Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review
Abstract
1. Introduction
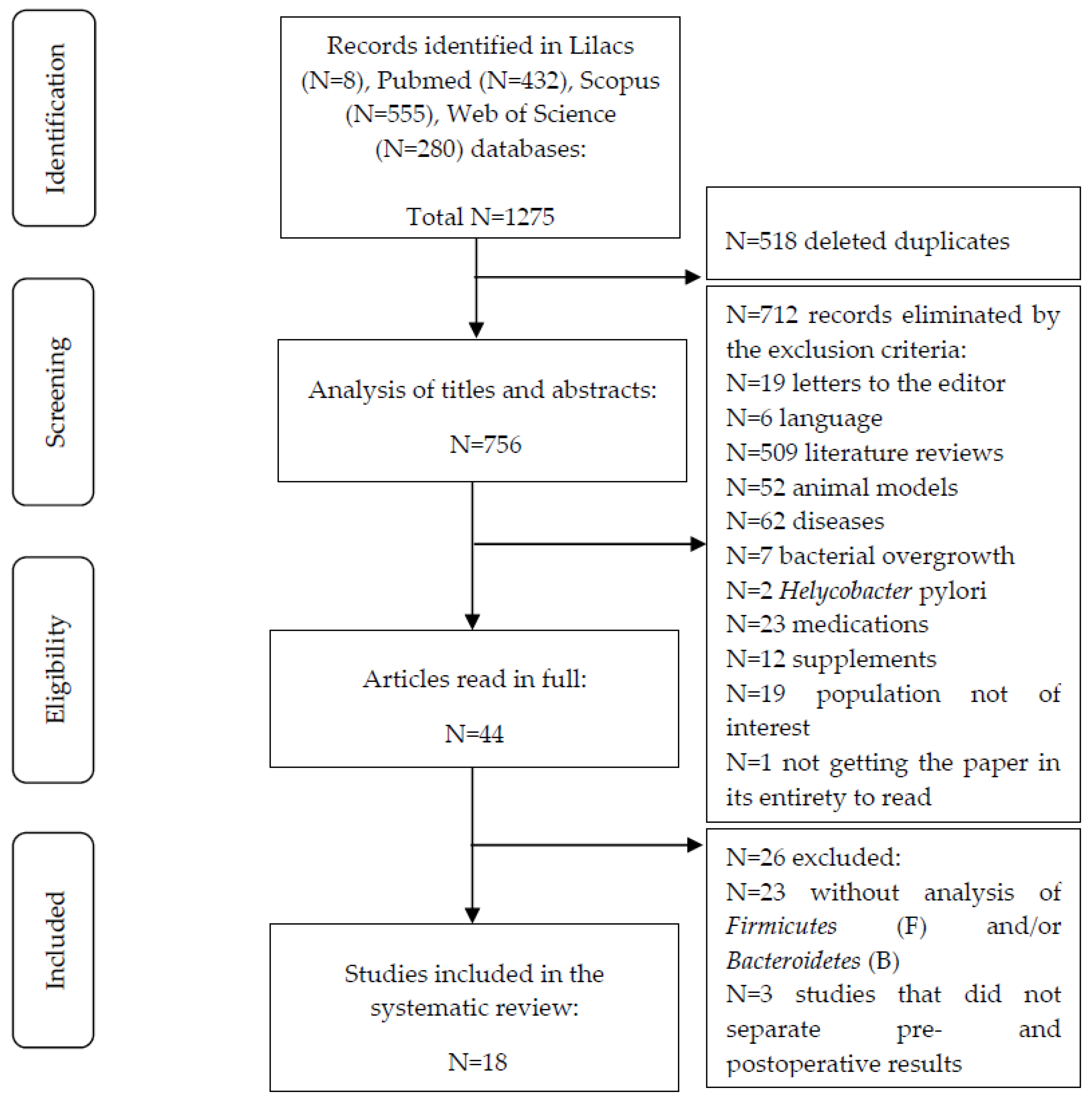
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO World Health Statistics 2016: Monitoring Health for the SDGs 2016. Available online: https://reliefweb.int/report/world/world-health-statistics-2016-monitoring-health-sdgs?gclid=Cj0KCQjwyt-ZBhCNARIsAKH1175VaFltqNiNjiLQjrWZswvD9yAIZmuL_W52N4Jyj5AZFvlZ6RDpBXIaAr8iEALw_wcB (accessed on 1 June 2021).
- ABESO Diretrizes Brasileiras de Obesidade 2016. Available online: https://abeso.org.br/wp-content/uploads/2019/12/Diretrizes-Download-Diretrizes-Brasileiras-de-Obesidade-2016.pdf (accessed on 1 March 2021).
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.A.; Pedreros, J.P.; Turiel, D.; Quezada, N.; Pimentel, F.; Escalona, A.; Garrido, D. Distinct Patterns in the Gut Microbiota after Surgical or Medical Therapy in Obese Patients. PeerJ 2017, 5, e3443. [Google Scholar] [CrossRef] [PubMed]
- Pajecki, D.; de Oliveira, L.C.; Sabino, E.C.; de Souza-Basqueira, M.; Dantas, A.C.B.; Nunes, G.C.; de Cleva, R.; Santo, M.A. Changes in the Intestinal Microbiota of Superobese Patients after Bariatric Surgery. Clinics 2019, 74, e1198. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Matteo, R.D.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A. Sleep Quality in Obesity: Does Adherence to the Mediterranean Diet Matter? Nutrients 2020, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: The Conductor in the Orchestra of Immune–Neuroendocrine Communication. Clin. Ther. 2015, 37, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, V.L.; Banan, B.; Ajouz, H.; Abumrad, N.N.; Flynn, C.R. Bile Acids and Bariatric Surgery. Mol. Aspects. Med. 2017, 56, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric Surgery in Obesity: Effects on Gut Microbiota and Micronutrient Status. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Dore, J.; Henegar, C.; et al. Differential Adaptation of Human Gut Microbiota to Bariatric Surgery-Induced Weight Loss: Links With Metabolic and Low-Grade Inflammation Markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef]
- Palmisano, S.; Campisciano, G.; Silvestri, M.; Guerra, M.; Giuricin, M.; Casagranda, B.; Comar, M.; de Manzini, N. Changes in Gut Microbiota Composition after Bariatric Surgery: A New Balance to Decode. J. Gastrointest. Surg. 2019, 24, 1736–1746. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clément, K. The Gut Microbiome, Diet, and Links to Cardiometabolic and Chronic Disorders. Nat. Rev. Nephrol. 2016, 12, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Ver. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Fernández, M.; Román-Sagüillo, S.; Porras, D.; García-Mediavilla, M.V.; Linares, P.; Ballesteros-Pomar, M.D.; Urioste-Fondo, A.; Álvarez-Cuenllas, B.; González-Gallego, J.; Sánchez-Campos, S.; et al. Long-Term Effects of Bariatric Surgery on Gut Microbiota Composition and Faecal Metabolome Related to Obesity Remission. Nutrients 2021, 13, 2519. [Google Scholar] [CrossRef]
- Chen, G.; Zhuang, J.; Cui, Q.; Jiang, S.; Tao, W.; Chen, W.; Yu, S.; Wu, L.; Yang, W.; Liu, F.; et al. Two Bariatric Surgical Procedures Differentially Alter the Intestinal Microbiota in Obesity Patients. Obes. Surg. 2020, 30, 2345–2361. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Gut Microbial Predictors of Type 2 Diabetes Remission Following Bariatric Surgery. Obes. Surg. 2020, 30, 3536–3548. [Google Scholar] [CrossRef]
- Faria, S.L.; Santos, A.; Magro, D.O.; Cazzo, E.; Assalin, H.B.; Guadagnini, D.; Vieira, F.T.; Dutra, E.S.; Saad, M.J.A.; Ito, M.K. Gut Microbiota Modifications and Weight Regain in Morbidly Obese Women After Roux-En-Y Gastric Bypass. Obes. Surg. 2020, 12, 4958–4966. [Google Scholar] [CrossRef] [PubMed]
- Farin, W.; Oñate, F.P.; Plassais, J.; Bonny, C.; Beglinger, C.; Woelnerhanssen, B.; Nocca, D.; Magoules, F.; Le Chatelier, E.; Pons, N.; et al. Impact of Laparoscopic Roux-En-Y Gastric Bypass and Sleeve Gastrectomy on Gut Microbiota: A Metagenomic Comparative Analysis. Surg. Obes. Relat. Dis. 2020, 16, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Koffert, J.; Lahti, L.; Nylund, L.; Salmine, S.; Hannukainen, J.C.; Salminen, P.; de Vos, W.M.; Nuutila, P. Partial restoration of normal intestinal microbiota in morbidly obese women six months after bariatric surgery. PeerJ 2020, 8, e10442. [Google Scholar] [CrossRef] [PubMed]
- Al Assal, K.; Prifti, E.; Belda, E.; Sala, P.; Clément, K.; Dao, M.-C.; Doré, J.; Levenez, F.; Taddei, C.R.; Fonseca, D.C.; et al. Gut Microbiota Profile of Obese Diabetic Women Submitted to Roux-En-Y Gastric Bypass and Its Association with Food Intake and Postoperative Diabetes Remission. Nutrients 2020, 12, 278. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Moreno-Indias, I.; de Hollanda, A.; Martín-Núñez, G.M.; Vidal, J.; Tinahones, F.J. Gut Microbiota Specific Signatures Are Related to the Successful Rate of Bariatric Surgery. Am. J. Transl. Res. 2019, 11, 942–952. [Google Scholar]
- Lee, C.J.; Florea, L.; Sears, C.L.; Maruthur, N.; Potter, J.J.; Schweitzer, M.; Magnuson, T.; Clark, J.M. Changes in Gut Microbiome after Bariatric Surgery Versus Medical Weight Loss in a Pilot Randomized Trial. Obes. Surg. 2019, 29, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.Y.; Lin, W.-D.; Huang, C.-K.; Hsin, M.-C.; Lin, W.-Y.; Pryor, A.D. Changes of Gut Microbiota between Different Weight Reduction Programs. Surg. Obes. Relat. Dis. 2019, 15, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Gómez-Pérez, A.M.; García-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut Microbiota Adaptation after Weight Loss by Roux-En-Y Gastric Bypass or Sleeve Gastrectomy Bariatric Surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895. [Google Scholar] [CrossRef]
- Cortez, R.V.; Petry, T.; Caravatto, P.; Pessôa, R.; Sanabani, S.S.; Martinez, M.B.; Sarian, T.; Salles, J.E.; Cohen, R.; Taddei, C.R. Shifts in Intestinal Microbiota after Duodenal Exclusion Favor Glycemic Control and Weight Loss: A Randomized Controlled Trial. Surg. Obes. Relat. Dis. 2018, 14, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R.; Irie, J.; Yamada-Goto, N.; Kikkawa, E.; Seki, Y.; Kasama, K.; Itoh, H. The Impact of Laparoscopic Sleeve Gastrectomy with Duodenojejunal Bypass on Intestinal Microbiota Differs from That of Laparoscopic Sleeve Gastrectomy in Japanese Patients with Obesity. Clin. Drug Investig. 2018, 38, 545–552. [Google Scholar] [CrossRef]
- Chen, H.; Qian, L.; Lv, Q.; Yu, J.; Wu, W.; Qian, H. Change in Gut Microbiota Is Correlated with Alterations in the Surface Molecule Expression of Monocytes after Roux-En-Y Gastric Bypass Surgery in Obese Type 2 Diabetic Patients. Am. J. Transl. Res. 2017, 9, 1243–1254. [Google Scholar]
- Sanmiguel, C.P.; Jacobs, J.; Gupta, A.; Ju, T.; Stains, J.; Coveleskie, K.; Lagishetty, V.; Balioukova, A.; Chen, Y.; Dutson, E.; et al. Surgically Induced Changes in Gut Microbiome and Hedonic Eating as Related to Weight Loss: Preliminary Findings in Obese Women Undergoing Bariatric Surgery. Psychosom. Med. 2017, 79, 880–887. [Google Scholar] [CrossRef]
- Murphy, R.; Tsai, P.; Jüllig, M.; Liu, A.; Plank, L.; Booth, M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes. Surg. 2016, 27, 917–925. [Google Scholar] [CrossRef]
- Ward, E.K.; Schuster, D.P.; Stowers, K.H.; Royse, A.K.; Ir, D.; Robertson, C.E.; Frank, D.N.; Austin, G.L. The Effect of PPI Use on Human Gut Microbiota and Weight Loss in Patients Undergoing Laparoscopic Roux-En-Y Gastric Bypass. Obes. Surg. 2014, 24, 1567–1571. [Google Scholar] [CrossRef]
- Campisciano, G.; Palmisano, S.; Cason, C.; Giuricin, M.; Silvestri, M.; Guerra, M.; Macor, D.; De Manzini, N.; Crocé, L.S.; Comar, M. Gut Microbiota Characterisation in Obese Patients before and after Bariatric Surgery. Benef. Microbes 2018, 9, 367–373. [Google Scholar] [CrossRef]
- Gralka, E.; Luchinat, C.; Tenori, L.; Ernst, B.; Thurnheer, M.; Schultes, B. Metabolomic Fingerprint of Severe Obesity Is Dynamically Affected by Bariatric Surgery in a Procedure-Dependent Manner. Am. J. Clin. Nutr. 2015, 102, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Shu, X.-O.; Howard, E.F.; Long, J.; English, W.J.; Flynn, C.R. Fecal Metagenomics and Metabolomics Reveal Gut Microbial Changes after Bariatric Surgery. Surg. Obes. Relat. Dis. 2020, 16, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Caixàs, A.; Ahlers, M.; Patel, K.; Gao, Z.; Dutia, R.; Blaser, M.J.; Clemente, J.C.; Laferrère, B. Longitudinal Changes of Microbiome Composition and Microbial Metabolomics after Surgical Weight Loss in Individuals with Obesity. Surg. Obes. Relat. Dis. 2019, 15, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-H.; Lin, T.-L.; Lee, W.-J.; Chen, S.-C.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C.; Chen, C.-Y. Impact of Metabolic Surgery on Gut Microbiota and Sera Metabolomic Patterns among Patients with Diabetes. Int. J. Mol. Sci. 2022, 23, 7797. [Google Scholar] [CrossRef] [PubMed]
- Graessler, J.; Qin, Y.; Zhong, H.; Zhang, J.; Licinio, J.; Wong, M.-L.; Xu, A.; Chavakis, T.; Bornstein, A.B.; Ehrhart-Bornstein, M.; et al. Metagenomic Sequencing of the Human Gut Microbiome before and after Bariatric Surgery in Obese Patients with Type 2 Diabetes: Correlation with Inflammatory and Metabolic Parameters. Pharm. J. 2013, 13, 514–522. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Defined Criteria |
|---|---|
| Population | Individuals over the age of 18 with obesity or who were overweight. |
| Intervention | Bariatric surgery: sleeve gastrectomy (SG) and Roux-en-Y gastric bypass. |
| Comparison | Comparison of the gut microbiota profile at different pre- and postsurgical stages. |
| Outcomes | Identification of the impact of the BS on the composition of the GM. |
| Designs | Cohort studies, prospective longitudinal, nonrandomized, randomized clinical trial, and randomized controlled clinical trials. |
| Authors, Country | Study Population at Baseline (Age, BMI) | Sample Size (Surgical Procedures, Sex) | Study Design | Sequencing/Genetic Analysis | Stool Collection Period | Time of Followup |
|---|---|---|---|---|---|---|
| Juaréz-Fernandes et al., 2021 [15], Spain | Age (years): 18–60 BMI (kg/m²): 45.46 ± 2.05 | (N = 9) RYGB: (N = 1) SG: (N = 6) BPD: (N = 2) (M:F): 2:7 | Longitudinal | 16S rRNA (V3–V4) gene sequencing | Before and four years after BS | 4 years |
| Chen et al., 2020 [16], China | Age (years): 30.92 ± 9.17 RYGB: 33.24 ± 10.13 SG: 29.50 ± 8.31 BMI (kg/m²): 40.84 ± 10.67 RYGB: 45.75 ± 14.26 SG: 37.84 ± 6.16 | (N = 87) RYGB: (N = 33) (M:F): 14:19 SG: (N = 54) (M:F): 13:41 | Longitudinal | 16S rDNA (V3-V4) sequencing, RT-PCR | Before and 3 months after BS | 9.60 ± 3.92 months |
| Davies et al., 2020 [17], New Zealand | Age (years): 20–56 RYGB *: 48.5 ± 5.5 SG *: 47.7 ± 6.9 BMI (kg/m²): 35–65 RYGB *: 38.2 ± 5.7 SG *: 40.0 ± 5.9 | (N = 44) RYGB: (N = 22) (M:F): 7:15 SG: (N = 22) (M:F): 14:8 | Randomized Controlled Trial | Genome shotgun sequencing | 2 days before and 1 year after BS | 12 months |
| Faria et al., 2020 [18], Brazil | Age (years): 18–65 BMI (kg/m²): 35–49.9 | (N = 34) CG (preoperative patients): (N = 8) F: 8 RYGB: (N = 26 ) Non-regain: (N = 12) Regain: (N = 14) F: 26 | Cross-sectional | 16S rRNA gene sequencing (V3–V4) | RYGB non-regain: before and 55 months after BS RYGB regain: before and 84 months after BS | At least 5 years RYGB non-regain *: 54.9 ± 34.5 months RYGB regain *: 83.8 ± 40.8 months |
| Farin et al., 2020 [19], France | Age (years): ≥18 BMI (kg/m²): ≥35 | (N = 197) RYGB: (N = 89) SG: (N = 108) Both sexes | Cohort | Shotgun metagenomic sequencing | 1 month before and 6 months after BS | 6 months |
| Koffert et al., 2020 [20], Finland | Age (years): 18–60 BMI (kg/m²): ≥35 40.9 ± 4.2 | (N = 27) RYGB: (N = 6) SG: (N = 7) Controls: (N = 14) F:27 | Clinical trial | 16S rRNA gene sequences | Before and 6 months after BS | 6 months |
| Al Assal et al., 2019 [21], Brazil | Age (years): 18–60 RYGB *: 45.80 ± 7.95 BMI (kg/m²): ≥35 RYGB *: 46.40 ± 5.48 | (N = 25) RYGB: (N = 25) F: 25 | Cohort | 16S rRNA gene sequencing (V4) | Before and 3 and 12 months after BS | 12 months |
| Gutiérrez-Repiso et al., 2019 [22], Spain | Age (years): ≥18 RYGB *: 43.33 ± 9.97 BMI* (kg/m²): 47.03 ± 6.01 | (N = 24) RYGB: (N = 24) Both sexes | Prospective cohort | 16S rRNA (V2, 3, 4, 6-7, 8, and 9) metagenomic sequencing | Before and 8.3 ± 1.7 * years after BS | 8.3 ± 1.7 * years |
| Lee et al., 2019 [23], USA | Age ** (years): 52.5 (32–62) RYGB **: 57 (43–60) SG **: 45 (41–53) BMI (kg/m²): 30–40 RYGB **: 35.1 (31.3–38.6) SG **: 35.8 (33.0–37.6) | (N = 12) MWL: (N = 4) RYGB: (N = 4) SG: (N = 4) F: 12 | Randomized controlled pilot trial | 16S rRNA (V3–V4) amplicon sequencing | RYGB: Before and 1.8 (0.9–5.6) ** after BS SG: Before and 2.3 (2.1–4.3) ** after BS | 3.4 (0.9–9.6) ** months RYGB **: 1.8 (0.9–5.6) SG**: 2.3 (2.1–4.3) |
| Lin et al., 2019 [24], USA | Age (years): 20–64 SG *: 36.2 ± 9.9 BMI (kg/m²): ≥30 SG*: 35.9 ± 4.0 | (N = 10) SG: (N = 10) (M:F): 4:6 | Longitudinal | 16S rRNA (V4) amplicon sequencing | Before and 1 and 3 months after BS | 3 months |
| Sánchez-Alcoholado et al., 2019 [25], Spain | Age (years): 26–63 BMI (kg/m²): RYGB: 43.7 ± 5.3 SG: 46.9 ± 6.6 | (N = 28) RYGB: (N = 14) (M:F): 4:10 SG: (N = 14) (M:F): 4:10 | Longitudinal | 16S rDNA genes next-generation sequencing | Before and 3 months after BS | 3 months |
| Cortez et al., 2018 [26], Brazil | Age (years): 18–64 DJBm *: 47 ± 8 BMI (kg/m²): 25.0–39.9 DJBm *: 29.7 ± 1.9 | (N = 21) Standard medical treatment: (N = 10) DJBm: (N = 11) Sex: not stated | Randomized controlled trial | 16S rRNA (V4) gene sequencing | Before and after 6 and 12 months | 12 months |
| Kikuchi et al., 2018 [27], Japan | Age (years): 18–65 LSG-DJB *: 48.0 ± 2.5 SG *: 40.7 ± 2.0 BMI (kg/m²): >30 | (N = 44) LSG-DJB: (N = 18) (M:F): 10:8 SG: (N = 22) (M:F): 11:11 LAGB: (N = 4) (M:F): 0:4 | Nonrandomized prospective observational clinical trial | 16S rDNA sequencing, RT-PCR | 1, 3 and 6 months | 6 months |
| Chen et al., 2017 [28], China | Age * (years): 51.5 ± 9.6 BMI (kg/m²): ≥40 RYGB *: 46.3 ± 4.7 | (N = 24) RYGB: (N = 24) (M:F): 14:10 | Cohort | 16S rDNA sequencing, RT-PCR | Before and 180 days after BS | 6 months |
| Medina et al., 2017 [5], Chile | Age (years): 18–60 BMI (kg/m²): 30–50 RYGB *: 37.1 ± 2.8 SG *: 35.2 ± 2.4 | (N = 19) MD: (N = 9) RYGB: (N = 5) SG: (N = 5) Sex: not stated | Cohort | 16S rRNA gene sequencing (V3–V4), RT-PCR | Before and 6 months after BBS | 12 months |
| Sanmiguel et al., 2017 [29], EUA | Age * (years): 39.5 ± 8.7 BMI * (kg/m²): 44.1 ± 5.6 | (N = 8) SG: (N = 8) F: 8 | Longitudinal | 16S rRNA gene sequencing (V4) | Before and 1 month after BS | 1 month |
| Murphy et al., 2016 [30], New Zealand | Age (years): RYGB *: 48.6 ± 6.1 SG *: 48.3 ± 6.1 BMI (kg/m²): RYGB *: 38.4 ± 5.2 SG *: 36.9 ± 5.1 | (N = 14) RYGB: (N = 7) (M:F): 3:4 SG: (N = 7) (M:F): 5:2 | Double-blind clinical trial | Shotgun metagenomic sequencing | Before and 1 year after BS | 12 months |
| Ward et al., 2014 [31], USA | Age (years): 18–70 BMI (kg/m²): ≥40 RYGB *: 47.1 ± 4.8 | (N = 8) RYGB: (N = 8) (M:F): 1:7 | Longitudinal | 16S rRNA gene sequencing (V4) | 1 month before and 6 months after BS | 6 months |
| Surgical Procedures | Bacteroidetes | Firmicutes | Firmicutes and Bacteroidetes Ratio | Specific Bacteria |
|---|---|---|---|---|
| RYGB | Increased: 6 months [5,26,28]; 12 months [17,26]. Decreased: 3 months [16]; 6 months [20]; 5–7 years [18]. | Increased: 12 months [17,30]. Stable: 3 months [16]. Decreased: 6 months [5,19,26]; 4 years [15]. | Decreased: 6 months [5]. | B: Increased in 6 months for Succiniclastum sp., Bacteroides, Bacteroides coprophilus, Bacteroides eggerthii [5], Bacteroides, Alistipes [20,26]. F: Increased in 6 months for Clostridiaceae, Clostridium, Veillonella, Granucatiella, Oscillospira [25], Streptococcus [20,21], Sporobacter termitidis [20], Veillonella [21], Gemella, Granulicatella [16], Lactobacillus, Enterococcus [28], Lactobacillales sp. [5], Dialister, Ruminococcus, Roseburia, Acidamicoccus [25], Streptococcus, Veillonella, Roseburia, Enterococcus faecalis [19]; in 9 months for Faecalibacterium prausnitzii [23]; in 4 years for Clostridiaceae [14]; in 5–7 years for Streptococcus, Enterococcus, Lachnobacterium [18]. Decreased in 3 months for Peptostreptococcaceae [25]; in 4 years for Coprococcus Acinetobacter, Coprococcus, Lachnospira, Lactococcus, Megamonas, Oribacterium, Phascolarctobacterium [14]; in 5–7 years for Faecalibacterium [18]. |
| SG | Increased: 1 and 3 months [27]; 12 months [17,29]. Decreased: 6 months [5,20]. | Increased: 6 months [5]. Stable: 3 months [16]. Decreased: 6 months [19]; 12 months [29]; 4 years [15]. | Trend of Increase: 1 and 3 months [27]. Increased: 6 months [5]. Decreased: 12 months [29]. | B: Decreased in 3 months for Butyricimonas [16]. Increased in 6 months for Alistipes [20]. F: Increased in 1 and 3 months for Streptococcus [27]; in 3 months for Gemella, Granulicatella, Faecalibacterium [16]; in 6 months for Streptococcus luteciae [5], Streptococcus spp. [20], Sporobacter termitidis [20], Clostridium, Anaerostipes hadrus, Flavonifractor plautii, Ruminococcus gnavus, Oscillibacter sp. KLE, Veillonela, Streptococcus [19]; in 12 months for Roseburia intestinalis, Streptococcus, Lactobacillus [30], Bulleidia [29]; in 4 years for Clostridiaceae, Acinetobacter, Coprococcus, Lachnospira, Lactococcus, Megamonas, Oribacterium, Phascolarctobacterium [15]. Decreased in 3 months for Clostridiaceae, Anaerostipes [25]; in 6 months for Ruminococcus gnavus, Faecalibacterium prausnitzii [19]; in 4 years for Coprococcus [15]. |
| Surgical Procedures | Actinobacteria | Proteobacteria | Diversity | Specific Bacteria |
|---|---|---|---|---|
| RYGB | Increased: 6 months [5]; 9 months [23]; 12 months [30]. | Increased: 6 months [5]; 9 months [23]; 12 months [17]; 4 years [15]; 5–7 years [18]. | Trend of increase: 9 months [23]; 12 months [21]. Increased: 3 months [16]; 6 months [14,19,26]; 12 months [26,30]; 4 years [15]; 5–7 years [18]. Stable before and after BS: 3 months [25]; 6 months [31]; 12 months [17]. Decreased: 8,3 ± 1,7 years [22]. | A: Increased in 6 months for Bifidobacterium [28]; in 3 months for Slackia. Decreased in 3 months for Bifidobacteriaceae, Bifidobacterium, Collinsella [25]; in 6 months for Bifidobacteria bifidum [19]. P: Increased in 3 months for Enterobacteriacea [25], Neisseria [21], Klebsiella, Haemophilus [16]; in 6 months for Citrobacter [5]; in 12 months for Enterobacteriales [17], Escherichia coli, Klebsiella pneumoniae, Haemophilus parainfluenzae [19]; in 4 years for Enterobacteriaceae, Sinobacteriaceae [15]; in 5–7 years for Succinivibrio, Klebsiella [18]. Decreased in 6 months for Escherichia [28]; in 4 years for Acinetobacter [15]. Verrucomicrobia (Akkermansia muciniphila): Increased in median 1.75 months [23]; in 6 and 12 months [26]; in 9.60 ± 3.92 months [16]; in non-regain group in 5 years. Stable in regain group (15% weight gain increase after the lowest weight after BS) in 5 years [18]. |
| SG | _ | Increased: 6 months [5]; 4 years [15]. | Increased: 3 months [16,24]; 6 months [19,20]; 4 years [15]. Stable before and after BS: 12 months [17]. Stable between RYGB and Sleeve: 3 months [25]. | A: Increased in 12 months for Atopobium [29]. Decreased in 3 months for Bifidobacteriaceae, Bifidobacterium [25], Actinomyces [16]; in 6 months for Bifidobacteria dentium [19]; in 12 months for Bifidobacteriaceae [29]. P: Increased in 3 months for Haemophilus, Klebsiella [16]; in 6 months for Enterobacteriales Bulleidia, Escherichia coli [5], Klebsiella pneumoniae, Haemophilus parainfluenzae [19]; in 4 years for Enterobacteriaceae, Sinobacteriaceae [14]. Decreased in 3 months for Oxalobacter, Sutterella, Desulfovibrio [16]; in 4 years for Acinetobacter [14]. Verrucomicrobia (Akkermansia muciniphila): Increased in 3 months [27]; in 6 months [5]; in 9.60 ± 3.92 months [16]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coimbra, V.O.R.; Crovesy, L.; Ribeiro-Alves, M.; Faller, A.L.K.; Mattos, F.; Rosado, E.L. Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review. Nutrients 2022, 14, 4979. https://doi.org/10.3390/nu14234979
Coimbra VOR, Crovesy L, Ribeiro-Alves M, Faller ALK, Mattos F, Rosado EL. Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review. Nutrients. 2022; 14(23):4979. https://doi.org/10.3390/nu14234979
Chicago/Turabian StyleCoimbra, Vívian O. R., Louise Crovesy, Marcelo Ribeiro-Alves, Ana Luísa K. Faller, Fernanda Mattos, and Eliane L. Rosado. 2022. "Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review" Nutrients 14, no. 23: 4979. https://doi.org/10.3390/nu14234979
APA StyleCoimbra, V. O. R., Crovesy, L., Ribeiro-Alves, M., Faller, A. L. K., Mattos, F., & Rosado, E. L. (2022). Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review. Nutrients, 14(23), 4979. https://doi.org/10.3390/nu14234979








