.org/10.3390/nu14235014
APA StyleCastro-Castaneda, C. R., Altamirano-Lamarque, F., Ortega-Macías, A. G., Santa Cruz-Pavlovich, F. J., Gonzalez-De la Rosa, A., Armendariz-Borunda, J., Santos, A., & Navarro-Partida, J. (2022). Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients, 14(23), 5014. https://doi.org/10.3390/nu14235014






