Effects of Intestinal Bacterial Hydrogen Gas Production on Muscle Recovery following Intense Exercise in Adult Men: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Preparation of the Test Beverage
2.4. Determination of VO2max
2.5. Respiratory Gas Analyses
2.6. Blood Sample Measurements
2.7. Determination of 8-Hydroxy-2′-deoxyguanosine (8-OHdG) Concentration
2.8. Fatigue and Muscle Soreness
2.9. Breath H2 Concentration Measurements
2.10. Fecal Microbiota Analysis
2.11. Statistical Analysis
3. Results
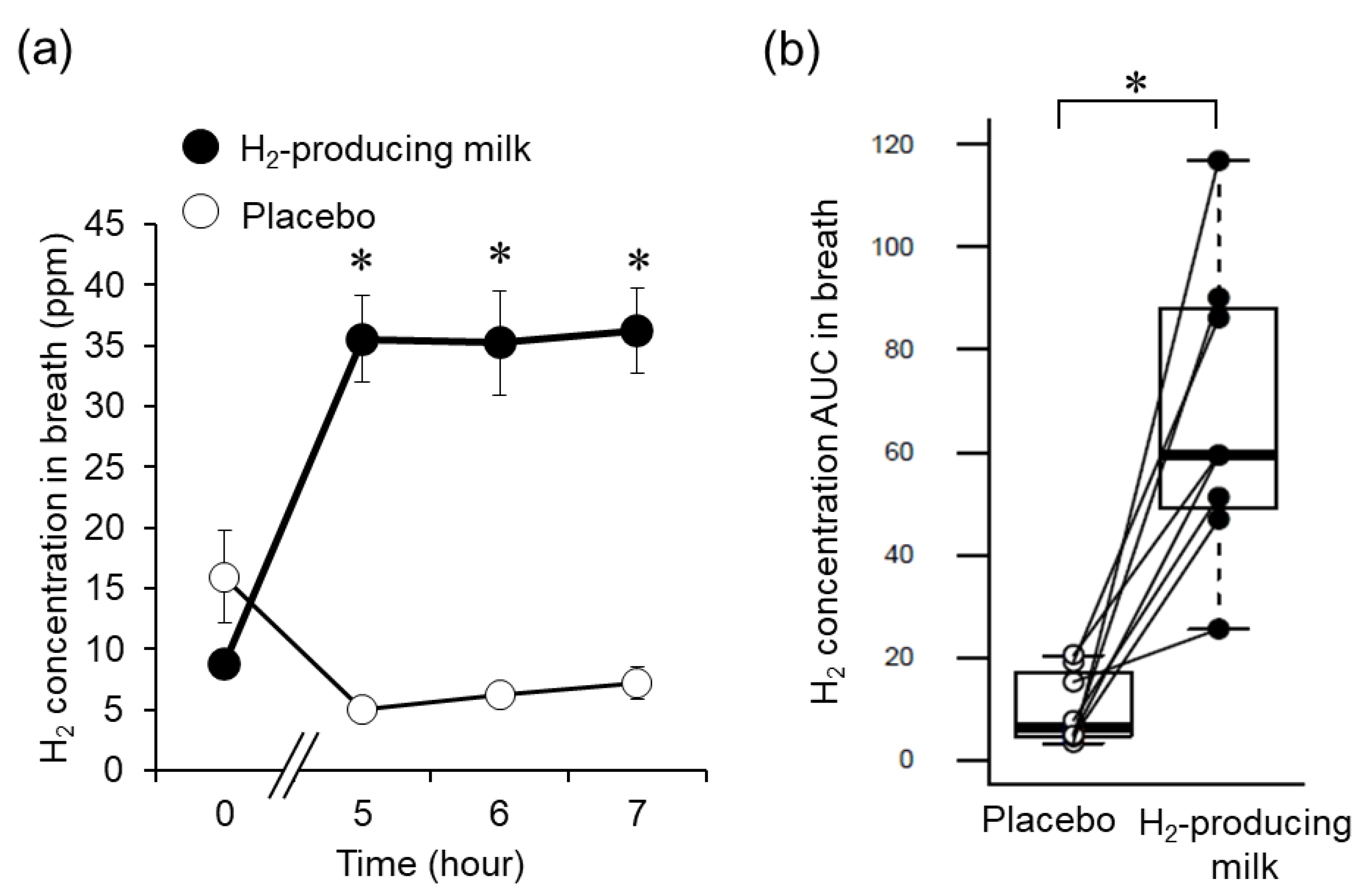
3.1. Breath H2 Concentration Changes
3.2. Muscle Soreness and Fatigue
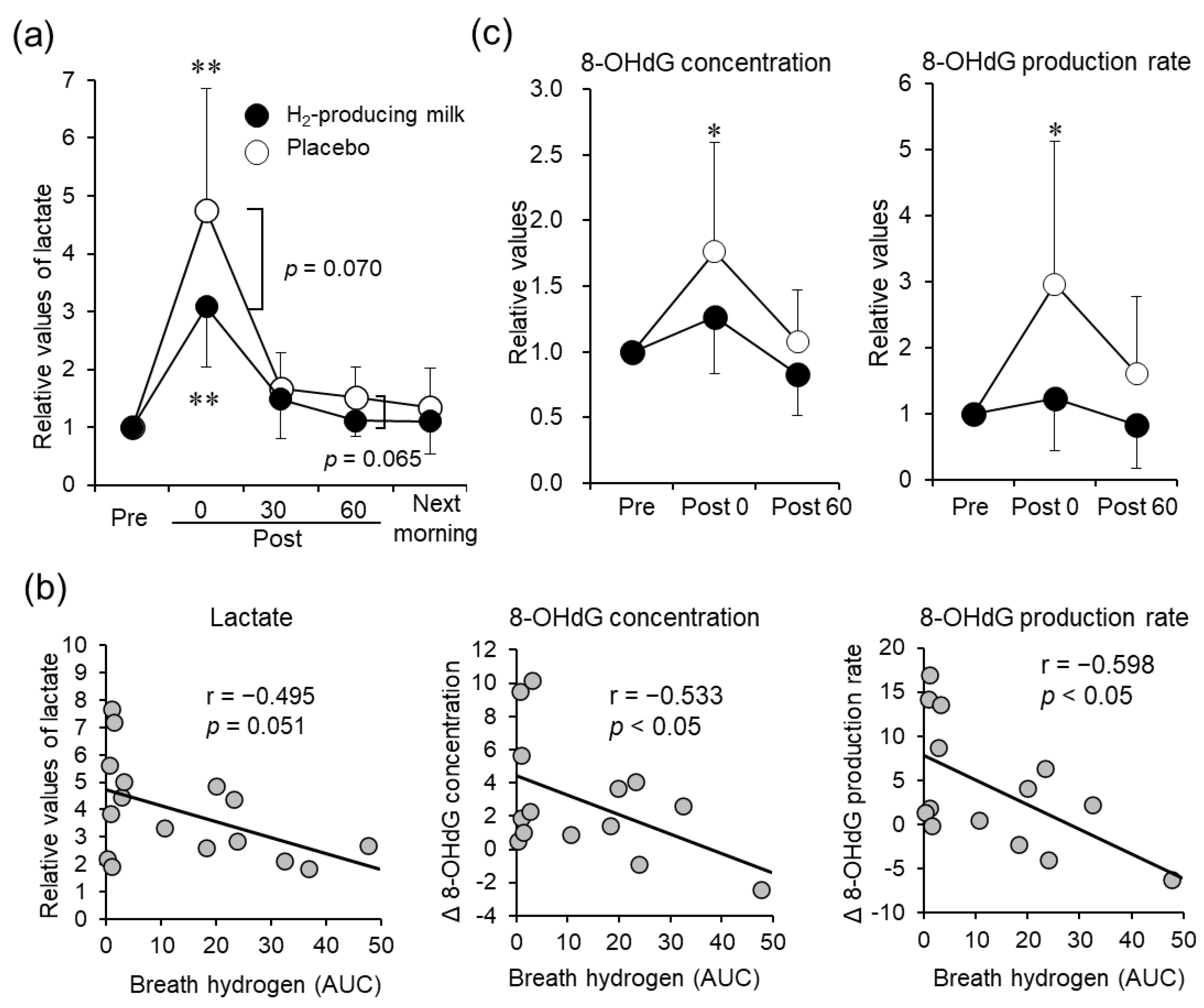
3.3. Blood Samples
3.4. Urinary 8-OHdG
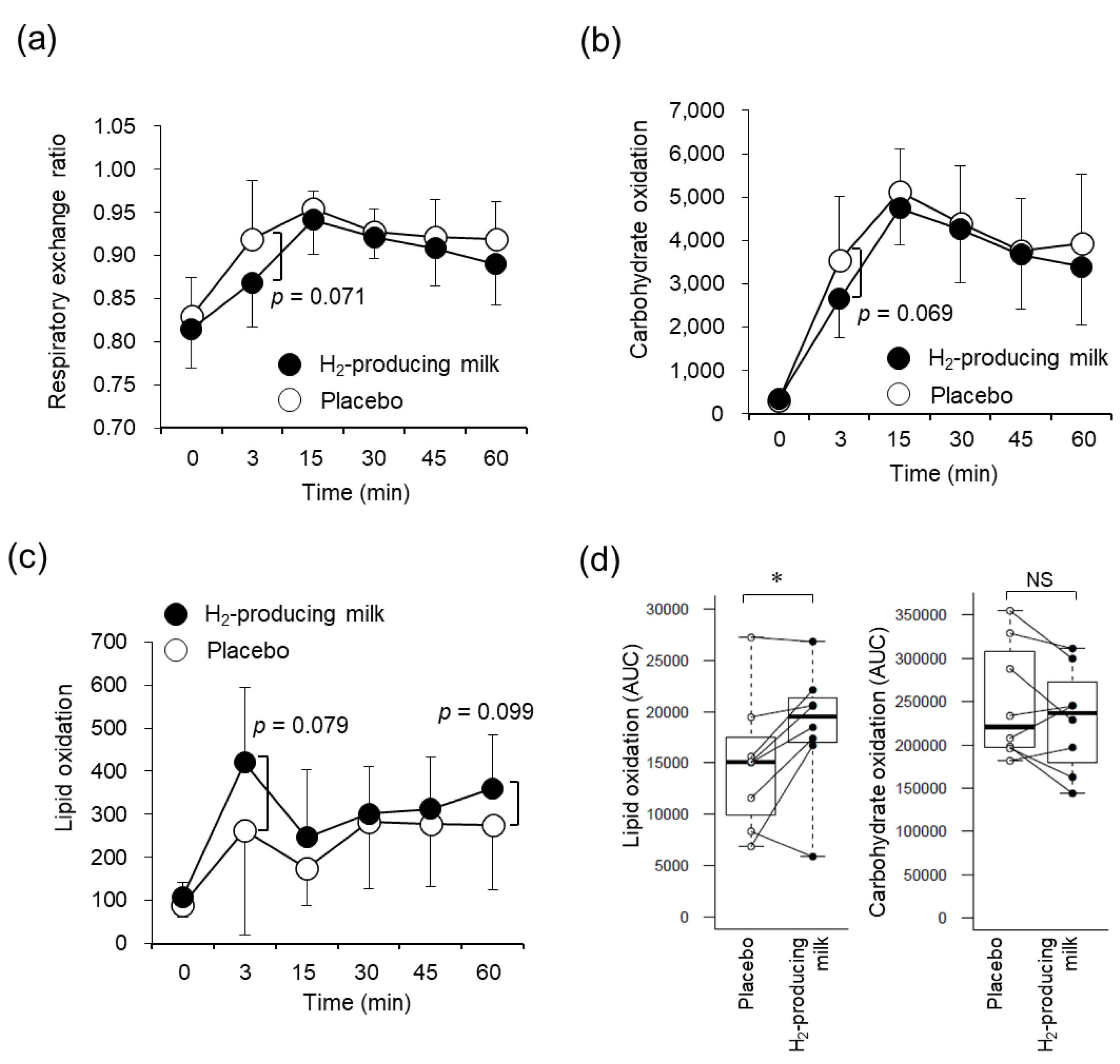
3.5. Respiratory Variables
3.6. Fecal Microbiota
4. Discussion
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Lovlin, R.; Cottle, W.; Pyke, I.; Kavanagh, M.; Belcastro, A.N. Are indices of free radical damage related to exercise intensity. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 313–316. [Google Scholar] [CrossRef]
- Sachdev, S.; Davies, K.J. Production, detection, and adaptive responses to free radicals in exercise. Free Radic. Biol. Med. 2008, 44, 215–223. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.E.; Hodges, N.J.; Bosch, J.A.; Aldred, S. Prolonged depletion of antioxidant capacity after ultraendurance exercise. Med. Sci. Sports Exerc. 2011, 43, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef]
- Kawamura, T.; Higashida, K.; Muraoka, I. Application of Molecular Hydrogen as a Novel Antioxidant in Sports Science. Oxid. Med. Cell. Longev. 2020, 2020, 2328768. [Google Scholar] [CrossRef]
- Da Ponte, A.; Giovanelli, N.; Nigris, D.; Lazzer, S. Effects of hydrogen rich water on prolonged intermittent exercise. J. Sports Med. Phys. Fitness 2018, 58, 612–621. [Google Scholar] [CrossRef]
- Shibayama, Y.; Dobashi, S.; Arisawa, T.; Fukuoka, T.; Koyama, K. Impact of hydrogen-rich gas mixture inhalation through nasal cannula during post-exercise recovery period on subsequent oxidative stress, muscle damage, and exercise performances in men. Med. Gas Res. 2020, 10, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Nakao, A.; Adachi, T.; Matsui, Y.; Miyakawa, S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med. Gas Res. 2012, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Vukomanovic, B.; Calleja-Gonzalez, J.; Hoffman, J.R. Effectiveness of oral and topical hydrogen for sports-related soft tissue injuries. Postgrad. Med. 2014, 126, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, N.; Ichimiya, H.; Iuchi, K.; Ohta, S. Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1alpha to enhance fatty acid metabolism. NPJ Aging Mech. Dis. 2016, 2, 16008. [Google Scholar] [CrossRef] [PubMed]
- Shimouchi, A.; Nose, K.; Yamaguchi, M.; Ishiguro, H.; Kondo, T. Breath hydrogen produced by ingestion of commercial hydrogen water and milk. Biomark. Insights 2009, 4, 27–32. [Google Scholar] [CrossRef]
- Matsumoto, M.; Fujita, A.; Yamashita, A.; Kameoka, S.; Shimomura, Y.; Kitada, Y.; Tamada, H.; Nakamura, S.; Tsubota, K. Effects of functional milk containing galactooligosaccharide, maltitol, and glucomannan on the production of hydrogen gas in the human intestine. J. Funct. Foods 2017, 35, 13–23. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Sonnenburg, J.L. Eating for two: How metabolism establishes interspecies interactions in the gut. Cell Host Microbe 2011, 10, 336–347. [Google Scholar] [CrossRef]
- Levitt, M.D. Production and excretion of hydrogen gas in man. N. Engl. J. Med. 1969, 281, 122–127. [Google Scholar] [CrossRef]
- Hammer, H.F. Colonic hydrogen absorption: Quantification of its effect on hydrogen accumulation caused by bacterial fermentation of carbohydrates. Gut 1993, 34, 818–822. [Google Scholar] [CrossRef]
- Kawashima, M.; Tsuno, S.; Matsumoto, M.; Tsubota, K. Hydrogen-producing milk to prevent reduction in tear stability in persons using visual display terminals. Ocul. Surf. 2019, 17, 714–721. [Google Scholar] [CrossRef]
- Kawano, H.; Mineta, M.; Asaka, M.; Miyashita, M.; Numao, S.; Gando, Y.; Ando, T.; Sakamoto, S.; Higuchi, M. Effects of different modes of exercise on appetite and appetite-regulating hormones. Appetite 2013, 66, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Elia, M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: Evaluation of errors with special reference to the detailed composition of fuels. Am. J. Clin. Nutr. 1988, 47, 608–628. [Google Scholar] [CrossRef]
- Dill, D.B.; Costill, D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974, 37, 247–248. [Google Scholar] [CrossRef]
- Yuen, K.H. The transit of dosage forms through the small intestine. Int. J. Pharm. 2010, 395, 9–16. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Baumert, P.; Lake, M.J.; Stewart, C.E.; Drust, B.; Erskine, R.M. Genetic variation and exercise-induced muscle damage: Implications for athletic performance, injury and ageing. Eur. J. Appl. Physiol. 2016, 116, 1595–1625. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, P.P.; Strzelec, B. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: A review. Folia Morphol. 2018, 77, 171–178. [Google Scholar] [CrossRef]
- Orhan, H.; van Holland, B.; Krab, B.; Moeken, J.; Vermeulen, N.P.; Hollander, P.; Meerman, J.H. Evaluation of a multi-parameter biomarker set for oxidative damage in man: Increased urinary excretion of lipid, protein and DNA oxidation products after one hour of exercise. Free Radic. Res. 2004, 38, 1269–1279. [Google Scholar] [CrossRef]
- Yasuda, N.; Bolin, C.; Cardozo-Pelaez, F.; Ruby, B.C. Effects of repeated bouts of long-duration endurance exercise on muscle and urinary levels of 8-hydroxy-2’-deoxyguanosine in moderately trained cyclists. J. Sports Sci. 2015, 33, 1692–1701. [Google Scholar] [CrossRef]
- Bejma, J.; Ji, L.L. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J. Appl. Physiol. 1999, 87, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Ichikawa, H.; Yoshida, N.; Yoshikawa, T. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic. Biol. Med. 2004, 37, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Zielinski, M.R.; Groschwitz, C.M.; Brown, A.S.; Gangemi, J.D.; Ghaffar, A.; Mayer, E.P. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2168–R2173. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Nogueira, J.E.; Amorim, M.R.; Pinto, A.P.; da Rocha, A.L.; da Silva, A.S.R.; Branco, L.G.S. Molecular hydrogen downregulates acute exhaustive exercise-induced skeletal muscle damage. Can. J. Physiol. Pharmacol. 2021, 99, 812–820. [Google Scholar] [CrossRef]
- Close, G.L.; Ashton, T.; McArdle, A.; Maclaren, D.P. The emerging role of free radicals in delayed onset muscle soreness and contraction-induced muscle injury. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 257–266. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Kibe, R.; Kurihara, S.; Sakai, Y.; Suzuki, H.; Ooga, T.; Sawaki, E.; Muramatsu, K.; Nakamura, A.; Yamashita, A.; Kitada, Y.; et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014, 4, 4548. [Google Scholar] [CrossRef] [PubMed]
- Costea, P.I.; Zeller, G.; Sunagawa, S.; Pelletier, E.; Alberti, A.; Levenez, F.; Tramontano, M.; Driessen, M.; Hercog, R.; Jung, F.E.; et al. Towards standards for human fecal sample processing in metagenomic studies. Nat. Biotechnol. 2017, 35, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Suda, W.; Kim, S.; Oshima, K.; Fukuda, S.; Ohno, H.; Morita, H.; Hattori, M. Robustness of gut microbiota of healthy adults in response to probiotic intervention revealed by high-throughput pyrosequencing. DNA Res. 2013, 20, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
| Measurements | Interaction | Time | Trial | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Post 30 | Post 60 | Next Morning | (ηp2) | (ηp2) | (ηp2) | ||
| General muscle soreness | HPM | 2.5 (4.5) | 27.0 (28.3) a | 25.0 (22.3) a | 10.0 (22.5) | 6.5 (16.0) | 0.418 | <0.001 | 0.113 |
| Placebo | 3.0 (7.3) | 41.5 (27.8) a | 29.0 (21.3) a | 18.5 (19.5) | 24.5 (17.5) b | (0.126) | (0.637) | (0.319) | |
| Lower limbs soreness | HPM | 3.0 (6.3) | 34.5 (33.8) a | 27.0 (31.5) | 11.0 (29.5) | 12.5 (30.5) | 0.351 | <0.001 | 0.400 |
| Placebo | 3.5 (6.5) | 48.5 (40.3) a | 28.5 (27.0) a | 21.5 (37.0) | 28.5 (32.8) a | (0.142) | (0.646) | (0.103) | |
| Fatigue | HPM | 2.0 (9.0) | 58.5 (22.0) b | 49.0 (26.3) a | 38.0 (38.0) | 27.0 (28.0) a | 0.878 | <0.001 | 0.680 |
| Placebo | 12.0 (14.3) | 67.0 (13.0) b | 43.0 (31.8) | 38.0 (24.3) | 38.5 (15.8) a | (0.041) | (0.744) | (0.026) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eda, N.; Tsuno, S.; Nakamura, N.; Sone, R.; Akama, T.; Matsumoto, M. Effects of Intestinal Bacterial Hydrogen Gas Production on Muscle Recovery following Intense Exercise in Adult Men: A Pilot Study. Nutrients 2022, 14, 4875. https://doi.org/10.3390/nu14224875
Eda N, Tsuno S, Nakamura N, Sone R, Akama T, Matsumoto M. Effects of Intestinal Bacterial Hydrogen Gas Production on Muscle Recovery following Intense Exercise in Adult Men: A Pilot Study. Nutrients. 2022; 14(22):4875. https://doi.org/10.3390/nu14224875
Chicago/Turabian StyleEda, Nobuhiko, Saki Tsuno, Nobuhiro Nakamura, Ryota Sone, Takao Akama, and Mitsuharu Matsumoto. 2022. "Effects of Intestinal Bacterial Hydrogen Gas Production on Muscle Recovery following Intense Exercise in Adult Men: A Pilot Study" Nutrients 14, no. 22: 4875. https://doi.org/10.3390/nu14224875
APA StyleEda, N., Tsuno, S., Nakamura, N., Sone, R., Akama, T., & Matsumoto, M. (2022). Effects of Intestinal Bacterial Hydrogen Gas Production on Muscle Recovery following Intense Exercise in Adult Men: A Pilot Study. Nutrients, 14(22), 4875. https://doi.org/10.3390/nu14224875






