The Role of Ketogenic Diet in the Treatment of Neurological Diseases
Abstract
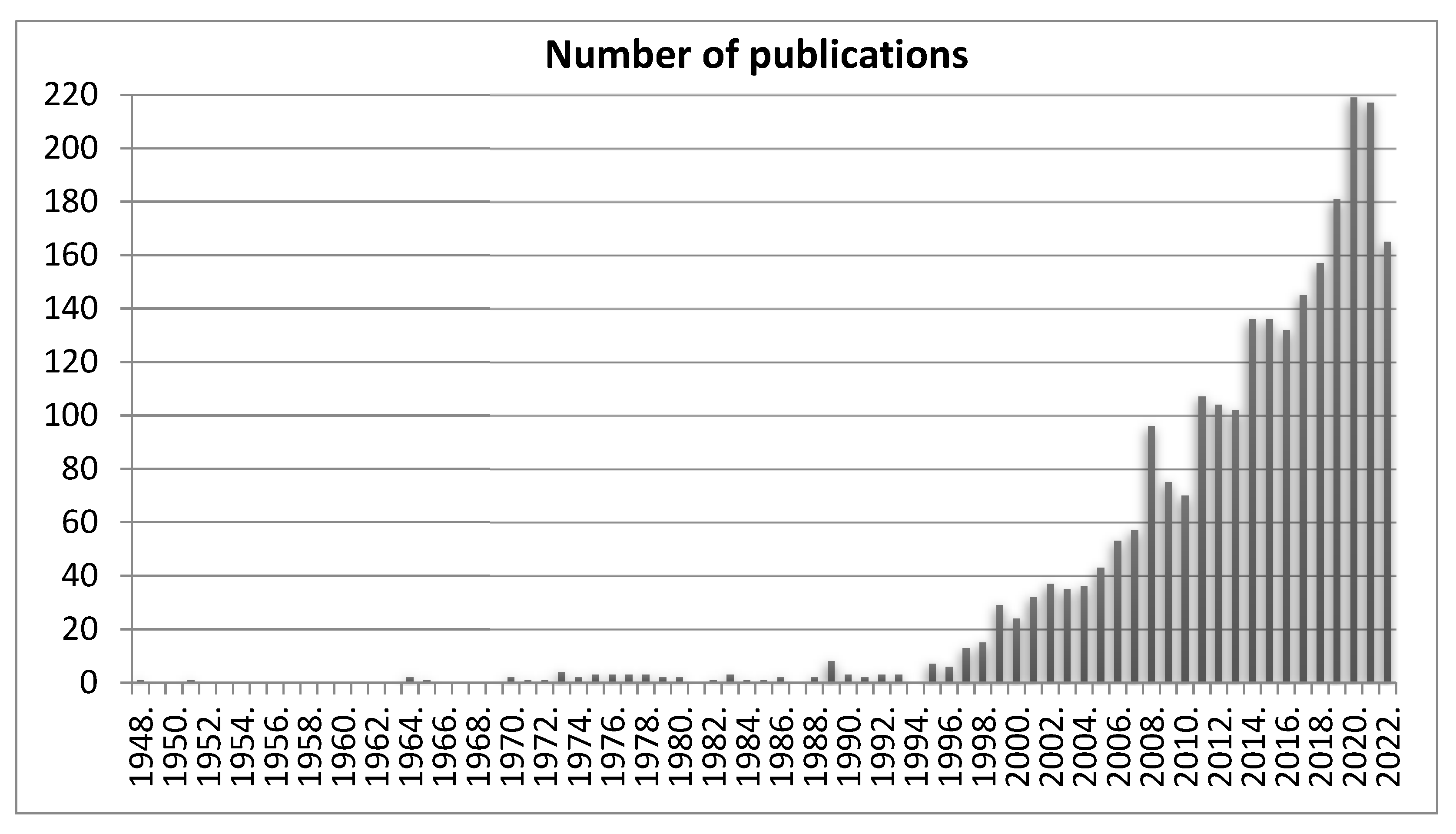
1. Introduction
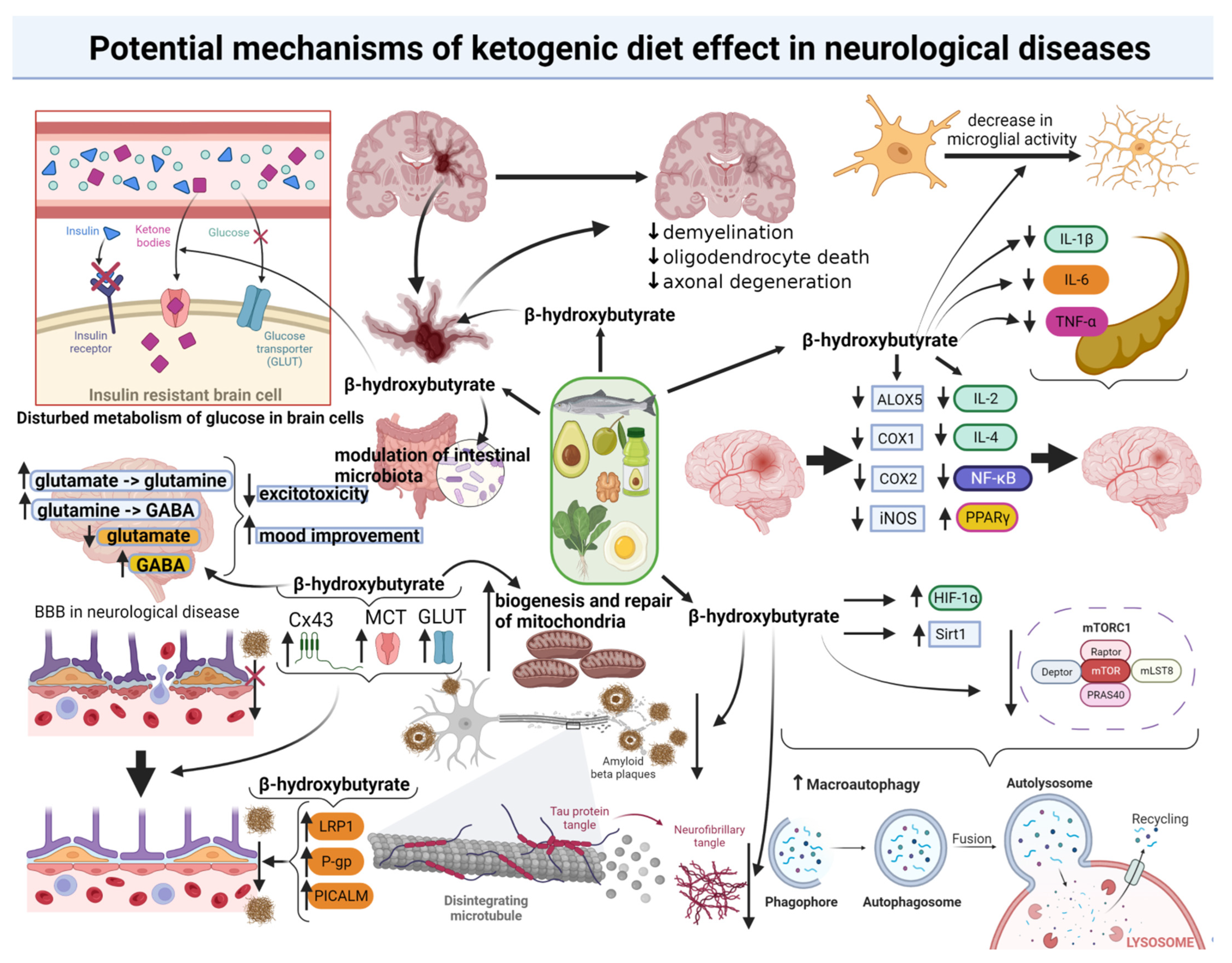
2. The Range and Mechanisms of the Ketogenic Diet Effect in Neurological Diseases
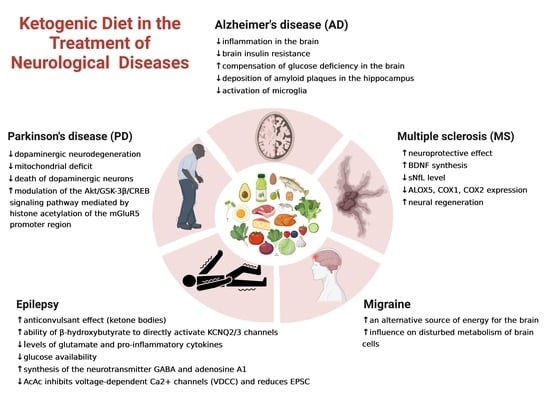
3. The Role of the Ketogenic Diet in the Treatment of Epilepsy
4. The Role of the Ketogenic Diet in the Therapy of Alzheimer’s Disease (AD)
5. The Role of the Ketogenic Diet in the Therapy of Parkinson’s Disease (PD)
6. The Role of the Ketogenic Diet in the Therapy of Multiple Sclerosis (MS)
7. Ketogenic Diet in the Therapy of Migraine
8. Limitations and Potential Adverse Health Impacts of the Ketogenic Diet
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L. The Evolution of Neuroepidemiology: Marking the 40-Year Anniversary of Publishing Studies on Epidemiology of Neurological Disorders. Neuroepidemiology 2022, 56, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Shehata, G.; Lord, K.; Grudzinski, M.; Elsayed, M.; Abdelnaby, R.; Elshabrawy, H. Neurological Complications of COVID-19: Underlying Mechanisms and Management. Int. J. Mol. Sci. 2021, 22, 4081. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1284437 patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef]
- Zarifkar, P.; Peinkhofer, C.; Benros, M.E.; Kondziella, D. Frequency of Neurological Diseases After COVID-19, Influenza A/B and Bacterial Pneumonia. Front. Neurol. 2022, 13, 904796. [Google Scholar] [CrossRef]
- Wise, J. COVID-19: Increased risk of some neurological and psychiatric disorders remains two years after infection, study finds. BMJ 2022, 378, o2048. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Gupta, L.; Khandelwal, D.; Kalra, S.; Gupta, P.; Dutta, D.; Aggarwal, S. Ketogenic diet in endocrine disorders: Current perspectives. J. Postgrad. Med. 2017, 63, 242–251. [Google Scholar]
- Phinney, S.D.; Bistrian, B.R.; Wolfe, R.R.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Physical and biochemical adaptation. Metabolism 1983, 32, 757–768. [Google Scholar] [CrossRef]
- Phinney, S.D. Ketogenic diets and physical performance. Nutr. Metab. 2004, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.M.; Hamdan, H.; Torisky, D.M.; Akers, J.D. A Low-Carbohydrate Ketogenic Diet Combined with 6-Weeks of Crossfit Training Improves Body Composition and Performance. Int. J. Sport. Exerc. Med. 2017, 3, 54. [Google Scholar] [CrossRef]
- Wilson, J.; Lowery, R. The Ketogenic Bible; Victory Belt Publishing Inc.: Las Vegas, NV, USA, 2017; ISBN-13 978-1-628601-04-6. [Google Scholar]
- Valenzuela, P.L.; Castillo García, A.; Lucia, A.; Naclerio, F. Ketogenic diets in strength-trained individuals: A narrative review. Nutrients 2021, 13, 3083. [Google Scholar] [CrossRef]
- Reichard, G.A., Jr.; Owen, O.E.; Haff, A.C.; Paul, P.; Bortz, W.M. Ketone-body production and oxidation in fasting obese humans. J. Clin. Investig. 1974, 53, 508–515. [Google Scholar] [CrossRef]
- Steinhauser, M.L.; Olenchock, B.A.; O’Keefe, J.; Lun, M.; Pierce, K.A.; Lee, H.; Pantano, L.; Klibanski, A.; Shulman, G.I.; Clish, C.B.; et al. The circulating metabolome of human starvation. J. Clin. Investig. 2018, 3, e121434. [Google Scholar] [CrossRef]
- Alharbi, A.; Al-Sowayan, N. The Effect of Ketogenic-Diet on Health. Food Nutr. Sci. 2020, 11, 301–313. [Google Scholar] [CrossRef]
- Dong, T.A.; Sandesara, P.B.; Dhindsa, D.S.; Mehta, A.; Arneson, L.C.; Dollar, A.L.; Taub, P.R.; Sperling, L.S. Intermittent Fasting: A Heart Healthy Dietary Pattern? Am. J. Med. 2020, 133, 901–907. [Google Scholar] [CrossRef]
- Williams, M.; Turos, E. The Chemistry of the Ketogenic Diet: Updates and Opportunities in Organic Synthesis. Int. J. Mol. Sci. 2021, 22, 5230. [Google Scholar] [CrossRef]
- Temkin, O. The Falling Sickness: A History of Epilepsy from the Greeks to the Beginnings of Modern Neurology; Johns Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49, 3–5. [Google Scholar] [CrossRef]
- Livingston, S. Comprehensive Management of Epilepsy in Infancy, Childhood, and Adolescence; Charles C Thomas: Springfield, IL, USA, 1972; pp. 378–405. [Google Scholar]
- Huisjen, D. Today’s Parallel Bible; Zondervan Corp: Grand Rapids, MI, USA, 2000; Volume 14–29, pp. 2306–2308. [Google Scholar]
- Wheless, J.W. History and Origin of the Ketogenic Diet, en Epilepsy and the Ketogenic Diet; Stafstrom, C.E., Rho, J.M., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 31–50. [Google Scholar]
- Guelpa, G.; Marie, A. La lutte contre l’e’pilepsie par la de’ sintoxication et par la re’e’ducation alimentaire. Rev. De Ther. Med. Chir. 1911, 78, 8–13. [Google Scholar]
- Wilder, R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307. [Google Scholar]
- Woodyatt, R.T. Objects and method of diet adjustment in diabetes. Arch. Intern. Med. 1921, 28, 125. [Google Scholar] [CrossRef]
- Peterman, M.G. The ketogenic diet in epilepsy. JAMA 1925, 84, 1979–1983. [Google Scholar] [CrossRef]
- Field, R.; Field, T.; Pourkazemi, F.; Rooney, K. Ketogenic diets and the nervous system: A scoping review of neurological outcomes from nutritional ketosis in animal studies. Nutr. Res. Rev. 2021, 35, 268–281. [Google Scholar] [CrossRef]
- Jensen, N.J.; Wodschow, H.Z.; Nilsson, M.; Rungby, J. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8767. [Google Scholar] [CrossRef]
- Shaito, A.; Hasan, H.; Habashy, K.J.; Fakih, W.; Abdelhady, S.; Ahmad, F.; Zibara, K.; El-Yazbi, A.F.; Kobeissy, F.H.; Eid, A.H. Western diet aggravates neuronal insult inpost-traumatic brain injury: Proposed pathways for interplay. EBioMedicine 2020, 57, 102829. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef]
- Gambardella, I.; Ascione, R.; D’Agostino, D.P.; Ari, C.; Worku, B.; Tranbaugh, R.F.; Ivascu, N.; Villena-Vargas, J.; Girardi, L.N. Systematic Review—Neuroprotection of ketosis in acute injury of the mammalian central nervous system: A meta-analysis. J. Neurochem. 2021, 158, 105–118. [Google Scholar] [CrossRef]
- Maalouf, M.; Rho, J.M.; Mattson, M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009, 59, 293–315. [Google Scholar] [CrossRef]
- Włodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, M.; Kitamura, Y.; Mori, S.; Sato, K.; Dohi, S.; Sato, T.; Matsuura, A.; Hiraide, A. β-Hydroxybutyrate, a Cerebral Function Improving Agent, Protects Rat Brain Against Ischemic Damage Caused by Permanent and Transient Focal Cerebral Ischemia. Jpn. J. Pharmacol. 2002, 89, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.; Giza, C. Induction of Monocarboxylate Transporter 2 Expression and Ketone Transport following Traumatic Brain Injury in Juvenile and Adult Rats. Dev. Neurosci. 2006, 28, 447–456. [Google Scholar] [CrossRef]
- Barkhoudarian, G.; Hovda, D.A.; Giza, C.C. The Molecular Pathophysiology of Concussive Brain Injury. Clin. Sports Med. 2011, 30, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, N.; Zhu, W.; Zhang, K.; Si, J.; Bi, M.; Lv, X.; Wang, J. Protective effect of β-hydroxybutyrate on glutamate induced cell death in HT22 cells. Int. J. Clin. Exp. Med. 2016, 9, 23433–23439. [Google Scholar]
- Dilimulati, D.; Zhang, F.; Shao, S.; Lv, T.; Lu, Q.; Cao, M.; Jin, Y.; Jia, F.; Zhang, X. Ketogenic Diet Modulates Neuroinflammation via Metabolites from Lactobacillus reuteri After Repetitive Mild Traumatic Brain Injury in Adolescent Mice. Cell. Mol. Neurobiol. 2022; 1–17, online ahead of print. [Google Scholar] [CrossRef]
- Arora, N.; Litofsky, N.S.; Golzy, M.; Aneja, R.; Staudenmyer, D.; Qualls, K.; Patil, S. Phase I single center trial of ketogenic diet for adults with traumatic brain injury. Clin. Nutr. ESPEN 2022, 47, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Wang, T.; Li, M.; Guan, T.; Guo, Y.; Zhang, X.; Zhang, G.; Kong, J. Ketogenic diet protects myelin and axons in diffuse axonal injury. Nutr. Neurosci. 2022, 25, 1534–1547. [Google Scholar] [CrossRef]
- Kim, D.Y.; Vallejo, J.; Rho, J.M. Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J. Neurochem. 2010, 114, 130–141. [Google Scholar] [CrossRef]
- Kim, D.Y.; Abdelwahab, M.G.; Lee, S.H.; O’Neill, D.; Thompson, R.J.; Duff, H.J.; Sullivan, P.G.; Rho, J.M. Ketones Prevent Oxidative Impairment of Hippocampal Synaptic Integrity through KATP Channels. PLoS ONE 2015, 10, e0119316. [Google Scholar] [CrossRef]
- Svart, M.; Gormsen, L.C.; Hansen, J.; Zeidler, D.; Gejl, M.; Vang, K.; Aanerud, J.; Moeller, N. Regional cerebral effects of ketone body infusion with 3-hydroxybutyrate in humans: Reduced glucose uptake, unchanged oxygen consumption and increased blood flow by positron emission tomography. A randomized, controlled trial. PLoS ONE 2018, 13, e0190556. [Google Scholar] [CrossRef]
- Prapong, T.; Buss, J.; Hsu, W.H.; Heine, P.; West Greenlee, H.; Uemura, E. Amyloid beta-peptide decreases neuronal glucose uptake despite causing increase in GLUT3 mRNA transcription and GLUT3 translocation to the plasma membrane. Exp. Neurol. 2002, 174, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.W.; Sanz-Blasco, S.; Dolatabadi, N.; Parker, J.; Chon, K.; Lee, M.S.; Soussou, W.; McKercher, S.R.; Ambasudhan, R.; Nakamura, T.; et al. Elevated glucose and oligomeric β-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat. Commun. 2016, 7, 10242. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.T. High carbohydrate diets and Alzheimer’s disease. Med. Hypotheses 2004, 62, 689–700. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jeon, S.-M.; Shin, S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Ketosis may promote brain macroautophagy by activating Sirt1 and hypoxia-inducible factor-1. Med. Hypotheses 2015, 85, 631–639. [Google Scholar] [CrossRef]
- Loos, B.; Klionsky, D.J.; Wong, E. Augmenting brain metabolism to increase macro- and chaperone-mediated autophagy for decreasing neuronal proteotoxicity and aging. Prog. Neurobiol. 2017, 156, 90–106. [Google Scholar] [CrossRef]
- Camberos-Luna, L.; Gerónimo-Olvera, C.; Montiel, T.; Rincon-Heredia, R.; Massieu, L. The Ketone Body, β-Hydroxybutyrate Stimulates the Autophagic Flux and Prevents Neuronal Death Induced by Glucose Deprivation in Cortical Cultured Neurons. Neurochem. Res. 2016, 41, 600–609. [Google Scholar] [CrossRef]
- Montiel, T.; Montes-Ortega, L.A.; Flores-Yáñez, S.; Massieu, L. Treatment with the Ketone Body D-β-hydroxybutyrate Attenuates Autophagy Activated by NMDA and Reduces Excitotoxic Neuronal Damage in the Rat Striatum In Vivo. Curr. Pharm. Des. 2020, 26, 1377–1387. [Google Scholar] [CrossRef]
- Liśkiewicz, D.; Liśkiewicz, A.; Nowacka-Chmielewska, M.M.; Grabowski, M.; Pondel, N.; Grabowska, K.; Student, S.; Barski, J.J.; Małecki, A. Differential Response of Hippocampal and Cerebrocortical Autophagy and Ketone Body Metabolism to the Ketogenic Diet. Front. Cell. Neurosci. 2021, 15, 733607. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef]
- Koh, S.; Dupuis, N.; Auvin, S. Ketogenic diet and Neuroinflammation. Epilepsy Res. 2020, 167, 106454. [Google Scholar] [CrossRef]
- Disabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.J.W.; Cervenka, M.C. Ketogenic Diets for Adult Neurological Disorders. Neurotherapeutics 2018, 15, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, B. Neuroprotective and Anti-inflammatory Activities of Ketogenic Diet on MPTP-induced Neurotoxicity. J. Mol. Neurosci. 2010, 42, 145–153. [Google Scholar] [CrossRef]
- Jeong, E.A.; Jeon, B.T.; Shin, H.J.; Kim, N.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Ketogenic diet-induced peroxisome proliferator-activated receptor-γ activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp. Neurol. 2011, 232, 195–202. [Google Scholar] [CrossRef]
- Fu, S.; Wang, J.; Xue, W.; Liu, H.; Liu, B.; Zeng, Y.; Li, S.; Huang, B.; Lv, Q.; Wang, W.; et al. Anti-inflammatory effects of BHBA inboth in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef]
- Guzmán, M.; Blázquez, C. Ketone body synthesis in the brain: Possible neuroprotective effects. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 287–292. [Google Scholar] [CrossRef]
- Jiang, Z.; Yin, X.; Wang, M.; Chen, T.; Wang, Y.; Gao, Z.; Wang, Z. Effects of Ketogenic Diet on Neuroinflammation in Neurodegenerative Diseases. Aging Dis. 2022, 13, 1146. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, C.; Wu, J.; Liu, P.; Deng, X.; Zhang, Y.; Peng, B.; Zhu, Y. Ketogenic diet ameliorates cognitive impairment and neuroinflammation in a mouse model of Alzheimer’s disease. CNS Neurosci. Ther. 2022, 28, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Kaufer, D. Blood-Brain Barrier Breakdown and Blood-Brain Communication in Neurological and Psychiatric Diseases. Cardiovasc. Psychiatry Neurol. 2011, 2011, 431470. [Google Scholar] [CrossRef]
- Xiao, M.; Xiao, Z.J.; Yang, B.; Lan, Z.; Fang, F. Blood-Brain Barrier: More Contributor to Disruption of Central Nervous System Homeostasis Than Victim in Neurological Disorders. Front. Neurosci. 2020, 14, 764. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.; Walker, L.; Rauchmann, B.; Perneczky, R. Dysfunction of the blood–brain barrier in Alzheimer’s disease: Evidence from human studies. Neuropathol. Appl. Neurobiol. 2022, 48, e12782. [Google Scholar] [CrossRef]
- Hasselbalch, S.G.; Knudsen, G.M.; Jakobsen, J.; Hageman, L.P.; Holm, S.; Paulson, O.B. Blood-brain barrier permeability of glucose and ketone bodies during short-term starvation in humans. Am. J. Physiol. Metab. 1995, 268, E1161–E1166. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Venkatesh, B. Clinical review: Ketones and brain injury. Crit. Care 2011, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-F.; Chan, K.-W.; Yeh, H.-I.; Kuo, J.; Liu, H.-J.; Wang, C.-Y. Ketone bodies upregulate endothelial connexin 43 (Cx43) gap junctions. Vet. J. 2013, 198, 696–701. [Google Scholar] [CrossRef]
- Versele, R.; Corsi, M.; Fuso, A.; Sevin, E.; Businaro, R.; Gosselet, F.; Fenart, L.; Candela, P. Ketone Bodies Promote Amyloid-β1–40 Clearance in a Human in Vitro Blood–Brain Barrier Model. Int. J. Mol. Sci. 2020, 21, 934. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Horyn, O.; Nissim, I.; Nissim, I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia 2008, 49, 73–75. [Google Scholar] [CrossRef]
- Lund, T.M.; Risa, O.; Sonnewald, U.; Schousboe, A.; Waagepetersen, H.S. Availability of neurotransmitter glutamate is diminished when β-hydroxybutyrate replaces glucose in cultured neurons. J. Neurochem. 2009, 110, 80–91. [Google Scholar] [CrossRef]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Penas, J.J. Epilepsia, cognicion y dieta cetogenica [Epilepsy, cognition and ketogenic diet]. Rev. Neurol. 2018, 66, S71–S75. (In Spanish) [Google Scholar] [PubMed]
- Włodarczyk, A.; Cubała, W.J.; Stawicki, M. Ketogenic diet for depression: A potential dietary regimen to maintain euthymia? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110257. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Leng, S.X.; Zhang, H. Ketogenic diet: An effective treatment approach for neurodegenerative diseases. Curr. Neuropharmacol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Bough, K.J.; Wetherington, J.; Hassel, B.; Pare, J.F.; Gawryluk, J.W.; Greene, J.G.; Shaw, R.; Smith, Y.; Geiger, J.D.; Dingledine, R.J. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-M.; Letchumanan, V.; Tan, L.T.-H.; Hong, K.-W.; Wong, S.-H.; Ab Mutalib, N.-S.; Lee, L.-H.; Law, J.W.-F. Ketogenic Diet: A Dietary Intervention via Gut Microbiome Modulation for the Treatment of Neurological and Nutritional Disorders (a Narrative Review). Nutrients 2022, 14, 3566. [Google Scholar] [CrossRef]
- Attaye, I.; van Oppenraaij, S.; Warmbrunn, M.V.; Nieuwdorp, M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2021, 14, 191. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiomeaxis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Silva, M.C.; Rocha, J.; Pires, C.S.; Ribeiro, L.C.; Brolese, G.; Leite, M.C.; Almeida, L.M.V.; Tramontina, F.; Ziegler, D.R.; Gonçalves, C.A. Transitory gliosis in the CA3 hippocampal region in rats fed on a ketogenic diet. Nutr. Neurosci. 2005, 8, 259–264. [Google Scholar] [CrossRef]
- Maalouf, M.; Sullivan, P.G.; Davis, L.; Kim, D.Y.; Rho, J.M. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 2007, 145, 256–264. [Google Scholar] [CrossRef]
- Beghi, E.; Hesdorffer, D. Prevalence of epilepsy—An unknown quantity. Epilepsia 2014, 55, 963–967. [Google Scholar] [CrossRef]
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 2017, 89, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- WHO. Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 22 September 2022).
- García-Ramos, R.; García Pastor, A.; Masjuan, J.; Sánchez, C.; Gil, A. FEEN: Informe sociosantario FEEN sobre la epilepsia en España [Feen report on epilepsy in Spain]. Neurologia 2011, 26, 548–555. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Xue-Ping, W.; Hai-Jiao, W.; Li-Na, Z.; Xu, D.; Ling, L. Risk factors for drug-resistant epilepsy. Medicine 2019, 98, e16402. [Google Scholar] [CrossRef] [PubMed]
- Martin-McGill, K.J.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst. Rev. 2020, 2020, CD001903. [Google Scholar] [CrossRef]
- Barborka, C.J. Epilepsy in adults: Results of treatment by ketogenic diet in one hundred cases. Arch. Neurol. Psychiatry 1930, 23, 904–914. [Google Scholar] [CrossRef]
- Bastible, C. The ketogenic treatment of epilepsy. Ir. J. Med. Sci. 1931, 6, 506–520. [Google Scholar] [CrossRef]
- Klein, P.; Janousek, J.; Barber, A.; Weissberger, R. Ketogenic diet treatment in adults with refractory epilepsy. Epilepsy Behav. 2010, 19, 575–579. [Google Scholar] [CrossRef]
- Schoeler, N.E.; Wood, S.; Aldridge, V.; Sander, J.W.; Cross, J.H.; Sisodiya, S.M. Ketogenic dietary therapies for adults with epilepsy: Feasibility and classification of response. Epilepsy Behav. 2014, 37, 77–81. [Google Scholar] [CrossRef]
- Klein, P.; Tyrlikova, I.; Mathews, G.C.; Wu, H.Z.; Ahmad, K.E.; Tan, K.; Blair, N.F. Dietary treatment in adults with refractory epilepsy: A review. Neurology 2014, 83, 1978–1985. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic Diet and Epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef]
- Herrero, J.R.; Villarroya, E.C.; Peñas, J.J.G.; Alcolea, B.G.; Fernández, B.G.; Macfarland, L.A.P.; Pedrón-Giner, C. Ketogenic dietary therapies for epilepsy: Experience in 160 patients over 18 years. 2022, 96, 511–522. An. De Pediatría 2022, 96, 511–522. [Google Scholar] [CrossRef]
- Pizzo, F.; Collotta, A.D.; Di Nora, A.; Costanza, G.; Ruggieri, M.; Falsaperla, R. Ketogenic diet in pediatric seizures: A randomized controlled trial review and meta-analysis. Expert Rev. Neurother. 2022, 22, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Sourbron, J.; Klinkenberg, S.; van Kuijk, S.M.J.; Lagae, L.; Lambrechts, D.; Braakman, H.M.H.; Majoie, M. Ketogenic diet for the treatment of pediatric epilepsy: Review and meta-analysis. Child’s Nerv. Syst. 2020, 36, 1099–1109. [Google Scholar] [CrossRef]
- Lyons, L.; Schoeler, N.E.; Langan, D.; Cross, H. Use of ketogenic diet therapy in infants with epilepsy: A systematic review and meta-analysis. Epilepsia 2020, 61, 1261–1281. [Google Scholar] [CrossRef]
- Rezaei, S.; Abdurahman, A.A.; Saghazadeh, A.; Badv, R.S.; Mahmoudi, M. Short-term and long-term efficacy of classical ketogenic diet and modified Atkins diet in children and adolescents with epilepsy: A systematic review and meta-analysis. Nutr. Neurosci. 2019, 22, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.B.; Filloux, F.M.; Alder, S.C.; Lyon, J.L.; Caplin, D.A. Efficacy of the Ketogenic Diet as a Treatment Option for Epilepsy: Meta-analysis. J. Child Neurol. 2006, 21, 193–198. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009, 50, 1109–1117. [Google Scholar] [CrossRef]
- Meira, I.D.; Romão, T.T.; Prado, H.J.P.D.; Krüger, L.T.; Pires, M.E.P.; Da Conceição, P.O. Ketogenic Diet and Epilepsy: What We Know So Far. Front. Neurosci. 2019, 13, 5. [Google Scholar] [CrossRef]
- Thio, L.L.; Wong, M.; Yamada, K.A. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology 2000, 54, 325. [Google Scholar] [CrossRef]
- Likhodii, S.S.; Burnham, W.M. Ketogenic diet: Does acetone stop seizures? Med. Sci. Monit. 2002, 8, HY19-24. [Google Scholar] [PubMed]
- Rho, J.M.; Anderson, G.D.; Donevan, S.D.; White, H.S. Acetoacetate, Acetone, and Dibenzylamine (a Contaminant in l-(+)-β-Hydroxybutyrate) Exhibit Direct Anticonvulsant Actions in Vivo. Epilepsia 2002, 43, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Gasior, M.; Rogawski, M.A.; Hartman, A. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol. 2006, 17, 431–439. [Google Scholar] [CrossRef]
- D’Agostino, D.P.; Pilla, R.; Held, H.E.; Landon, C.S.; Puchowicz, M.; Brunengraber, H.; Ari, C.; Arnold, P.; Dean, J.B. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R829–R836. [Google Scholar] [CrossRef]
- Likhodii, S.S.; Serbanescu, I.; Cortez, M.A.; Murphy, P.; Snead, O.C.; Burnham, W.M. Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann. Neurol. 2003, 54, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Thio, L.L.; Rensing, N.; Maloney, S.; Wozniak, D.F.; Xiong, C.; Yamada, K.A. A ketogenic diet does not impair rat behavior or long-term potentiation. Epilepsia 2010, 51, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Manville, R.; Papanikolaou, M.; Abbott, G.W. M-Channel Activation Contributes to the Anticonvulsant Action of the Ketone Body β-Hydroxybutyrate. J. Pharmacol. Exp. Ther. 2020, 372, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.E.; Todorova, M.T.; Seyfried, T.N. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J. Neurochem. 2003, 86, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, P.R. Ketonemia and Seizures: Metabolic and Anticonvulsant Effects of Two Ketogenic Diets in Childhood Epilepsy. Pediatr. Res. 1976, 10, 536–540. [Google Scholar] [CrossRef]
- Garriga-Canut, M.; Schoenike, B.; Qazi, R.; Bergendahl, K.; Daley, T.J.; Pfender, R.M.; Morrison, J.F.; Ockuly, J.C.; Stafstrom, C.E.; Sutula, T.P.; et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP–dependent metabolic regulation of chromatin structure. Nat. Neurosci. 2006, 9, 1382–1387. [Google Scholar] [CrossRef]
- Mironov, S.; Richter, D. Intracellular signalling pathways modulate KATP channels in inspiratory brainstem neurones and their hypoxic activation: Involvement of metabotropic receptors, G-proteins and cytoskeleton. Brain Res. 2000, 853, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-P.; Chen, S.-R.; Pan, H.-L. Adenosine inhibits paraventricular pre-sympathetic neurons through ATP-dependent potassium channels. J. Neurochem. 2010, 113, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, E.; Patel, V.; Tideman, S.; Frech, R.; Frigerio, R.; Narayanan, J. Efficacy of supplemental MCT oil on seizure reduction of adult drug-resistant epilepsy—A single-center open-label pilot study. Nutr. Neurosci. 2022; 1–5, online ahead of print. [Google Scholar] [CrossRef]
- Kadowaki, A.; Sada, N.; Juge, N.; Wakasa, A.; Moriyama, Y.; Inoue, T. Neuronal inhibition and seizure suppression by acetoacetate and its analog, 2-phenylbutyrate. Epilepsia 2017, 58, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Szot, P.; Weinshenker, D.; Rho, J.M.; Storey, T.W.; Schwartzkroin, P.A. Norepinephrine is required for the anticonvulsant effect of the ketogenic diet. Dev. Brain Res. 2001, 129, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Bergqvist, C.; Hunter, J.V.; Jin, D.; Wang, D.-J.; Wehrli, S.; Zimmerman, R.A. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy? Exploration of GABA levels in a ketogenic diet. Magn. Reson. Med. 2003, 49, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Yudkoff, M.; Daikhin, Y.; Melø, T.M.; Nissim, I.; Sonnewald, U.; Nissim, I. The Ketogenic Diet and Brain Metabolism of Amino Acids: Relationship to the Anticonvulsant Effect. Annu. Rev. Nutr. 2007, 27, 415–430. [Google Scholar] [CrossRef]
- Gavrilovici, C.; Rho, J.M. Metabolic epilepsies amenable to ketogenic therapies: Indications, contraindications, and underlying mechanisms. J. Inherit. Metab. Dis. 2021, 44, 42–53. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- Dupuis, N.; Curatolo, N.; Benoist, J.-F.; Auvin, S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia 2015, 56, e95–e98. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Liu, Q.; Kong, G.; Zhou, J.; Jiang, J.; Zhu, Q. Ketogenic metabolism inhibits histone deacetylase (HDAC) and reduces oxidative stress after spinal cord injury in rats. Neuroscience 2017, 366, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Kwon, H.E.; Kim, H.D. Updates on the ketogenic diet therapy for pediatric epilepsy. Biomed. J. 2022, 45, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C. World Alzheimer Report 2018; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- WHO. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 20 September 2022).
- Scheltens, P.; Blennow, K.; Breteler, M.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Barczak, A. Wczesne rozpoznanie choroby Alzheimera—Wskazówki dla lekarza POZ. Lek. POZ 2022, 8, 57–62. [Google Scholar]
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimer Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef]
- Lilamand, M.; Mouton-Liger, F.; Paquet, C. Ketogenic diet therapy in Alzheimer’s disease: An updated review. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 372–378. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Wands, J.R. Alzheimer’s Disease is Type 3 Diabetes—Evidence Reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.-Y.; Kazi, H.; Han, L.-Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef]
- Ferreira, L.S.S.; Fernandes, C.S.; Vieira, M.N.N.; De Felice, F.G. Insulin Resistance in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 830. [Google Scholar] [CrossRef] [PubMed]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef] [PubMed]
- Mietelska-Porowska, A.; Domańska, J.; Want, A.; Więckowska-Gacek, A.; Chutorański, D.; Koperski, M.; Wojda, U. Induction of Brain Insulin Resistance and Alzheimer’s Molecular Changes by Western Diet. Int. J. Mol. Sci. 2022, 23, 4744. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.-X. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008, 582, 359–364. [Google Scholar] [CrossRef]
- Lying-Tunell, U.; Lindblad, B.S.; Malmlund, H.O.; Persson, B. Cerebral blood flow and metabolic rate of oxygen, glucose, lactate, pyruvate, ketone bodies and amino acids. Acta Neurol. Scand. 1981, 63, 337–350. [Google Scholar] [CrossRef]
- Castellano, C.-A.; Nugent, S.; Paquet, N.; Tremblay, S.; Bocti, C.; Lacombe, G.; Imbeault, H.; Turcotte, E.; Fulop, T.; Cunnane, S.C. Lower Brain 18F-Fluorodeoxyglucose Uptake But Normal 11C-Acetoacetate Metabolism in Mild Alzheimer’s Disease Dementia. J. Alzheimer’s Dis. 2015, 43, 1343–1353. [Google Scholar] [CrossRef]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO). European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245, PMCID: PMC8138199. [Google Scholar] [CrossRef] [PubMed]
- Sakr, H.F.; Sirasanagandla, S.R.; Das, S.; Bima, A.I.; Elsamanoudy, A.Z. Low-Carbohydrate Ketogenic Diet for Improvement of Glycemic Control: Mechanism of Action of Ketosis and Beneficial Effects. Curr. Diabetes Rev. 2022; ahead of print. [Google Scholar] [CrossRef]
- Michalczyk, M.M.; Klonek, G.; Maszczyk, A.; Zajac, A. The Effects of a Low Calorie Ketogenic Diet on Glycaemic Control Variables in Hyperinsulinemic Overweight/Obese Females. Nutrients 2020, 12, 1854. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sullivan, D.K.; Keller, J.E.; Burns, J.M.; Swerdlow, R.H. Potential for Ketotherapies as Amyloid-Regulating Treatment in Individuals at Risk for Alzheimer’s Disease. Front. Neurosci. 2022, 16, 899612. [Google Scholar] [CrossRef]
- Hernandez, A.R.; Hernandez, C.M.; Truckenbrod, L.M.; Campos, K.T.; McQuail, J.A.; Bizon, J.L.; Burke, S.N. Age and Ketogenic Diet Have Dissociable Effects on Synapse-Related Gene Expression Between Hippocampal Subregions. Front Aging Neurosci. 2019, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Wang, D.-D.; Sun, Y.-X.; Zhao, D.-J.; Ni, H. Neuro-Behavioral Status and the Hippocampal Expression of Metabolic Associated Genes in Wild-Type Rat Following a Ketogenic Diet. Front. Neurol. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Koppel, S.J.; Pei, D.; Wilkins, H.M.; Weidling, I.W.; Wang, X.; Menta, B.W.; Perez-Ortiz, J.; Kalani, A.; Manley, S.; Novikova, L.; et al. A ketogenic diet differentially affects neuron and astrocyte transcription. J. Neurochem. 2021, 157, 1930–1945. [Google Scholar] [CrossRef]
- Hughes, S.D.; Kanabus, M.; Anderson, G.; Hargreaves, I.P.; Rutherford, T.; Donnell, M.O.; Cross, J.H.; Rahman, S.; Eaton, S.; Heales, S.J.R. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J. Neurochem. 2014, 129, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Swerdlow, R.H. Amyloid precursor protein processing and bioenergetics. Brain Res. Bull. 2017, 133, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hasan-Olive, M.; Lauritzen, K.H.; Ali, M.; Rasmussen, L.J.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem. Res. 2019, 44, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Seira, O.; Kolehmainen, K.; Liu, J.; Streijger, F.; Haegert, A.; Lebihan, S.; Boushel, R.; Tetzlaff, W. Ketogenesis controls mitochondrial gene expression and rescues mitochondrial bioenergetics after cervical spinal cord injury in rats. Sci. Rep. 2021, 11, 16359. [Google Scholar] [CrossRef]
- Croteau, E.; Castellano, C.-A.; Richard, M.A.; Fortier, M.; Nugent, S.; Lepage, M.; Duchesne, S.; Whittingstall, K.; Turcotte, E.; Bocti, C.; et al. Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 551–561. [Google Scholar] [CrossRef]
- Juby, A.G.; Blackburn, T.E.; Mager, D.R. Use of medium chain triglyceride (MCT) oil in subjects with Alzheimer’s disease: A randomized, double-blind, placebo-controlled, crossover study, with an open-label extension. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2022, 8, e12259. [Google Scholar] [CrossRef]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.; Hyde, K.; Chapman, D.; Craft, S. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef]
- Ota, M.; Matsuo, J.; Ishida, I.; Hattori, K.; Teraishi, T.; Tonouchi, H.; Ashida, K.; Takahashi, T.; Kunugi, H. Effect of a ketogenic meal on cognitive function in elderly adults: Potential for cognitive enhancement. Psychopharmacology 2016, 233, 3797–3802. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. d-β-Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444. [Google Scholar] [CrossRef] [PubMed]
- Studzinski, C.M.; MacKay, W.A.; Beckett, T.L.; Henderson, S.T.; Murphy, M.P.; Sullivan, P.G.; Burnham, W.M. Induction of ketosis may improve mitochondrial function and decrease steady-state amyloid-β precursor protein (APP) levels in the aged dog. Brain Res. 2008, 1226, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Bergman, C.; Lee, J.-H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef]
- Nafar, F.; Clarke, J.; Mearow, K. Coconut oil protects cortical neurons from amyloid beta toxicity by enhancing signaling of cell survival pathways. Neurochem. Int. 2017, 105, 64–79. [Google Scholar] [CrossRef]
- Neal, E.G.; Cross, J.H. Efficacy of dietary treatments for epilepsy. J. Hum. Nutr. Diet. 2010, 23, 113–119. [Google Scholar] [CrossRef]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the ketogenic agentAC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. (Lond) 2009, 6, 31. [Google Scholar] [CrossRef]
- Newport, M.T.; VanItallie, T.B.; Kashiwaya, Y.; King, M.T.; Veech, R.L. A new way to produce hyperketonemia: Use of ketone ester in a case of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 99–103. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef]
- Raina, P.; Santaguida, P.; Ismaila, A.; Patterson, C.; Cowan, D.; Levine, M.; Booker, L.; Oremus, M. Effectiveness of Cholinesterase Inhibitors and Memantine for Treating Dementia: Evidence Review for a Clinical Practice Guideline. Ann. Intern. Med. 2008, 148, 379–397. [Google Scholar] [CrossRef]
- Siemers, E.; Holdridge, K.C.; Sundell, K.L.; Liu-Seifert, H. Function and clinical meaningfulness of treatments for mild Alzheimer’s disease. Alzheimer’s Dementia Diagn. Assess. Dis. Monit. 2016, 2, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.M. Understanding disability in Parkinson’s disease. Mov. Disord. 2010, 25 (Suppl. S1), S131–S135. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin Geriatr Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Lotankar, S.; Prabhavalkar, K.S.; Bhatt, L.K. Biomarkers for Parkinson’s Disease: Recent Advancement. Neurosci. Bull. 2017, 33, 585–597. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Estimates 2020: Disease Burden by Cause, Age, Sex by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Evans, J.R.; Mason, S.L.; Williams-Gray, C.H.; Foltynie, T.; Brayne, C.; Robbins, T.W.; Barker, R.A. The natural history of treated Parkinson’s disease in an incident, community based cohort. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1112–1118. [Google Scholar] [CrossRef]
- Shaafi, S.; Najmi, S.; Aliasgharpour, H.; Mahmoudi, J.; Sadigh-Etemad, S.; Farhoudi, M.; Baniasadi, N. The efficacy of the ketogenic diet on motor functions in Parkinson’s disease: A rat model. Iran. J. Neurol. 2016, 15, 63–69. [Google Scholar]
- Pietrzak, D.; Kasperek, K.; Rękawek, P.; Piątkowska-Chmiel, I. The Therapeutic Role of Ketogenic Diet in Neurological Disorders. Nutrients. 2022, 14, 1952. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, X.; Cheng, Z.; Dong, Q.; Ruan, G. The Anti-Inflammatory Effect of Preventive Intervention with Ketogenic Diet Mediated by the Histone Acetylation of mGluR5 Promotor Region in Rat Parkinson’s Disease Model: A Dual-Tracer PET Study. Park. Dis. 2022, 2022, 3506213. [Google Scholar] [CrossRef]
- Masino, S.A.; Rho, J.M. Mechanisms of Ketogenic Diet Action. In Jasper’s Basic Mechanisms of the Epilepsies; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012; pp. 1157–1182. [Google Scholar]
- Norwitz, N.G.; Hu, M.T.; Clarke, K. The Mechanisms by Which the Ketone Body D-β-Hydroxybutyrate May Improve the Multiple Cellular Pathologies of Parkinson’s Disease. Front Nutr. 2019, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Aureli, C.; Cassano, T.; Masci, A.; Francioso, A.; Martire, S.; Cocciolo, A.; Chichiarelli, S.; Romano, A.; Gaetani, S.; Mancini, P.; et al. 5-S-cysteinyldopamine neurotoxicity: Influence on the expression of α-synuclein and ERp57 in cellular and animal models of Parkinson’s disease. J. Neurosci. Res. 2014, 92, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B.; Nonas, C.; di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson Disease with Diet-Induced Hyperketonemia: A Feasibility Study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef] [PubMed]
- Irfannuddin, I.; Sarahdeaz, S.F.P.; Murti, K.; Santoso, B.; Koibuchi, N. The effect of ketogenic diets on neurogenesis and apoptosis in the dentate gyrus of the male rat hippocampus. J. Physiol. Sci. 2021, 71, 3. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Phillips, M.C.; Murtagh, D.K.; Gilbertson, L.J.; Asztely, F.J.; Lynch, C.D. Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef]
- Tidman, M.M.; White, D.; White, T. Effects of an low carbohydrate/healthy fat/ketogenic diet on biomarkers of health and symptoms, anxiety and depression in Parkinson’s disease: A pilot study. Neurodegener. Dis. Manag. 2022, 12, 57–66. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Summer, S.S.; Sullivan, P.G.; Duker, A.P.; Isaacson, R.S.; Espay, A.J. Nutritional ketosis for mild cognitive impairment in Parkinson’s disease: A controlled pilot trial. Clin. Park. Relat. Disord. 2019, 1, 41–47. [Google Scholar] [CrossRef]
- Tidman, M. Effects of a Ketogenic Diet on Symptoms, Biomarkers, Depression, and Anxiety in Parkinson’s Disease: A Case Study. Cureus 2022, 14, e23684. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, H.; Fidan, V.; Toktas, H.; Binay, O.; Celik, H. Effect of ketogenic diet versus regular diet on voice quality of patients with Parkinson’s disease. Acta Neurol. Belg. 2021, 121, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.H.; Lord, S. Postural Control in Multiple Sclerosis: Implications for Fall Prevention. Curr. Neurol. Neurosci. Rep. 2010, 10, 407–412. [Google Scholar] [CrossRef]
- Hebert, J.R.; Corboy, J.R.; Manago, M.M.; Schenkman, M. Effects of Vestibular Rehabilitation on Multiple Sclerosis–Related Fatigue and Upright Postural Control: A Randomized Controlled Trial. Phys. Ther. 2011, 91, 1166–1183. [Google Scholar] [CrossRef] [PubMed]
- The Multiple Sclerosis International Federation (MSIF). Atlas of MS. In Mapping Multiple Sclerosis around the World Key Epidemiology Findings, 3rd ed.; MSIF: London, UK, 2020. [Google Scholar]
- Updated Atlas of MS Shows Over 2.8 Million People Worldwide Have Multiple Sclerosis—With Nearly 1 Million in the US. Available online: https://www.nationalmssociety.org/About-the-Society/News/Updated-Atlas-of-MS-Shows-Over-2-8-million-People (accessed on 20 September 2022).
- Lin, W.-S.; Lin, S.-J.; Liao, P.-Y.; Suresh, D.; Hsu, T.-R.; Wang, P.-Y. Role of Ketogenic Diets in Multiple Sclerosis and Related Animal Models: An Updated Review. Adv. Nutr. Int. Rev. J. 2022, 13, 2002–2014. [Google Scholar] [CrossRef]
- Di Majo, D.; Cacciabaudo, F.; Accardi, G.; Gambino, G.; Giglia, G.; Ferraro, G.; Candore, G.; Sardo, P. Ketogenic and Modified Mediterranean Diet as a Tool to Counteract Neuroinflammation in Multiple Sclerosis: Nutritional Suggestions. Nutrients 2022, 14, 2384. [Google Scholar] [CrossRef]
- Cellerino, A.; Carroll, P.; Thoenen, H.; Barde, Y.A. Reduced Size of Retinal Ganglion Cell Axons and Hypomyelination in Mice Lacking Brain-Derived Neurotrophic Factor. Mol. Cell. Neurosci. 1997, 9, 397–408. [Google Scholar] [CrossRef]
- Veer, A.V.; Du, Y.; Fischer, T.Z.; Boetig, D.R.; Wood, M.R.; Dreyfus, C.F. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J. Neurosci. Res. 2009, 87, 69–78. [Google Scholar] [CrossRef]
- Lee, D.-H.; Geyer, E.; Flach, A.-C.; Jung, K.; Gold, R.; Flügel, A.; Linker, R.A.; Lühder, F. Central nervous system rather than immune cell-derived BDNF mediates axonal protective effects early in autoimmune demyelination. Acta Neuropathol. 2012, 123, 247–258. [Google Scholar] [CrossRef]
- Paoli, A.; Cenci, L.; Pompei, P.; Sahin, N.; Bianco, A.; Neri, M.; Caprio, M.; Moro, T. Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders. Nutrients 2021, 13, 374. [Google Scholar] [CrossRef]
- Vauzour, D. Polyphenols and brain health. OCL 2017, 24, A202. [Google Scholar] [CrossRef]
- Benlloch, M.; López-Rodríguez, M.M.; Cuerda-Ballester, M.; Drehmer, E.; Carrera, S.; Ceron, J.J.; Tvarijonaviciute, A.; Chirivella, J.; Fernández-García, D.; de la Rubia Ortí, J.E. Satiating effect of a ketogenic diet and its impact on muscle improvement and oxidation state in multiple sclerosis patients. Nutrients 2019, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.; Steffen, F.; Zipp, F.; Bittner, S. Impact of Dietary Intervention on Serum Neurofilament Light Chain in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 9, e1102. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.; Karber, M.; Kuhn, H. Ketogenic diets attenuate cyclooxygenase and lipoxygenase gene expression in multiple sclerosis. eBioMedicine 2018, 36, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.N.; Lehner-Gulotta, D.; Woolbright, E.; Banwell, B.; Bergqvist, A.G.C.; Chen, S.; Coleman, R.; Conaway, M.; Goldman, M.D. Phase II study of ketogenic diets in relapsing multiple sclerosis: Safety, tolerability and potential clinical benefits. J. Neurol. Neurosurg. Psychiatry 2022, 93, 637–644. [Google Scholar] [CrossRef]
- Brenton, J.N.; Banwell, B.; Bergqvist, A.C.; Lehner-Gulotta, D.; Gampper, L.; Leytham, E.; Coleman, R.; Goldman, M.D. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e565. [Google Scholar] [CrossRef]
- Lee, J.E.; Titcomb, T.J.; Bisht, B.; Rubenstein, L.M.; Louison, R.; Wahls, T.L. A Modified MCT-Based Ketogenic Diet Increases Plasma β-Hydroxybutyrate but Has Less Effect on Fatigue and Quality of Life in People with Multiple Sclerosis Compared to a Modified Paleolithic Diet: A Waitlist-Controlled, Randomized Pilot Study. J. Am. Coll. Nutr. 2021, 40, 13–25. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef]
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef]
- Roos-Araujo, D.; Stuart, S.; Lea, R.A.; Haupt, L.M.; Griffiths, L.R. Epigenetics and migraine; complex mitochondrial interactions contributing to disease susceptibility. Gene 2014, 543, 1–7. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Pinto, A.; Ienca, R.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Parisi, V.; Serrao, M.; Spagnoli, A.; Vestri, A.; et al. A Randomized Double-Blind, Cross-Over Trial of very Low-Calorie Diet in Overweight Migraine Patients: A Possible Role for Ketones? Nutrients 2019, 11, 1742. [Google Scholar] [CrossRef]
- Valente, M.; Garbo, R.; Filippi, F.; Antonutti, A.; Ceccarini, V.; Tereshko, Y.; Di Lorenzo, C.; Gigli, G.L. Migraine Prevention through Ketogenic Diet: More than Body Mass Composition Changes. J. Clin. Med. 2022, 11, 4946. [Google Scholar] [CrossRef] [PubMed]
- Lovati, C.; D’Alessandro, C.M.; Della Ventura, S.; Muzio, F.; Pantoni, L. Ketogenic diet in refractory migraine: Possible efficacy and role of ketone bodies—A pilot experience. Neurol. Sci. 2022, 43, 6479–6485. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, D.; Benedetto, C.; Corvisieri, S.; Del Favero, C.; Orlandi, F.; Allais, G.; Sinigaglia, S.; Fadda, M. Effectiveness of ketogenic diet in treatment of patients with refractory chronic migraine. Neurol. Sci. 2021, 42, 3865–3870. [Google Scholar] [CrossRef] [PubMed]
- Caminha, M.C.; Moreira, A.B.; Matheus, F.C.; Rieger, D.K.; Moreira, J.D.; Dalmarco, E.M.; Demarchi, I.G.; Lin, K. Efficacy and tolerability of the ketogenic diet and its variations for preventing migraine in adolescents and adults: A systematic review. Nutr. Rev. 2022, 80, 1634–1647. [Google Scholar] [CrossRef]
- Bostock, E.C.S.; Kirkby, K.C.; Taylor, B.V.; Hawrelak, J.A. Consumer Reports of “Keto Flu” Associated With the Ketogenic Diet. Front. Nutr. 2020, 7, 20. [Google Scholar] [CrossRef] [PubMed]
| Disease | No. of Patients | Intervention in the Study Group | Intervention in the Control Group | Result | Reference |
|---|---|---|---|---|---|
| Alzheimer’s disease | 26 | 12-week ketogenic diet; 58% fat (including 26% saturated and 32% unsaturated), 29% protein, 7% roughage and 6% carbohydrates net. + a multivitamin preparation * | 12-week diet according to New Zealand’s principles of healthy nutrition; 11% fat (3% saturated, 8% unsaturated), 19% protein, 8% roughage, and 62% carbohydrates net. + a multivitamin preparation * | Compared with the control group, the ketogenic diet improved the following: cognitive functions (by 2.12 pts on the ACE-III scale), everyday functioning (by 3.13 pts on the ADCS-ADL scale), quality of life (by 3.37 pts on the QOL-AD scale) | [171] |
| Parkinson’s disease | 47 | 8-week ketogenic diet, 1750 kcal, 152 g fat (67 g saturated), 75 g protein, 16 g carbohydrates net and 11 g roughage + a possibility of additional “loading” of 500 kcal (50 g fat (22 g saturated), 6 g protein, 5 g carbohydrates net and 4 g roughage) | 8-week low-fat diet 1750 kcal, 42 g fat (10 g saturated), 75 g protein, 246 g carbohydrates net and 33 g roughage + a possibility of additional “loading” of 500 kcal (4 g fat (1 g saturated), 6 g protein, 102 g carbohydrates net and 11 g roughage) | Improvement in Part I of the UPDRS by 48% compared with 11% improvement in the control group, a greater improvement of non-motor signs in the ketogenic group, improvement of motor signs in both groups | [192] |
| Multiple sclerosis | 60 | 6-month ketogenic diet, >160 g fat, ≤100 g protein, <50 g carbohydrates net, high (unspecified) roughage consumption | 6-month diet according to the principles of a healthy diet in the German population | Reduced expression of proinflammatory ALOX5 compared with the control group, impaired expression also of other proinflammatory enzymes (COX1, COX2), significant inverse correlation between expression of proinflammatory ALOX5 and COX2 and the MSQoL-54 marker | [211] |
| Migraine | 35 | 4-week very-low-calorie ketogenic diet (VLCKD), 20 g fat, ≥75 g protein, 30–50 g carbohydrates + a preparation with microelements | 4-week very-low-calorie non-ketogenic diet (VLCnKD), 20 g fat, ≅50 g protein, ≥70 g carbohydrates + a preparation with microelements | Reduction in the mean number of days with migraine monthly by 3.73 days, compared with the control group, in the number of migraine attacks over that time by 3.02; a greater percentage of patients in the ketogenic group, in whom a reduction by at least 50% of the days with migraine, was obtained (74.28% vs. 8.57% of the participants) | [218] |
| Epilepsy | 145 | 12-month ketogenic diet enriched with MCT, 70–75% energy from fat (30% long-chain fatty acids, 40–45% MCT), 10% energy from protein, 15% energy from carbohydrates | 12-month classic ketogenic diet; in most cases, the fat to carbohydrates and protein ratio was 4:1; protein was maintained in amounts recommended by the WHO | Effectiveness of both types of ketogenic diets: a reduction of the mean number of initial attacks in the 3rd, 6th and 12th months in the classic version was 66.5%,48.5%, and 40,5%, respectively, while in the MCT version, it was 68.9%, 67.6%, and 53.2%, respectively | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. https://doi.org/10.3390/nu14235003
Dyńka D, Kowalcze K, Paziewska A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients. 2022; 14(23):5003. https://doi.org/10.3390/nu14235003
Chicago/Turabian StyleDyńka, Damian, Katarzyna Kowalcze, and Agnieszka Paziewska. 2022. "The Role of Ketogenic Diet in the Treatment of Neurological Diseases" Nutrients 14, no. 23: 5003. https://doi.org/10.3390/nu14235003
APA StyleDyńka, D., Kowalcze, K., & Paziewska, A. (2022). The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients, 14(23), 5003. https://doi.org/10.3390/nu14235003