Erythrocyte Membrane Docosahexaenoic Acid (DHA) and Lipid Profile in Preterm Infants at Birth and Over the First Month of Life: A Comparative Study with Infants at Term
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Samples
2.3. Analysis of Fatty Acids
2.4. Panel of Fatty Acids
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Fatty Acid Composition of Erythrocyte Membrane
3.3. Fatty Acid Rations and Enzyme Activity Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.H. Polyunsaturated fatty acids in children. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 153–161. [Google Scholar] [CrossRef]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane. Database. Syst. Rev. 2017, 2017, CD000376. [Google Scholar] [CrossRef] [PubMed]
- Gerster, H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int. J. Vitam. Nutr. Res. 1998, 68, 159–173. [Google Scholar] [PubMed]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.; Rodríguez González-Herrero, M.E.; Romeo Villadóniga, S.; Domingo, J.C. Antioxidant activity and neuroprotective role of docosahexaenoic acid (DHA) supplementation in eye diseases that can lead to blindness: A narrative review. Antioxidants 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Mesa-Herrera, F.; Marín, R. DHA and its elaborated modulation of antioxidant defenses of the brain: Implications in aging and ad neurodegeneration. Antioxidants 2021, 10, 907. [Google Scholar] [CrossRef]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.; Ciappolino, V.; Agostoni, C. DHA effects in brain development and function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Mallick, R.; Basak, S.; Duttaroy, A.K. Docosahexaenoic acid,22:6n-3: Its roles in the structure and function of the brain. Int. J. Dev. Neurosci. 2019, 79, 21–31. [Google Scholar] [CrossRef]
- Meldrum, S.; Simmer, K. Docosahexaenoic acid and neurodevelopmental outcomes of term infants. Ann. Nutr. Metab. 2016, 69 (Suppl. S1), 22–28. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Kadri, L.; Bacle, A.; Khoury, S.; Vandebrouck, C.; Bescond, J.; Faivre, J.F.; Ferreira, T.; Sebille, S. Polyunsaturated phospholipids increase cell resilience to mechanical constraints. Cells 2021, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Kinnun, J.J.; Leng, X.; Williams, J.A.; Wassall, S.R. How polyunsaturated fatty acids modify molecular organization in membranes: Insight from NMR studies of model systems. Biochim. Biophys. Acta 2015, 1848 Pt B, 211–219. [Google Scholar] [CrossRef]
- Haggarty, P. Placental regulation of fatty acid delivery and its effect on fetal growth–A review. Placenta 2002, 23, S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef]
- Koletzko, B.; Larqué, E.; Demmelmair, H. Placental transfer of long-chain polyunsaturated fatty acids (LC-PUFA). J. Perinat. Med. 2007, 35 (Suppl. S1), S5–S11. [Google Scholar] [CrossRef]
- Mukherjee, M.; Yuil-Valdes, A.; Nordgren, T.M.; Ulu, A.; Harris Jackson, K.; Anderson-Berry, A. Intrauterine transfer of polyunsaturated fatty acids in mother-infant dyads as analyzed at time of delivery. Nutrients 2021, 13, 996. [Google Scholar] [CrossRef]
- Baack, M.L.; Puumala, S.E.; Messier, S.E.; Pritchett, D.K.; Harris, W.S. What is the relationship between gestational age and docosahexaenoic acid (DHA) and arachidonic acid (ARA) levels? Prostaglandins Leukot. Essent. Fatty Acids 2015, 100, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.T.; Martin, C.R. Fatty acid requirements for the preterm infant. Semin. Fetal Neonatal Med. 2017, 22, 8–14. [Google Scholar] [CrossRef]
- Fares, S.; Sethom, M.M.; Kacem, S.; Khouaja-Mokrani, C.; Feki, M.; Kaabachi, N. Plasma arachidonic and docosahexaenoic acids in Tunisian very low birth weight infants: Status and association with selected neonatal morbidities. J. Health Popul. Nutr. 2015, 33, 1. [Google Scholar] [CrossRef][Green Version]
- Hellström, A.; Pivodic, A.; Gränse, L.; Lundgren, P.; Sjöbom, U.; Nilsson, A.K.; Söderling, H.; Hård, A.L.; Smith, L.E.H.; Löfqvist, C.A. Association of docosahexaenoic acid and arachidonic acid serum levels with retinopathy of prematurity in preterm infants. JAMA Netw. Open 2021, 4, e2128771. [Google Scholar] [CrossRef] [PubMed]
- Castillo, F.; Castillo-Ferrer, F.J.; Cordobilla, B.; Domingo, J.C. Inadequate content of docosahexaenoic acid (DHA) of donor human milk for feeding preterm infants: A comparison with mother’s own milk at different stages of lactation. Nutrients 2021, 13, 1300. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; MacKay, D.; Friel, J.K. Fatty acid composition in feeds and plasma of Canadian premature infants. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Berenhauser, A.C.; Pinheiro do Prado, A.C.; da Silva, R.C.; Gioielli, L.A.; Block, J.M. Fatty acid composition in preterm and term breast milk. Int. J. Food Sci. Nutr. 2012, 63, 318–325. [Google Scholar] [CrossRef]
- Juber, B.A.; Jackson, K.H.; Johnson, K.B.; Harris, W.S.; Baack, M.L. Breast milk DHA levels may increase after informing women: A community-based cohort study from South Dakota USA. Int. Breastfeed. J 2017, 12, 7. [Google Scholar] [CrossRef]
- Jackson, K.H.; Harris, W.S. Should there be a target level of docosahexaenoic acid in breast milk? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 92–96. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid. Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Andersen, S.B.; Hellgren, L.I.; Larsen, M.K.; Verder, H.; Leuritzen, L. Long-chain polyunsaturated fatty acids in breast-milk and erythrocytes and neurodevelopmental outcomes in Danish late-preterm infants. J. Pregnancy Child Health 2015, 2, 3. [Google Scholar] [CrossRef]
- Lapillonne, A.; Brossard, N.; Claris, O.; Reygrobellet, B.; Salle, B.L. Erythrocyte fatty acid composition in term infants fed human milk or a formula enriched with a low eicosapentanoic acid fish oil for 4 months. Eur. J. Pediatr. 2000, 159, 49–53. [Google Scholar] [CrossRef]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Göpel, W.; Härtel, C.; German Neonatal Network, German Center for Lung Research and Priming Immunity at the beginning of life (PRIMAL) Consortium. Preterm birth and sustained inflammation: Consequences for the neonate. Semin. Immunopathol. 2020, 42, 451–468. [Google Scholar] [CrossRef]
- Cappelletti, M.; Della Bella, S.; Ferrazzi, E.; Mavilio, D.; Divanovic, S. Inflammation and preterm birth. J. Leukoc. Biol. 2016, 99, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Areia, A.L.; Mota-Pinto, A. Inflammation and preterm birth: A systematic review. Reprod. Med. 2022, 3, 101–111. [Google Scholar] [CrossRef]
- Papandreou, P.; Gioxari, A.; Ntountaniotis, D.; Korda, O.N.; Skouroliakou, M.; Siahanidou, T. Administration of an intravenous fat emulsion enriched with medium-chain triglyceride/ω-3 fatty acids is beneficial towards anti-inflammatory related fatty acid profile in preterm neonates: A randomized, double-blind clinical trial. Nutrients 2020, 12, 3526. [Google Scholar] [CrossRef] [PubMed]
- Skouroliakou, M.; Konstantinou, D.; Agakidis, C.; Kaliora, A.; Kalogeropoulos, N.; Massara, P.; Antoniadi, M.; Panagiotakos, D.; Karagiozoglou-Lampoudi, T. Parenteral MCT/ω-3 polyunsaturated fatty acid-enriched intravenous fat emulsion is associated with cytokine and fatty acid profiles consistent with attenuated inflammatory response in preterm neonates: A randomized, double-blind clinical trial. Nutr. Clin. Pract. 2016, 31, 235–244. [Google Scholar] [CrossRef]
- Lapillonne, A.; Groh-Wargo, S.; Gonzalez, C.H.; Uauy, R. Lipid needs of preterm infants: Updated recommendations. J. Pediatr. 2013, 162 (Suppl. S3), S37–S47. [Google Scholar] [CrossRef]
- Gibson, R.A.; Muhlhausler, B.; Makrides, M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child Nutr. 2011, 7 (Suppl. S2), 17–26. [Google Scholar] [CrossRef]
- Shulkin, M.; Pimpin, L.; Bellinger, D.; Kranz, S.; Fawzi, W.; Duggan, C.; Mozaffarian, D. n-3 Fatty acid supplementation in mothers, preterm infants, and term infants and childhood psychomotor and visual development: A systematic review and meta-analysis. J. Nutr. 2018, 148, 409–418. [Google Scholar] [CrossRef]
- Boehm, G.; Borte, M.; Böhles, H.J.; Müller, H.; Kohn, G.; Moro, G. Docosahexaenoic and arachidonic acid content of serum and red blood cell membrane phospholipids of preterm infants fed breast milk, standard formula or formula supplemented with n-3 and n-6 long-chain polyunsaturated fatty acids. Eur. J. Pediatr. 1996, 155, 410–416. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Uauy, R. Essentiality of dietary omega 3 fatty acids for premature infants: Plasma and red blood cell fatty acid composition. Lipids 1992, 27, 886–895. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Wheaton, D.K.; James, K.J.; Tuazon, M.; Diersen-Schade, D.A.; Harris, C.L.; Stolz, S.; Berseth, C.L. Docosahexaenoic acid in red blood cells of term infants receiving two levels of long-chain polyunsaturated fatty acids. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 287–292. [Google Scholar] [CrossRef]
- Sauerwald, U.C.; Fink, M.M.; Demmelmair, H.; Schoenaich, P.V.; Rauh-Pfeiffer, A.A.; Koletzko, B. Effect of different levels of docosahexaenoic acid supply on fatty acid status and linoleic and α-linolenic acid conversion in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.T.; Sullivan, T.R.; McPhee, A.J.; Stark, M.J.; Makrides, M.; Gibson, R.A. A dose response randomised controlled trial of docosahexaenoic acid (DHA) in preterm infants. Prostaglandins Leukot. Essent. Fatty Acids 2015, 99, 1–6. [Google Scholar] [CrossRef]
- Smith, S.L.; Rouse, C.A. Docosahexaenoic acid and the preterm infant. Matern. Health. Neonatol. Perinatol. 2017, 3, 22. [Google Scholar] [CrossRef]
- Tanaka, K.; Tanaka, S.; Shah, N.; Ota, E.; Namba, F. Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Devarshi, P.P.; Grant, R.W.; Ikonte, C.J.; Hazels Mitmesser, S. Maternal omega-3 nutrition, placental transfer and fetal brain development in gestational diabetes and preeclampsia. Nutrients 2019, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Innis, S.M. Controversial nutrients that potentially affect preterm neurodevelopment: Essential fatty acids and iron. Pediatr. Res. 2005, 57, 99R–103R. [Google Scholar] [CrossRef] [PubMed]
- Najm, S.; Löfqvist, C.; Hellgren, G.; Engström, E.; Lundgren, P.; Hård, A.L.; Lapillonne, A.; Sävman, K.; Nilsson, A.K.; Andersson, M.X.; et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: A randomized controlled trial. Clin. Nutr. ESPEN 2017, 20, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Simmer, K.; Deshmukh, M.; Mori, T.A.; Croft, K.D.; Kristensen, J. Fish oil (SMOFlipid) and olive oil lipid (Clinoleic) in very preterm neonates. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 177–182. [Google Scholar] [CrossRef]
- D’Ascenzo, R.; Savini, S.; Biagetti, C.; Bellagamba, M.P.; Marchionni, P.; Pompilio, A.; Cogo, P.E.; Carnielli, V.P. Higher docosahexaenoic acid, lower arachidonic acid and reduced lipid tolerance with high doses of a lipid emulsion containing 15% fish oil: A randomized clinical trial. Clin. Nutr. 2014, 33, 1002–1009. [Google Scholar] [CrossRef]
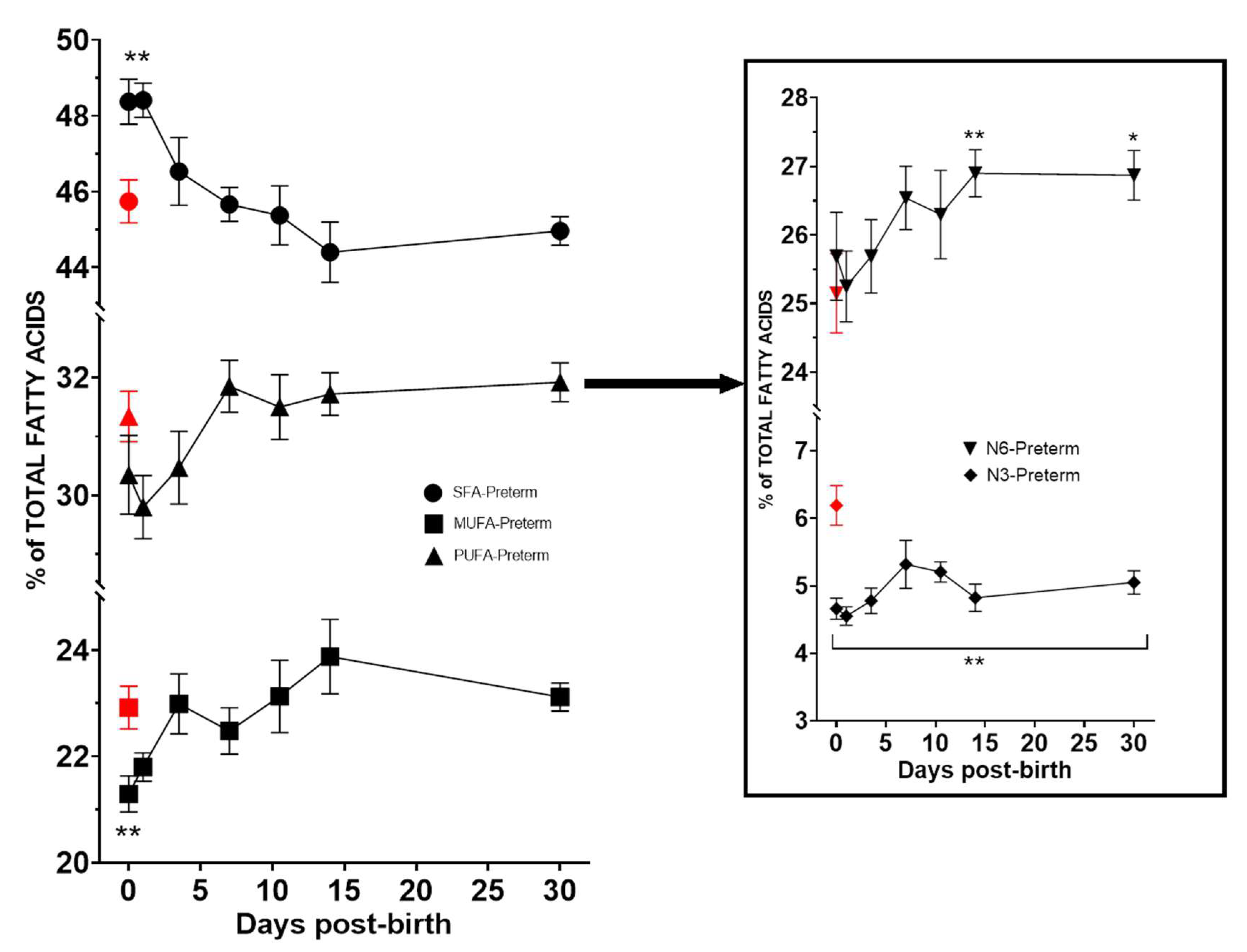

| Variables | Neonates | |
|---|---|---|
| Preterm (n = 65) | Term (n = 10) | |
| Infant characteristics | ||
| Gestational age, weeks, mean (SD) (range) | 28.7 (2.2) (24.4–32.3) | 38.5 (1.7) (37.2–41) |
| Birth weight, g, mean (SD) (range) | 1098 (338.7) (470–1910) | 2798 (354.6) (2170–4050) |
| Male sex, n (%) | 37 (59.6) | 6 (60) |
| Cesarean section, n (%) | 51 (78.5) | 2 (20) |
| Maternal characteristics | ||
| Age, years, mean (SD) (range) | 32.6 (6.4) (17–48) | 31.8 (4.2) (22–39) |
| Gravida, median (range) | 1 (0–14) | 2 (0–3) |
| Country of origin, n (%) | ||
| Spain | 45 (69.2) | 7 (70) |
| Other European countries | 5 (7.7) | 0 |
| South America | 3 (4.6) | 0 |
| North Africa | 4 (6.2) | 0 |
| Sub-Saharan Africa | 1 (1.5) | 2 (20) |
| Asia | 7 (10.8) | 1 (10) |
| Nutritional management | ||
| Age starting enteral feeding, hours, mean (SD) | 22.5 (6–144) | |
| Age at full enteral feeding, days, mean (SD) | 9.8 (5–18) | |
| Total parenteral nutrition, n (%) | 64 (98.5) | |
| Days of total parenteral nutrition, mean (SD) | 7.4 (0–36) | |
| Fatty Acid Family (% Molar of Total Fatty Acid) | Term Infants at Birth (n = 10) | Preterm Infants at Birth (n = 30) | p Value | Preterm Infants at 30 Days (n = 17) | p Value |
|---|---|---|---|---|---|
| SFAs | 45.74 (1.79) | 48.37 (3.25) | 0.003 | 44.96 (1.55) | 0.365 |
| MUFAs | 22.92 (1.27) | 21.29 (1.86) | 0.005 | 23.12 (1.09) | 0.421 |
| PUFAs | 31.34 (1.35) | 30.35 (3.66) | 0.938 | 31.92 (1.36) | 0.333 |
| n-6 PUFAs | 24.06 (1.19) | 25.69 (3.50) | 0.004 | 26.87 (1.50) | <0.0001 |
| n-3 PUFAs | 6.19 (0.92) | 4.66 (0.86) | <0.0001 | 5.05 (0.71) | 0.001 |
| Fatty Acid (% Molar of Total Fatty Acid) | Term Infants at Birth (n = 10) | Preterm Infants at Birth (n = 30) | p Value | Preterm Infants at 30 Days (n = 17) | p Value |
|---|---|---|---|---|---|
| SFAs | |||||
| Palmitic acid (C16:0) | 23.53 (1.59) | 23.16 (1.86) | 0.114 | 22.13 (1.49) | 0.079 |
| Stearic acid (C18:0) | 16.16 (1.54) | 17.09 (0.90) | 0.036 | 15.95 (0.70) | 0.702 |
| Lignoceric acid (C24:0) | 3.57 (0.38) | 5.43 (0.88) | <0.0001 | 4.03 (0.86) | 0.114 |
| MUFAs | |||||
| Oleic acid (C18:1 n9) | 14.52 (1.09) | 12.05 (1.44) | <0.0001 | 14.60 (0.87) | 0.666 |
| Nervonic acid (C24:1 n9) | 5.38 (0.90) | 5.44 (0.88) | 0.275 | 5.30 (0.53) | 0.980 |
| n-6 PUFAs | |||||
| LA (C18:2 n6) | 9.05 (1.22) | 3.91 (0.71) | <0.0001 | 8.60 (1.88) | 0.242 |
| ARA (C20:4 n6) | 11.30 (1.24) | 15.35 (2.32) | <0.0001 | 12.79 (1,53) | 0.017 |
| n-3 PUFAs | |||||
| ALA (C18:3 n3) | 0.13 (0.04) | 0.06 (0.04) | <0.0001 | 0.11 (0.05) | 0.216 |
| EPA (C20:5 n3) | 0.88 (0.62) | 0.16 (0.08) | <0.0001 | 0.58 (0.36) | 0.407 |
| DPA (C22:5 n3) | 1.31 (0.33) | 0.30 (0.09) | <0.0001 | 0.80 (0.27) | 0.0002 |
| DHA (C22:6 n3) | 3.86 (0.34) | 4.15 (0.79) | 0.236 | 3.56 (0.47) | 0.076 |
| Term Infants at Birth (n = 10) | Preterm Infants at Birth (n = 30) | p Value | Preterm Infants at 30 Days (n = 17) | p Value | |
|---|---|---|---|---|---|
| Fatty acid ratios: | |||||
| Omega-3 index | 4.73 (0.60) | 4.30 (0.81) | 0.150 | 4.14 (0.53) | 0.015 |
| n-6 PUFA/n-3 PUFA | 4.16 (0.80) | 5.70 (1.40) | 0.0003 | 5.43 (0.91) | 0.0018 |
| ARA/DHA | 2.90 (0.38) | 3.81 (0.87) | 0.0003 | 3.63 (0.51) | 0.0006 |
| ARA/EPA | 25.34 (23.46) | 132.10 (91.86) | <0.0001 | 36.06 (22.85) | 0.242 |
| EPA/DHA | 0.23 (0.17) | 0.038 (0.020) | <0.0001 | 0.18 (0.18) | 0.464 |
| LA/ARA | 0.81 (0.17) | 0.26 (0.07) | <0.0001 | 0.70 (0.25) | 0.053 |
| AIFAI, % | 57.38 (11.62) | 44.02 (8.71) | <0.0001 | 49.54 (7.50) | 0.028 |
| EFASTI | 1.37 (0.10) | 1.44 (0.25) | 0.739 | 1.38 (0.10) | 0.814 |
| DHASI | 8.70 (2.51) | 4.32 (1.65) | <0.0001 | 5.50 (1.87) | 0.001 |
| DHADI | 0.28 (0.07) | 0.38 (0.06) | 0.0006 | 0.28 (0.07) | 0.719 |
| Enzyme activity indexes: | |||||
| 16-SCD | 0.037 (0.024) | 0.034 (0.010) | 0.453 | 0.025 (0.011) | 0.177 |
| 18-SCD | 0.88 (0.16) | 0.71 (0.10) | 0.0019 | 0.92 (0.07) | 0.173 |
| D5D | 6.88 (1.41) | 6.97 (1.87) | >0.999 | 5.93 (1.20) | 0.023 |
| D6D | 0.18 (0.10) | 0.60 (0.13) | <0.0001 | 0.27 (0.08) | 0.001 |
| Elongase | 0.69 (0.10) | 0.74 (0.14) | 0.120 | 0.72 (0.05) | 0.143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo Salinas, F.; Montaner Ramón, A.; Castillo Ferrer, F.-J.; Domingo-Carnice, A.; Cordobilla, B.; Domingo, J.C. Erythrocyte Membrane Docosahexaenoic Acid (DHA) and Lipid Profile in Preterm Infants at Birth and Over the First Month of Life: A Comparative Study with Infants at Term. Nutrients 2022, 14, 4956. https://doi.org/10.3390/nu14234956
Castillo Salinas F, Montaner Ramón A, Castillo Ferrer F-J, Domingo-Carnice A, Cordobilla B, Domingo JC. Erythrocyte Membrane Docosahexaenoic Acid (DHA) and Lipid Profile in Preterm Infants at Birth and Over the First Month of Life: A Comparative Study with Infants at Term. Nutrients. 2022; 14(23):4956. https://doi.org/10.3390/nu14234956
Chicago/Turabian StyleCastillo Salinas, Félix, Alicia Montaner Ramón, Félix-Joel Castillo Ferrer, Adrià Domingo-Carnice, Begoña Cordobilla, and Joan Carles Domingo. 2022. "Erythrocyte Membrane Docosahexaenoic Acid (DHA) and Lipid Profile in Preterm Infants at Birth and Over the First Month of Life: A Comparative Study with Infants at Term" Nutrients 14, no. 23: 4956. https://doi.org/10.3390/nu14234956
APA StyleCastillo Salinas, F., Montaner Ramón, A., Castillo Ferrer, F.-J., Domingo-Carnice, A., Cordobilla, B., & Domingo, J. C. (2022). Erythrocyte Membrane Docosahexaenoic Acid (DHA) and Lipid Profile in Preterm Infants at Birth and Over the First Month of Life: A Comparative Study with Infants at Term. Nutrients, 14(23), 4956. https://doi.org/10.3390/nu14234956






