Long-Term Diet Quality and Risk of Diabetes in a National Survey of Chinese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Dietary Data Collection
2.3. DBI-16
2.4. Definition of Diabetes
2.5. Other Variables
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the DBI-16 Sub-Components on the Contribution
3.2. Baseline Characteristics of the Study Participants
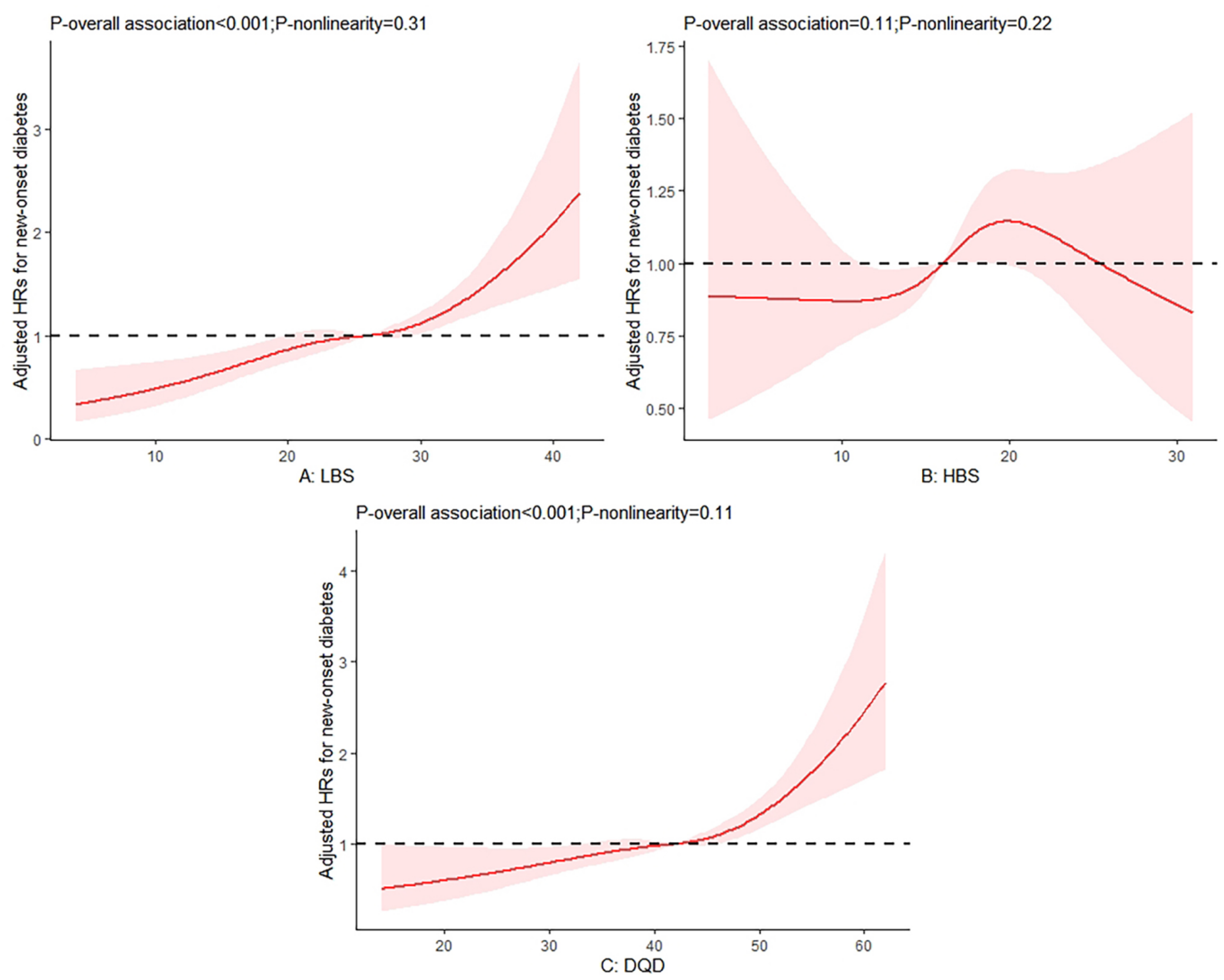
3.3. Associations of Diet Quality with the Risks of Diabetes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas. Available online: https://diabetesatlas.org/ (accessed on 27 September 2022).
- Ma, R.C.W. Epidemiology of diabetes and diabetic complications in China. Diabetologia 2018, 61, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.L. Diabetes mellitus survey in China. Chin. Med. J. 1982, 95, 423–430. [Google Scholar] [PubMed]
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef]
- Papamichou, D.; Panagiotakos, D.B.; Itsiopoulos, C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr. Metab. Cardiovasc. Dis. NMCD 2019, 29, 531–543. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Hoffmann, G.; Schwingshackl, L. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2020, 120, 1998–2031.e1915. [Google Scholar] [CrossRef]
- He, Y.N. Chinese Diet Balance Index: Approach and Application. Master’s Thesis, Peking Union Medical College, Beijing, China, 2004. [Google Scholar]
- Yang, C.; Zhao, A.; Lan, H.; Ren, Z.; Zhang, J.; Szeto, I.M.; Wang, P.; Zhang, Y. Association Between Dietary Quality and Postpartum Depression in Lactating Women: A Cross-Sectional Survey in Urban China. Front. Nutr. 2021, 8, 705353. [Google Scholar] [CrossRef]
- Wang, X.; Liu, A.; Du, M.; Wu, J.; Wang, W.; Qian, Y.; Zheng, H.; Liu, D.; Nan, X.; Jia, L.; et al. Diet quality is associated with reduced risk of hypertension among Inner Mongolia adults in northern China. Public Health Nutr. 2020, 23, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- He, Y.N.; Fang, Y.H.; Xia, J. Update of the Chinese diet balance index: DBI_16. J. Acta. Nutr. Sin. 2018, 40, 526–530. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, G.Y.; Pan, X.C. China Food Composition Table; Institute of Nutrition and Food Safety of the Chinese Center for Disease Control and Prevention. China Food Composition 2002; Peking University Medical Press: Beijing, China, 2002. [Google Scholar]
- Yang, Y.X. China Food Composition Table; Institute of Nutrition and Food Safety of the Chinese Center for Disease Control and Prevention. China Food Composition 2004; Peking University Medical Press: Beijing, China, 2005. [Google Scholar]
- Xu, X.; Hall, J.; Byles, J.; Shi, Z. Assessing dietary quality of older Chinese people using the Chinese Diet Balance Index (DBI). PLoS ONE 2015, 10, e0121618. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Steffen, L.M.; Selvin, E.; Rebholz, C.M. Diet quality, change in diet quality and risk of incident CVD and diabetes. Public Health Nutr. 2020, 23, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- Jacobs, S.; Harmon, B.E.; Boushey, C.J.; Morimoto, Y.; Wilkens, L.R.; Le Marchand, L.; Kröger, J.; Schulze, M.B.; Kolonel, L.N.; Maskarinec, G. A priori-defined diet quality indexes and risk of type 2 diabetes: The Multiethnic Cohort. Diabetologia 2015, 58, 98–112. [Google Scholar] [CrossRef]
- Chen, G.C.; Koh, W.P.; Neelakantan, N.; Yuan, J.M.; Qin, L.Q.; van Dam, R.M. Diet Quality Indices and Risk of Type 2 Diabetes Mellitus: The Singapore Chinese Health Study. Am. J. Epidemiol. 2018, 187, 2651–2661. [Google Scholar] [CrossRef]
- Yu, D.; Zheng, W.; Cai, H.; Xiang, Y.B.; Li, H.; Gao, Y.T.; Shu, X.O. Long-term Diet Quality and Risk of Type 2 Diabetes Among Urban Chinese Adults. Diabetes Care 2018, 41, 723–730. [Google Scholar] [CrossRef]
- Pan, X.F.; Pan, A. Dietary transitions and cardiometabolic health in China. Lancet. Diabetes Endocrinol. 2019, 7, 502–503. [Google Scholar] [CrossRef]
- Popkin, B.M. Synthesis and implications: China’s nutrition transition in the context of changes across other low- and middle-income countries. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2014, 15, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, Z.; Wang, H.; Zhao, L.; Jiang, H.; Zhang, B.; Ding, G. Nutrition transition and related health challenges over decades in China. Eur. J. Clin. Nutr. 2021, 75, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.; Hosseini, Z.; Lafrenière, J.; Conklin, A.I. The role of dietary diversity in preventing metabolic-related outcomes: Findings from a systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021, 22, e13174. [Google Scholar] [CrossRef] [PubMed]
- Toi, P.L.; Anothaisintawee, T.; Chaikledkaew, U.; Briones, J.R.; Reutrakul, S.; Thakkinstian, A. Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review. Nutrients 2020, 12, 2722. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ruan, W.; Peng, Y.; Wang, D. Soy and the risk of type 2 diabetes mellitus: A systematic review and meta-analysis of observational studies. Diabetes Res. Clin. Pract. 2018, 137, 190–199. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2017, 9, 982. [Google Scholar] [CrossRef]
- Tang, J.; Wan, Y.; Zhao, M.; Zhong, H.; Zheng, J.S.; Feng, F. Legume and soy intake and risk of type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 111, 677–688. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Dietary Guidelines for Chinese Residents (2022); Chinese Nutrition Society: Beijing, China, 2022. [Google Scholar]
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ 2019, 366, l2368. [Google Scholar] [CrossRef]
- Hu, G.; Jousilahti, P.; Peltonen, M.; Lindström, J.; Tuomilehto, J. Urinary sodium and potassium excretion and the risk of type 2 diabetes: A prospective study in Finland. Diabetologia 2005, 48, 1477–1483. [Google Scholar] [CrossRef]
- Radzeviciene, L.; Ostrauskas, R. Adding Salt to Meals as a Risk Factor of Type 2 Diabetes Mellitus: A Case-Control Study. Nutrients 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: A meta-analysis of 10 prospective studies and 136,846 participants. Metab. Clin. Exp. 2014, 63, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Missbach, B.; König, J.; Hoffmann, G. Adherence to a Mediterranean diet and risk of diabetes: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin. J. Diabetes 2021, 13, 315–409. [Google Scholar]
| LBS | % | HBS | % | DQD | % |
|---|---|---|---|---|---|
| Dairy | 21.37 | Cereal | 48.48 | Cereal | 19.40 |
| Diet variety | 20.74 | Salt | 18.97 | Dairy | 13.00 |
| Fruit | 19.13 | Oil | 16.37 | Diet variety | 12.61 |
| Fish | 10.18 | Meat | 11.01 | Fruit | 11.64 |
| Soybean | 9.97 | Egg | 3.92 | Salt | 7.44 |
| Egg | 7.55 | Alcohol | 0.82 | Meat | 6.54 |
| Vegetable | 6.74 | Sugar | 0.42 | Oil | 6.41 |
| Meat | 3.65 | Fish | 6.19 | ||
| Cereal | 0.66 | Egg | 6.13 | ||
| Soybean | 6.06 | ||||
| Vegetable | 4.10 | ||||
| Alcohol | 0.32 | ||||
| Sugar | 0.17 |
| Characteristics | LBS Levels | p Value | HBS levels | p Value | DQD Levels | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L1 | L2 | L3 | L4 | L1&2 | L3 | L4 | ||||
| N | 384 | 3847 | 4858 | 305 | 591 | 6133 | 2565 | 105 | 1718 | 6508 | 1168 | |||
| Score range | 0–12 | 13–24 | 25–36 | >36 | 0–9 | 10–18 | 19–27 | >27 | 0–34 | 35–50 | >50 | |||
| Age(years) | 53.0 [39.8; 60.0] | 48.0 [38.0; 57.0] | 48.0 [39.0; 58.0] | 51.0 [41.0; 62.0] | <0.001 | 49.0 [38.0; 59.0] | 48.0 [39.0; 57.0] | 49.0 [39.0; 58.0] | 47.0 [38.0; 55.0] | 0.070 | 49.0 [38.0; 58.0] | 48.0 [39.0;57.0] | 51.0 [41.0;59.0] | <0.001 |
| Male (%) | 38.8 | 45.6 | 47.1 | 47.2 | 0.012 | 40.1 | 44.7 | 50.4 | 62.9 | <0.001 | 39.2 | 47.2 | 50.4 | <0.001 |
| Urban site (%) | 88.5 | 58.5 | 26.2 | 22.0 | <0.001 | 63.1 | 38.0 | 45.7 | 48.6 | <0.001 | 71.1 | 37.5 | 22.8 | <0.001 |
| Education level (%) | <0.001 | <0.001 | <0.001 | |||||||||||
| Primary school | 8.6 | 24.4 | 47.5 | 65.2 | 19.1 | 38.7 | 37.3 | 28.6 | 14.1 | 39.7 | 55.7 | |||
| Middle school | 23.7 | 30.7 | 32.6 | 22.6 | 27.2 | 31.2 | 31.9 | 33.3 | 27.8 | 32.3 | 29.8 | |||
| High school or above | 67.7 | 44.9 | 19.9 | 12.1 | 53.6 | 30.1 | 30.8 | 38.1 | 58.1 | 28.0 | 14.5 | |||
| Occupation (%) # | <0.001 | <0.001 * | <0.001 | |||||||||||
| Farmer or worker | 13.3 | 33.8 | 69.5 | 84.7 | 30.8 | 57.0 | 54.7 | 47.0 | 22.7 | 57.1 | 80.3 | |||
| Service staff | 15.7 | 22.4 | 13.4 | 8.1 | 17.4 | 16.2 | 17.6 | 20.5 | 18.5 | 17.8 | 8.3 | |||
| Managers or technicians | 62.9 | 37.2 | 11.8 | 6.4 | 43.7 | 21.6 | 21.3 | 27.7 | 53.1 | 18.8 | 8.5 | |||
| Other | 8.1 | 6.6 | 5.3 | 0.8 | 8.1 | 5.3 | 6.4 | 4.8 | 5.7 | 6.3 | 3.0 | |||
| Smoking (%) | 22.1 | 29.6 | 33.5 | 34.4 | <0.001 | 25.5 | 30.5 | 34.8 | 41.0 | <0.001 | 23.9 | 32.3 | 38.1 | <0.001 |
| Alcohol drinking(%) | 34.9 | 35.8 | 33.5 | 29.2 | 0.034 | 30.3 | 32.8 | 38.1 | 56.2 | <0.001 | 33.8 | 34.2 | 36.0 | 0.408 |
| BMI(kg/m2) | 23.9 [21.9; 26.1] | 23.7 [21.4; 25.9] | 23.0 [21.0; 25.2] | 23.0 [20.5; 24.8] | <0.001 | 23.4 [21.2; 25.6] | 23.2 [21.1; 25.4] | 23.7 [21.5; 25.9] | 23.4 [21.4; 26.0] | <0.001 | 23.6 [21.5; 25.9] | 23.4 [21.2;25.5] | 23.0 [21.0;25.2] | <0.001 |
| Physical activity | <0.001 | <0.001 | ||||||||||||
| Inadequate | 33.1 | 34.7 | 31.9 | 33.1 | 36.0 | 33.7 | 31.8 | 18.1 | 36.0 | 32.8 | 30.7 | |||
| Low | 8.3 | 4.5 | 2.6 | 0.7 | 7.3 | 3.0 | 3.9 | 8.6 | 6.2 | 2.9 | 3.2 | |||
| Moderate or high | 58.6 | 60.8 | 65.5 | 66.2 | 56.7 | 63.3 | 64.3 | 73.3 | 57.8 | 64.2 | 66.2 | |||
| Dietary intake | ||||||||||||||
| Total energy (kcal/day) | 1774 [1481; 2144] | 1970 [1638; 2327] | 2050 [1692; 2418] | 1789 [1395; 2211] | <0.001 | 1973 [1692; 2314] | 2055 [1749; 2392] | 1842 [1399; 2312] | 1425 [1145; 1673] | <0.001 | 1962 [1674; 2274] | 2027 [1675;2402] | 1877 [1432;2325] | <0.001 |
| Fat(g/day) | 78.2 [66.9; 91.4] | 77.4 [66.6; 90.6] | 67.4 [53.8; 80.7] | 55.8 [38.9; 71.2] | <0.001 | 83.2 [71.6; 96.4] | 70.7 [57.0; 84.5] | 72.7 [60.0; 85.8] | 80.9 [70.9; 92.5] | <0.001 | 80.1 [68.3; 93.3] | 71.7 [58.5;84.9] | 61.7 [47.5;77.4] | <0.001 |
| Carbohydrate (g/day) | 243 [213; 270] | 257 [227; 283] | 290 [257; 322] | 323 [286; 360] | <0.001 | 240 [212; 267] | 279 [244; 312] | 272 [239; 302] | 238 [210; 265] | <0.001 | 246 [218; 273] | 277 [244;308] | 307 [266;340] | <0.001 |
| Protein(g/day) | 82.2 [73.8; 92.4] | 69.3 [63.0; 77.4] | 59.9 [54.6; 66.3] | 53.6 [48.5; 59.2] | 0.000 | 71.7 [61.5; 84.0] | 63.2 [56.8; 70.9] | 64.9 [57.7; 73.3] | 70.9 [63.1; 78.7] | <0.001 | 74.0 [66.3; 84.3] | 63.3 [57.3;70.3] | 56.8 [50.5;63.2] | 0.000 |
| Hypertension (%) | 22.7 | 11.4 | 9.2 | 7.5 | <0.001 | 14.4 | 9.8 | 11.7 | 10.5 | 0.001 | 15.0 | 9.6 | 9.4 | <0.001 |
| Levels of the DBI | Score Range | N | Cases/Person-Years | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| LBS | ||||||
| L1 | 0–12 | 384 | 12/1614 | Ref | Ref | Ref |
| L2 | 13–24 | 3847 | 250/23,585 | 2.35 [1.31, 4.21] | 2.27 [1.27, 4.08] | 2.43 [1.36, 4.37] |
| L3 | 25–36 | 4858 | 365/35,228 | 2.67 [1.48, 4.84] | 2.58 [1.43, 4.68] | 3.05 [1.69, 5.53] |
| L4 | >36 | 305 | 30/1552 | 3.97 [1.99, 7.92] | 3.86 [1.94, 7.70] | 4.90 [2.46, 9.78] |
| HBS | ||||||
| L1 | 0–9 | 591 | 33/2428 | Ref | Ref | Ref |
| L2 | 10–18 | 6133 | 406/44,459 | 1.02 [0.71, 1.46] | 0.99 [0.69, 1.43] | 1.06 [0.74, 1.51] |
| L3 | 19–27 | 2565 | 213/14,700 | 1.35 [0.93, 1.96] | 1.33 [0.92, 1.93] | 1.30 [0.90, 1.88] |
| L4 | >27 | 105 | 5/391 | 0.93 [0.36, 2.38] | 0.94 [0.37, 2.42] | 0.99 [0.39, 2.55] |
| DQD | ||||||
| L1&2 | 0–34 | 1718 | 95/8969 | Ref | Ref | Ref |
| L3 | 35–50 | 6508 | 445/45,987 | 1.23 [0.97, 1.56] | 1.21 [0.96, 1.54] | 1.28 [1.01, 1.61] |
| L4 | >50 | 1168 | 117/7023 | 1.88 [1.40, 2.53] | 1.88 [1.39, 2.52] | 2.10 [1.57, 2.82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Y.; Zhang, Z.; Liu, A. Long-Term Diet Quality and Risk of Diabetes in a National Survey of Chinese Adults. Nutrients 2022, 14, 4841. https://doi.org/10.3390/nu14224841
Hua Y, Zhang Z, Liu A. Long-Term Diet Quality and Risk of Diabetes in a National Survey of Chinese Adults. Nutrients. 2022; 14(22):4841. https://doi.org/10.3390/nu14224841
Chicago/Turabian StyleHua, Yumeng, Ziwei Zhang, and Aiping Liu. 2022. "Long-Term Diet Quality and Risk of Diabetes in a National Survey of Chinese Adults" Nutrients 14, no. 22: 4841. https://doi.org/10.3390/nu14224841
APA StyleHua, Y., Zhang, Z., & Liu, A. (2022). Long-Term Diet Quality and Risk of Diabetes in a National Survey of Chinese Adults. Nutrients, 14(22), 4841. https://doi.org/10.3390/nu14224841





