Intra-Amniotic Administration—An Emerging Method to Investigate Necrotizing Enterocolitis, In Vivo (Gallus gallus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Animals and Study Design
Intra-Amniotic Administration
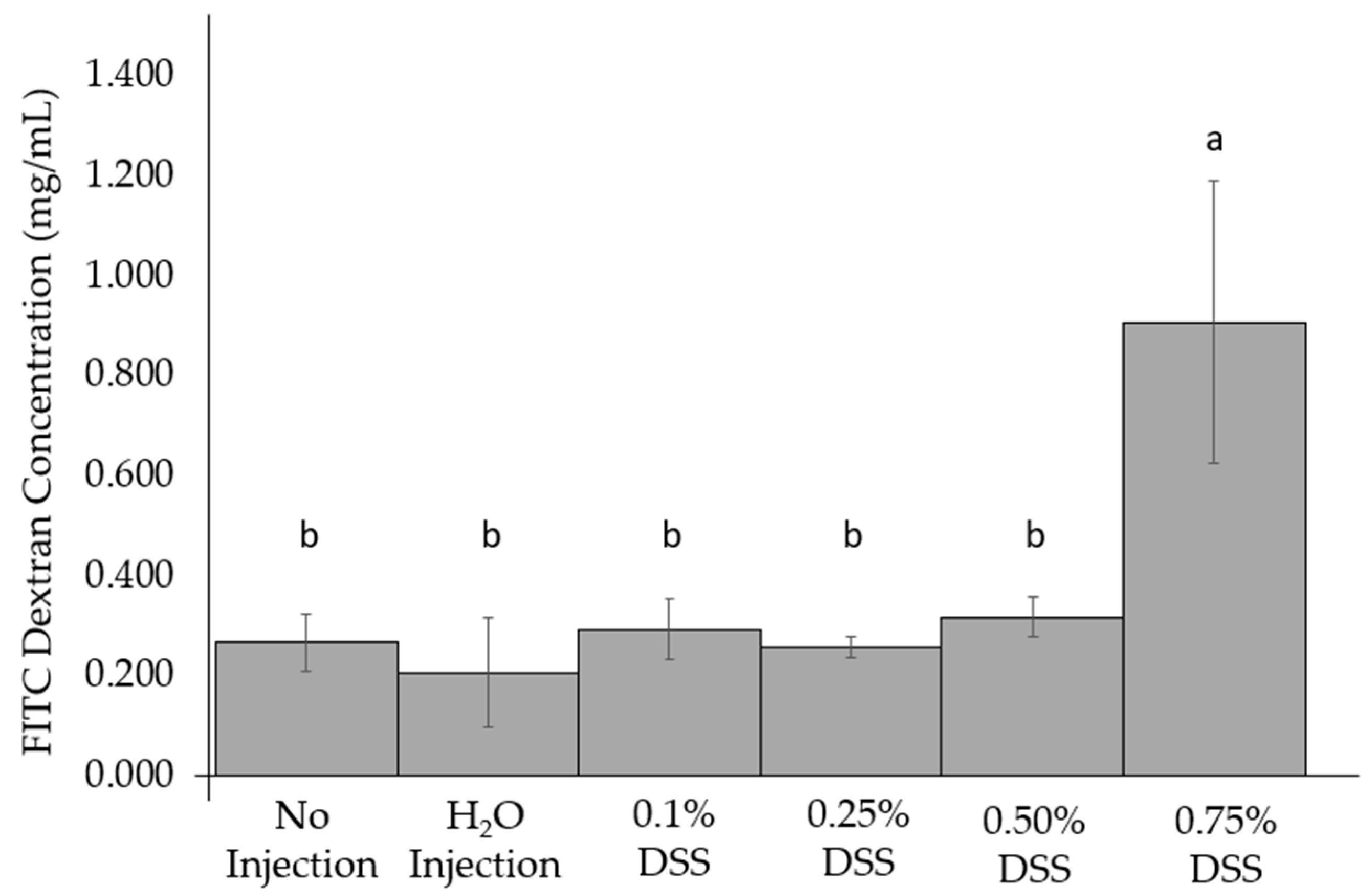
2.3. Intestinal Permeability Test: Fluorescein Isothiocyanate Dextran (FITC-Dextran) Test
2.4. Glycogen Analysis as a Measurement of Energetic Status
2.5. Isolation of the Total RNA from the Duodenum Samples
2.6. Real-Time Polymerase Chain Reaction (RT-PCR)
2.7. Intestinal Primer Design and Real-Time Quantitative PCR Design
2.8. Intestinal Content DNA Isolation, Bacterial Primer Design, and PCR Amplification of Bacterial 16S rDNA
2.9. Morphological Examination
2.10. Statistical Analysis
3. Results
3.1. Gross Physical Findings
3.2. Hb Concentration and Hepatic Glycogen Levels
3.3. Change of Intestinal Permeability across the Groups
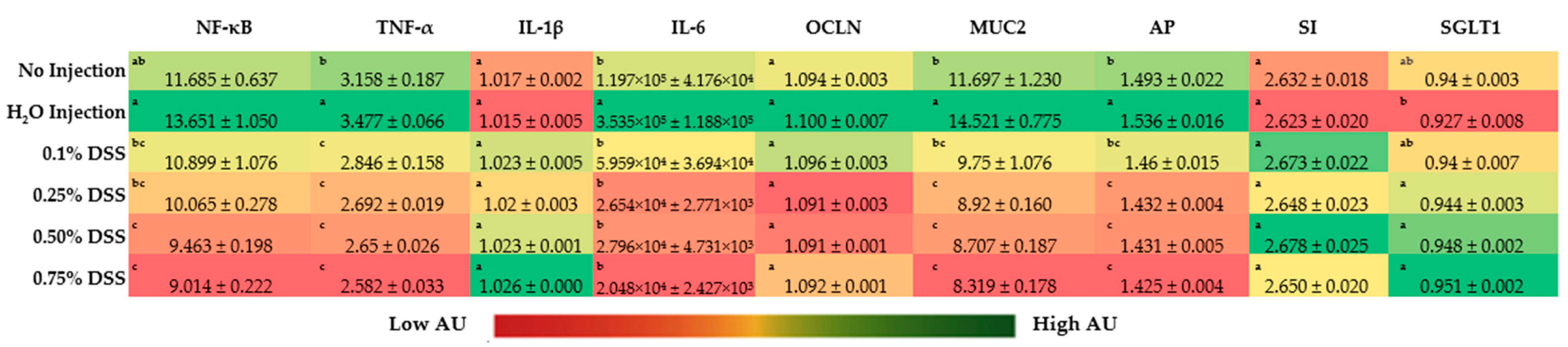
3.4. Duodenal Gene Expression
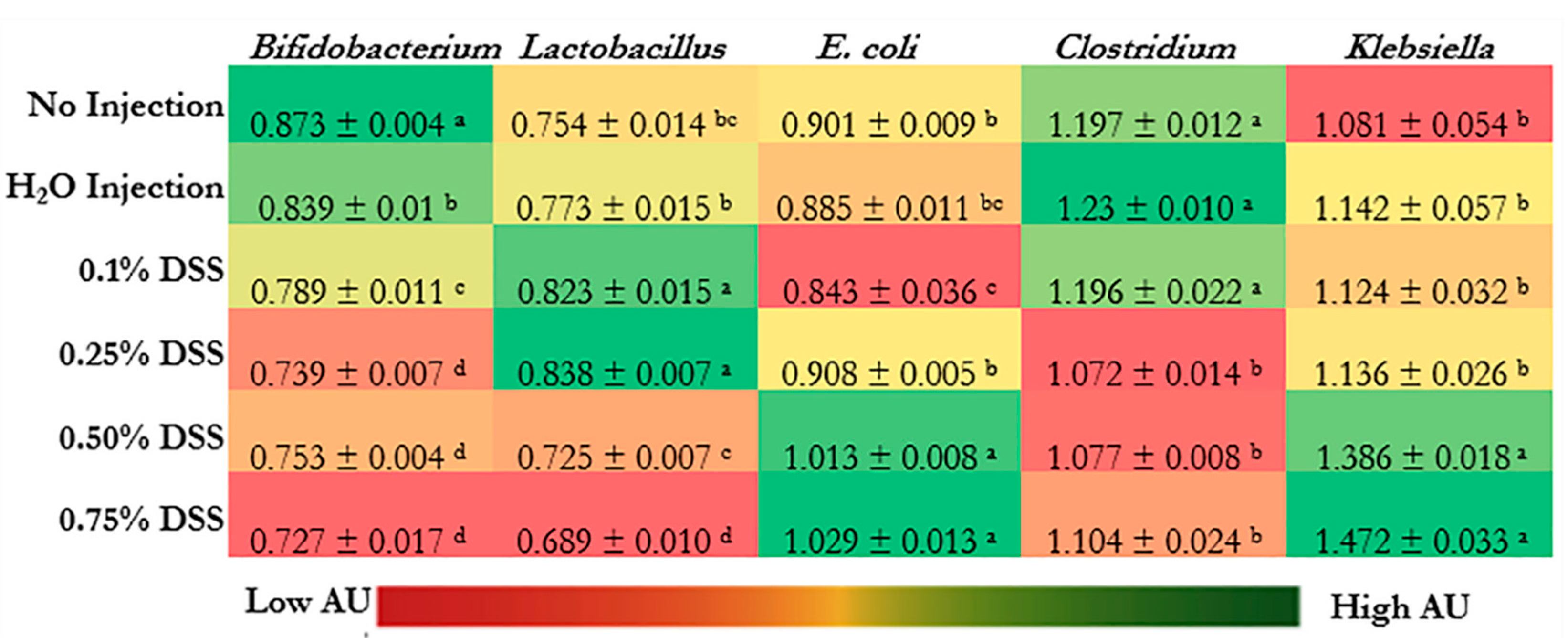
3.5. Microbial Dysbiosis
3.6. Intestinal Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alsaied, A.; Islam, N.; Thalib, L. Global Incidence of Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. BMC Pediatr. 2020, 20, 344. [Google Scholar] [CrossRef]
- Kosloske, A. Epidemiology of Necrotizing Enterocolitis. Acta Paediatr. 1994, 83, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.; Rees, C.M.; Hall, N.J. Current Research on the Epidemiology, Pathogenesis, and Management of Necrotizing Enterocolitis. Neonatology 2017, 111, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Faraday, C.; Hamad, S.; Jones, K.D.; Sim, K.; Cherian, S.; James, A.; Godambe, S.; New, H.V.; Kroll, J.S.; Clarke, P. Characteristics and Incidence of Transfusion-Associated Necrotizing Enterocolitis in the UK. J. Matern. Fetal. Neonatal. Med. 2020, 33, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Tiwari, S.; Jain, U. Potential Biomarkers for Effective Screening of Neonatal Sepsis Infections: An Overview. Microb. Pathog. 2017, 107, 234–242. [Google Scholar] [CrossRef]
- Doğan, G.; İpek, H. The Development of Necrotizing Enterocolitis Publications: A Holistic Evolution of Global Literature with Bibliometric Analysis. Eur. J. Pediatr. Surg. 2020, 30, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Battersby, C.; Santhalingam, T.; Costeloe, K.; Modi, N. Incidence of Neonatal Necrotising Enterocolitis in High-Income Countries: A Systematic Review. Arch. Dis. Child. Fetal. Neonatal Ed. 2018, 103, F182–F189. [Google Scholar] [CrossRef] [PubMed]
- De Plaen, I.G. Inflammatory Signaling in Necrotizing Enterocolitis. Clin. Perinat. 2013, 40, 109–124. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Sherman, M.P.; Prince, L.S.; Bader, D.; Weitkamp, J.-H.; Slaughter, J.C.; McElroy, S.J. Paneth Cell Ablation in the Presence of Klebsiella Pneumoniae Induces Necrotizing Enterocolitis (NEC)-like Injury in Immature Murine Small Intestine. Dis. Models Mech. 2012, 5, 522–532. [Google Scholar] [CrossRef]
- Bazacliu, C.; Neu, J. Pathophysiology of Necrotizing Enterocolitis: An Update. Curr. Pediatr. Rev. 2019, 15, 68–87. [Google Scholar] [CrossRef]
- Meister, A.L.; Doheny, K.K.; Travagli, R.A. Necrotizing Enterocolitis: It’s Not All in the Gut. Exp. Biol. Med. 2020, 245, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Rich, B.S.; Dolgin, S.E. Necrotizing Enterocolitis. Pediatr. Rev. 2017, 38, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Mϋller, M.J.; Paul, T.; Seeliger, S. Necrotizing Enterocolitis in Premature Infants and Newborns. NPM 2016, 9, 233–242. [Google Scholar] [CrossRef]
- Adams-Chapman, I. Necrotizing Enterocolitis and Neurodevelopmental Outcome. Clin. Perinatol. 2018, 45, 453–466. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef]
- Tirone, C.; Pezza, L.; Paladini, A.; Tana, M.; Aurilia, C.; Lio, A.; D’Ippolito, S.; Tersigni, C.; Posteraro, B.; Sanguinetti, M.; et al. Gut and Lung Microbiota in Preterm Infants: Immunological Modulation and Implication in Neonatal Outcomes. Front. Immunol. 2019, 10, 2910. [Google Scholar] [CrossRef]
- Schnabl, K.-L.; Van Aerde, J.-E.; Thomson, A.-B.; Clandinin, M.-T. Necrotizing Enterocolitis: A Multifactorial Disease with No Cure. World J. Gastroenterol. 2008, 14, 2142–2161. [Google Scholar] [CrossRef] [PubMed]
- Alganabi, M.; Lee, C.; Bindi, E.; Li, B.; Pierro, A. Recent Advances in Understanding Necrotizing Enterocolitis. F1000Research 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Jilling, T.; Simon, D.; Lu, J.; Meng, F.J.; Li, D.; Schy, R.; Thomson, R.B.; Soliman, A.; Arditi, M.; Caplan, M.S. The Roles of Bacteria and TLR4 in Rat and Murine Models of Necrotizing Enterocolitis. J. Immunol. 2006, 177, 3273–3282. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Bass, J.; Krantis, A. An Intraluminal Model of Necrotizing Enterocolitis in the Developing Neonatal Piglet. J. Pediatr. Surg. 1995, 30, 1138–1142. [Google Scholar] [CrossRef]
- Kappel, S.S.; Sangild, P.T.; Hilsted, L.; Hartmann, B.; Thymann, T.; Aunsholt, L. Gastric Residual to Predict Necrotizing Enterocolitis in Preterm Piglets As Models for Infants. J. Parenter. Enter. Nutr. 2021, 45, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Barlow, B.; Santulli, T.V.; Heird, W.C.; Pitt, J.; Blanc, W.A.; Schullinger, J.N. An Experimental Study of Acute Neonatal Enterocolitis—The Importance of Breast Milk. J. Pediatr. Surg. 1974, 9, 587–595. [Google Scholar] [CrossRef]
- Sodhi, C.; Richardson, W.; Gribar, S.; Hackam, D.J. The Development of Animal Models for the Study of Necrotizing Enterocolitis. Dis. Model. Mech. 2008, 1, 94–98. [Google Scholar] [CrossRef]
- Sulistyo, A.; Rahman, A.; Biouss, G.; Antounians, L.; Zani, A. Animal Models of Necrotizing Enterocolitis: Review of the Literature and State of the Art. Innov. Surg. Sci. 2018, 3, 87–92. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Gong, H.; Pope, B.; Schlievert, P.; McElroy, S.J. Paneth Cell Disruption-Induced Necrotizing Enterocolitis Requires Live Bacteria and Occurs Independent of TLR4 Signaling. Dis. Model. Mech. 2017, 10, 727–736. [Google Scholar] [CrossRef]
- Lyu, C.; Jiang, S.; Kong, M.; Chen, X.; Zhang, L. Vitamin D Protects against Necrotising Enterocolitis in Newborn Mice by Activating the ERK Signalling Pathway. Mol. Med. Rep. 2020, 22, 2107–2114. [Google Scholar] [CrossRef]
- MohanKumar, K.; Kaza, N.; Jagadeeswaran, R.; Garzon, S.A.; Bansal, A.; Kurundkar, A.R.; Namachivayam, K.; Remon, J.I.; Bandepalli, C.R.; Feng, X.; et al. Gut Mucosal Injury in Neonates Is Marked by Macrophage Infiltration in Contrast to Pleomorphic Infiltrates in Adult: Evidence from an Animal Model. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G93–G102. [Google Scholar] [CrossRef]
- Roura, E.; Koopmans, S.-J.; Lallès, J.-P.; Le Huerou-Luron, I.; de Jager, N.; Schuurman, T.; Val-Laillet, D. Critical Review Evaluating the Pig as a Model for Human Nutritional Physiology. Nutr. Res. Rev. 2016, 29, 60–90. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Merrifield, C.A.; Lewis, M.; Claus, S.P.; Beckonert, O.P.; Dumas, M.-E.; Duncker, S.; Kochhar, S.; Rezzi, S.; Lindon, J.C.; Bailey, M.; et al. A Metabolic System-Wide Characterisation of the Pig: A Model for Human Physiology. Mol. BioSyst. 2011, 7, 2577. [Google Scholar] [CrossRef]
- Holgersen, K.; Gao, X.; Narayanan, R.; Gaur, T.; Carey, G.; Barton, N.; Pan, X.; Muk, T.; Thymann, T.; Sangild, P.T. Supplemental Insulin-Like Growth Factor-1 and Necrotizing Enterocolitis in Preterm Pigs. Front. Pediatr 2020, 8, 602047. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Thymann, T.; Goericke-Pesch, S.K.; Ren, S.; Wei, W.; Skovgaard, K.; Damborg, P.; Brunse, A.; van Gorp, C.; Kramer, B.W.; et al. Prenatal Intra-Amniotic Endotoxin Induces Fetal Gut and Lung Immune Responses and Postnatal Systemic Inflammation in Preterm Pigs. Am. J. Pathol. 2018, 188, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Bass, J.; Krantis, A. Use of L-Arginine in the Treatment of Experimental Necrotizing Enterocolitis. J. Pediatr. Surg. 1995, 30, 235–241. [Google Scholar] [CrossRef]
- Waligora-Dupriet, A.J.; Dugay, A.; Auzeil, N.; Nicolis, I.; Rabot, S.; Huerre, M.R.; Butel, M.J. Short-Chain Fatty Acids and Polyamines in the Pathogenesis of Necrotizing Enterocolitis: Kinetics Aspects in Gnotobiotic Quails. Anaerobe 2009, 15, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Waligora-Dupriet, A.-J.; Dugay, A.; Auzeil, N.; Huerre, M.; Butel, M.-J. Evidence for Clostridial Implication in Necrotizing Enterocolitis through Bacterial Fermentation in a Gnotobiotic Quail Model. Pediatr. Res. 2005, 58, 629–635. [Google Scholar] [CrossRef]
- Baer, J.; Lansford, R.; Cheng, K. Japanese Quail as a Laboratory Animal Model. In Laboratory Animal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1087–1108. ISBN 978-0-12-409527-4. [Google Scholar]
- Ares, G.J.; McElroy, S.J.; Hunter, C.J. The Science and Necessity of Using Animal Models in the Study of Necrotizing Enterocolitis. Semin. Pediatr. Surg. 2018, 27, 29–33. [Google Scholar] [CrossRef]
- Dodgson, J.B.; Romanov, M.N. Use of Chicken Models for the Analysis of Human Disease. Curr. Protoc. Hum. Genet. 2004, 40, 15.5.1–15.5.12. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Li, C.; Liang, Z.; Huang, Z.; Wang, Q.; Li, S.; Zhao, Z. Fat Mass- and Obesity-Associated (FTO) Gene Promoted Myoblast Differentiation through the Focal Adhesion Pathway in Chicken. 3 Biotech 2020, 10, 403. [Google Scholar] [CrossRef]
- Cui, C.; Han, S.; Tang, S.; He, H.; Shen, X.; Zhao, J.; Chen, Y.; Wei, Y.; Wang, Y.; Zhu, Q.; et al. The Autophagy Regulatory Molecule CSRP3 Interacts with LC3 and Protects Against Muscular Dystrophy. Int. J. Mol. Sci. 2020, 21, 749. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Rasmussen, M.K.; Young, J.F.; Bertram, H.C. High Resolution Magic Angle Spinning NMR Spectroscopy Reveals That Pectoralis Muscle Dystrophy in Chicken Is Associated with Reduced Muscle Content of Anserine and Carnosine. Food Chem. 2017, 217, 151–154. [Google Scholar] [CrossRef]
- Thu, H.M.; Myat, T.W.; Win, M.M.; Thant, K.Z.; Rahman, S.; Umeda, K.; Nguyen, S.V.; Icatlo, F.C., Jr.; Higo-Moriguchi, K.; Taniguchi, K.; et al. Chicken Egg Yolk Antibodies (IgY) for Prophylaxis and Treatment of Rotavirus Diarrhea in Human and Animal Neonates: A Concise Review. Korean J. Food Sci. Anim. Resour. 2017, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sood, U.; Gupta, V.; Kumar, R.; Lal, S.; Fawcett, D.; Rattan, S.; Poinern, G.E.J.; Lal, R. Chicken Gut Microbiome and Human Health: Past Scenarios, Current Perspectives, and Futuristic Applications. Indian J. Microbiol. 2020, 60, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Lacharme-Lora, L.; Owen, S.V.; Blundell, R.; Canals, R.; Wenner, N.; Perez-Sepulveda, B.; Fong, W.Y.; Gilroy, R.; Wigley, P.; Hinton, J.C.D. The Use of Chicken and Insect Infection Models to Assess the Virulence of African Salmonella Typhimurium ST313. PLoS Negl. Trop. Dis. 2019, 13, e0007540. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.A.; Branton, S.L.; Evans, J.D.; Collier, S.D. Fluorescent Microspheres as a Positive Indicator in an Intratracheal Infection Model. J. Microbiol. Methods 2020, 172, 105886. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Magori, K.; Kasper, L.H. The Chicken or the Egg Dilemma: Intestinal Dysbiosis in Multiple Sclerosis. Ann. Transl. Med. 2017, 5, 145. [Google Scholar] [CrossRef]
- Sundick, R.S.; Bagchi, N.; Brown, T.R. The Obese Strain Chicken as a Model for Human Hashimoto’s Thyroiditis. Exp Clin Endocrinol. Diabetes 2009, 104, 4–6. [Google Scholar] [CrossRef]
- Teixeira, A.R.L.; Nitz, N.; Bernal, F.M.; Hecht, M.M. Parasite Induced Genetically Driven Autoimmune Chagas Heart Disease in the Chicken Model. J. Vis. Exp. 2012, e3716. [Google Scholar] [CrossRef]
- Hu, J.; Ishihara, M.; Chin, A.I.; Wu, L. Establishment of Xenografts of Urological Cancers on Chicken Chorioallantoic Membrane (CAM) to Study Metastasis. Precis. Clin. Med. 2019, 2, 140–151. [Google Scholar] [CrossRef]
- Kunz, P.; Schenker, A.; Sähr, H.; Lehner, B.; Fellenberg, J. Optimization of the Chicken Chorioallantoic Membrane Assay as Reliable in Vivo Model for the Analysis of Osteosarcoma. PLoS ONE 2019, 14, e0215312. [Google Scholar] [CrossRef]
- Hawkridge, A.M. The Chicken Model of Spontaneous Ovarian Cancer. Prot. Clin. Appl. 2014, 8, 689–699. [Google Scholar] [CrossRef]
- Reed, S.; Neuman, H.; Glahn, R.P.; Koren, O.; Tako, E. Characterizing the Gut (Gallus gallus) Microbiota Following the Consumption of an Iron Biofortified Rwandan Cream Seeded Carioca (Phaseolus vulgaris L.) Bean-Based Diet. PLoS ONE 2017, 12, e0182431. [Google Scholar] [CrossRef]
- Reed, S.; Knez, M.; Uzan, A.; Stangoulis, J.; Glahn, R.P.; Koren, O.; Tako, E. Alterations in the Gut (Gallus gallus) Microbiota Following the Consumption of Zinc Biofortified Wheat (Triticum aestivum)-Based Diet. J. Agric. Food Chem. 2018, 66, 6291–6300. [Google Scholar] [CrossRef] [PubMed]
- Carboni, J.; Reed, S.; Kolba, N.; Eshel, A.; Koren, O.; Tako, E. Alterations in the Intestinal Morphology, Gut Microbiota, and Trace Mineral Status Following Intra-Amniotic Administration (Gallus gallus) of Teff (Eragrostis Tef) Seed Extracts. Nutrients 2020, 12, 3020. [Google Scholar] [CrossRef] [PubMed]
- Shterzer, N.; Rothschild, N.; Sbehat, Y.; Stern, E.; Nazarov, A.; Mills, E. Large Overlap Between the Intestinal and Reproductive Tract Microbiomes of Chickens. Front. Microbiol. 2020, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Juste Contin Gomes, M.; Stampini Duarte Martino, H.; Tako, E. Effects of Iron and Zinc Biofortified Foods on Gut Microbiota In Vivo (Gallus gallus): A Systematic Review. Nutrients 2021, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.T.; Johnson, A.A.T.; Kolba, N.; Bonneau, J.P.; Glahn, R.P.; Ozeri, L.; Koren, O.; Tako, E. Nicotianamine-Chelated Iron Positively Affects Iron Status, Intestinal Morphology and Microbial Populations in Vivo (Gallus gallus). Sci. Rep. 2020, 10, 2297. [Google Scholar] [CrossRef]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.; Koren, O.; Tako, E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P. Iron Status of the Late Term Broiler (Gallus gallus) Embryo and Hatchling. Int. J. Poult. Sci. 2011, 10, 42–48. [Google Scholar] [CrossRef]
- Warkentin, T.; Kolba, N.; Tako, E. Low Phytate Peas (Pisum sativum L.) Improve Iron Status, Gut Microbiome, and Brush Border Membrane Functionality In Vivo (Gallus gallus). Nutrients 2020, 12, 2563–2581. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Kolba, N.; Tako, E. Yacon (Smallanthus sonchifolius) Flour Soluble Extract Improve Intestinal Bacterial Populations, Brush Border Membrane Functionality and Morphology in Vivo (Gallus gallus). 2020, 137, 109705-109716. Food Res. Int. 2020, 137, 109705–109716. [Google Scholar] [CrossRef]
- Dias, D.M.; Kolba, N.; Hart, J.J.; Ma, M.; Sha, S.T.; Lakshmanan, N.; Nutti, M.R.; Martino, H.S.D.; Glahn, R.P.; Tako, E. Soluble Extracts from Carioca Beans (Phaseolus vulgaris L.) Affect the Gut Microbiota and Iron Related Brush Border Membrane Protein Expression in Vivo (Gallus gallus). Food Res. Int. 2019, 123, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kolba, N.; Liang, J.; Tako, E. Alterations in Gut Microflora Populations and Brush Border Functionality Following Intra-Amniotic Administration (Gallus gallus) of Wheat Bran Prebiotic Extracts. Food Funct. 2019, 10, 4834–4843. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Tako, E. The In Ovo Feeding Administration (Gallus gallus)—An Emerging In Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients 2018, 10, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Kolba, N.; Jung, Y.; Cheng, J.; Tako, E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus gallus). Nutrients 2022, 141, 220–233. [Google Scholar] [CrossRef]
- Agarwal, N.; Kolba, N.; Khen, N.; Even, C.; Turjeman, S.; Koren, O.; Tako, E. Quinoa Soluble Fiber and Quercetin Alter the Composition of the Gut Microbiome and Improve Brush Border Membrane Morphology In Vivo (Gallus gallus). Nutrients 2022, 14, 448–462. [Google Scholar] [CrossRef]
- Pereira da Silva, B.; Kolba, N.; Duarte Martino, H.S.; Hart, J.J.; Tako, E. Soluble Extracts from Chia Seed (Salvia hispanica L.) Affect Brush Border Membrane Functionality, Morphology and Intestinal Bacterial Populations In Vivo (Gallus gallus). Nutrients 2019, 11, 2457–2474. [Google Scholar] [CrossRef]
- Haahr, M.; Haahr, S. Random Sequence Generator; Dublin, Ireland, 2022. [Google Scholar]
- Barekatain, R.; Chrystal, P.V.; Howarth, G.S.; McLaughlan, C.J.; Gilani, S.; Nattrass, G.S. Performance, Intestinal Permeability, and Gene Expression of Selected Tight Junction Proteins in Broiler Chickens Fed Reduced Protein Diets Supplemented with Arginine, Glutamine, and Glycine Subjected to a Leaky Gut Model. Poult. Sci. 2019, 98, 6761–6771. [Google Scholar] [CrossRef]
- Dreiling, C.E.; Brown, D.E.; Casale, L.; Kelly, L. Muscle Glycogen: Comparison of Iodine Binding and Enzyme Digestion Assays and Application to Meat Samples. Meat Sci. 1987, 20, 167–177. [Google Scholar] [CrossRef]
- Morais Dias, D.; Kolba, N.; Binyamin, D.; Ziv, O.; Regini Nutti, M.; Stampini Duarte Martino, H.; Glahn, R.P.; Koren, O.; Tako, E. Iron Biofortified Carioca Bean (Phaseolus vulgaris L.)-Based Brazilian Diet Delivers More Absorbable Iron and Affects the Gut Microbiota In Vivo (Gallus gallus). Nutrients 2018, 10, 1970–1990. [Google Scholar] [CrossRef]
- Pacifici, S.; Song, J.; Zhang, C.; Wang, Q.; Glahn, R.; Kolba, N.; Tako, E. Intra Amniotic Administration of Raffinose and Stachyose Affects the Intestinal Brush Border Functionality and Alters Gut Microflora Populations. Nutrients 2017, 9, 304. [Google Scholar] [CrossRef]
- Hou, T.; Kolba, N.; Glahn, R.; Tako, E. Intra-Amniotic Administration (Gallus gallus) of Cicer Arietinum and Lens Culinaris Prebiotics Extracts and Duck Egg White Peptides Affects Calcium Status and Intestinal Functionality. Nutrients 2017, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.S.; Johnson, P.A. Occludin Expression and Regulation in Small Follicles of the Layer and Broiler Breeder Hen. Gen. Comp. Endocrinol. 2017, 248, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hartono, K.; Reed, S.; Ankrah, N.A.; Glahn, R.P.; Tako, E. Alterations in Gut Microflora Populations and Brush Border Functionality Following Intra-Amniotic Daidzein Administration. RSC Adv. 2015, 5, 6407–6412. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Welch, R.M.; Lei, X.; Yasuda, K.; Miller, D.D. Dietary Inulin Affects the Expression of Intestinal Enterocyte Iron Transporters, Receptors and Storage Protein and Alters the Microbiota in the Pig Intestine. Br. J. Nutr. 2008, 99, 472–480. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Zhong, T.; Pandya, Y.; Joerger, R.D. 16S RRNA-Based Analysis of Microbiota from the Cecum of Broiler Chickens. Appl. Environ. Microbiol. 2002, 68, 124–137. [Google Scholar] [CrossRef]
- Brisse, S.; Verhoef, J. Phylogenetic Diversity of Klebsiella Pneumoniae and Klebsiella Oxytoca Clinical Isolates Revealed by Randomly Amplified Polymorphic DNA, GyrA and ParC Genes Sequencing and Automated Ribotyping. Int. J. Syst. Evol. Microbiol. 2001, 51, 915–924. [Google Scholar] [CrossRef]
- Younis, A.I.; Elbialy, A.I. Molecular Detection of Genus Klebsiella and Genotypic Identification of Klebsiella Pneumoniae and Klebsiella Oxytoca by Duplex Polymerase Chain Reaction in Poultry. Glob. Vet. 2017, 18, 234–241. [Google Scholar]
- Tako, E.; Glahn, R.P.; Knez, M.; Stangoulis, J.C. The Effect of Wheat Prebiotics on the Gut Bacterial Population and Iron Status of Iron Deficient Broiler Chickens. Nutr. J. 2014, 13, 1. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Kolba, N.; Agarwal, N.; Kim, D.; Eshel, A.; Koren, O.; Tako, E. Modifications in the Intestinal Functionality, Morphology and Microbiome Following Intra-Amniotic Administration (Gallus gallus) of Grape (Vitis vinifera) Stilbenes (Resveratrol and Pterostilbene). Nutrients 2021, 13, 3247. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Martino, H.S.D.; Kolba, N.; Cheng, J.; Agarwal, N.; De Moura Rocha, M.; Tako, E. Zinc Biofortified Cowpea (Vigna unguiculata L. Walp.) Soluble Extracts Modulate Assessed Cecal Bacterial Populations and Gut Morphology in Vivo (Gallus gallus). Front. BioScience Landmark 2022, 27, 140–153. [Google Scholar] [CrossRef]
- Agarwal, N.; Shukla, V.; Kolba, N.; Jackson, C.; Cheng, J.; Padilla-Zakour, O.I.; Tako, E. Comparing the Effects of Concord Grape (Vitis labrusca L.) Puree, Juice, and Pomace on Intestinal Morphology, Functionality, and Bacterial Populations In Vivo (Gallus gallus). Nutrients 2022, 14, 3539. [Google Scholar] [CrossRef] [PubMed]
- Agrizzi Verediano, T.; Stampini Duarte Martino, H.; Kolba, N.; Fu, Y.; Cristina Dias Paes, M.; Tako, E. Black Corn (Zea mays L.) Soluble Extract Showed Anti-Inflammatory Effects and Improved the Intestinal Barrier Integrity in Vivo (Gallus gallus). Food Res. Int. 2022, 157, 111227–111239. [Google Scholar] [CrossRef] [PubMed]
- Kolba, N.; Zarei, A.; Cheng, J.; Agarwal, N.; Dadmohammadi, Y.; Khazdooz, L.; Abbaspourrad, A.; Tako, E. Alterations in Intestinal Brush Border Membrane Functionality and Bacterial Populations Following Intra-Amniotic Administration (Gallus gallus) of Nicotinamide Riboside and Its Derivatives. Nutrients 2022, 14, 3130–3150. [Google Scholar] [CrossRef]
- Uni, Z.; Ganot, S.; Sklan, D. Posthatch Development of Mucosal Function in the Broiler Small Intestine. Poult. Sci. 1998, 77, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Kolba, N.; Sisser, P.; Turjeman, S.; Even, C.; Koren, O.; Tako, E. Intraamniotic Administration (Gallus gallus) of Genistein Alters Mineral Transport, Intestinal Morphology, and Gut Microbiota. Nutrients 2022, 14, 3473. [Google Scholar] [CrossRef]
- Spinner, J.A.; Morris, S.A.; Nandi, D.; Costarino, A.T.; Marino, B.S.; Rossano, J.W.; Shamszad, P. Necrotizing Enterocolitis and Associated Mortality in Neonates With Congenital Heart Disease: A Multi-Institutional Study. Pediatr. Crit. Care Med. 2020, 21, 228–234. [Google Scholar] [CrossRef]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision Microbiome Reconstitution Restores Bile Acid Mediated Resistance to Clostridium Difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The Effect of Palmitic Acid on Inflammatory Response in Macrophages: An Overview of Molecular Mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- Bousseboua, H.; Le Coz, Y.; Dabard, J.; Szylit, O.; Raibaud, P.; Popoff, M.R.; Ravisse, P. Experimental Cecitis in Gnotobiotic Quails Monoassociated with Clostridium Butyricum Strains Isolated from Patients with Neonatal Necrotizing Enterocolitis and from Healthy Newborns. Infect. Immun. 1989, 57, 932–936. [Google Scholar] [CrossRef]
- Zou, X.; Ji, J.; Wang, J.; Qu, H.; Shu, D.M.; Guo, F.Y.; Luo, C.L. Dextran Sulphate Sodium (DSS) Causes Intestinal Histopathology and Inflammatory Changes Consistent with Increased Gut Leakiness in Chickens. Br. Poult. Sci. 2018, 59, 166–172. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran Sodium Sulfate Colitis Murine Model: An Indispensable Tool for Advancing Our Understanding of Inflammatory Bowel Diseases Pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Perše, M.; Cerar, A. Dextran Sodium Sulphate Colitis Mouse Model: Traps and Tricks. J. Biomed. Biotechnol. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ginzel, M.; Feng, X.; Kuebler, J.F.; Klemann, C.; Yu, Y.; von Wasielewski, R.; Park, J.-K.; Hornef, M.W.; Vieten, G.; Ure, B.M.; et al. Dextran Sodium Sulfate (DSS) Induces Necrotizing Enterocolitis-like Lesions in Neonatal Mice. PLoS ONE 2017, 12, e0182732. [Google Scholar] [CrossRef] [PubMed]
- Denning, T.L.; Bhatia, A.M.; Kane, A.F.; Patel, R.M.; Denning, P.W. Pathogenesis of NEC: Role of the Innate and Adaptive Immune Response. Semin. Perinatol. 2017, 41, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Menconi, A.; Hernandez-Velasco, X.; Vicuña, E.A.; Kuttappan, V.A.; Faulkner, O.B.; Tellez, G.; Hargis, B.M.; Bielke, L.R. Histopathological and Morphometric Changes Induced by a Dextran Sodium Sulfate (DSS) Model in Broilers. Poult. Sci. 2015, 94, 906–911. [Google Scholar] [CrossRef]
- Simon, K.; Arts, J.A.J.; de Vries Reilingh, G.; Kemp, B.; Lammers, A. Effects of Early Life Dextran Sulfate Sodium Administration on Pathology and Immune Response in Broilers and Layers. Poult. Sci. 2016, 95, 1529–1542. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Animal Models of Ischemia-Reperfusion-Induced Intestinal Injury: Progress and Promise for Translational Research. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G63–G75. [Google Scholar] [CrossRef]
- Joldrichsen, M.R.; Kim, E.; Cormet-Boyaka, E.; Boyaka, P.N. Paneth Cells Regulate Diet-Induced Obesity and Trafficking of Inflammatory Immune Cells into Adipose Tissues. J. Immunol. 2020, 204, 83.3. [Google Scholar]
- Bergstrom, K.S.B.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa. PLoS Pathog. 2010, 6, e1000902. [Google Scholar] [CrossRef]
- Song, C.S.; Park, D.I.; Yoon, M.Y.; Seok, H.S.; Park, J.H.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I. Association Between Red Cell Distribution Width and Disease Activity in Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2012, 57, 1033–1038. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Shi, X.; Richardson, W.M.; Grant, Z.S.; Shapiro, R.A.; Prindle, T.; Branca, M.; Russo, A.; Gribar, S.C.; Ma, C.; et al. Toll-Like Receptor-4 Inhibits Enterocyte Proliferation via Impaired β-Catenin Signaling in Necrotizing Enterocolitis. Gastroenterology 2010, 138, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.E.; Zheng, B.; Koelink, P.J.; van de Kant, H.J.G.; Haazen, L.C.J.M.; van Roest, M.; Garssen, J.; Folkerts, G.; Kraneveld, A.D. New Perspective on Dextran Sodium Sulfate Colitis: Antigen-Specific T Cell Development during Intestinal Inflammation. PLoS ONE 2013, 8, e69936. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria Penetrate the Normally Impenetrable Inner Colon Mucus Layer in Both Murine Colitis Models and Patients with Ulcerative Colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. Amino Acid Transport Across Mammalian Intestinal and Renal Epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. BioMed Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef]
- Gephart, S.M.; Gordon, P.V.; Penn, A.H.; Gregory, K.E.; Swanson, J.R.; Maheshwari, A.; Sylvester, K. Changing the Paradigm of Defining, Detecting, and Diagnosing NEC: Perspectives on Bell’s Stages and Biomarkers for NEC. Semin. Pediatr. Surg. 2018, 27, 3–10. [Google Scholar] [CrossRef]
- Caplan, M.S.; Sun, X.-M.; Hsueh, W.; Hageman, J.R. Role of Platelet Activating Factor and Tumor Necrosis Factor-Alpha in Neonatal Necrotizing Enterocolitis. J. Pediatr. 1990, 116, 960–964. [Google Scholar] [CrossRef]
- Klinke, M.; Wiskemann, H.; Bay, B.; Schäfer, H.-J.; Pagerols Raluy, L.; Reinshagen, K.; Vincent, D.; Boettcher, M. Cardiac and Inflammatory Necrotizing Enterocolitis in Newborns Are Not the Same Entity. Front. Pediatr. 2021, 8, 593926. [Google Scholar] [CrossRef]
- Miyake, H.; Li, B.; Lee, C.; Koike, Y.; Chen, Y.; Seo, S.; Pierro, A. Liver Damage, Proliferation, and Progenitor Cell Markers in Experimental Necrotizing Enterocolitis. J. Pediatr. Surg. 2018, 53, 909–913. [Google Scholar] [CrossRef]
- Zmora, O.; Gutzeit, O.; Segal, L.; Boulos, S.; Millo, Z.; Ginsberg, Y.; Khatib, N.; Dabbah-Assad, F.; Fainaru, O.; Weiner, Z.; et al. Prophylactic Antenatal N-Acetyl Cysteine Administration Combined with Postnatal Administration Can Decrease Mortality and Injury Markers Associated with Necrotizing Enterocolitis in a Rat Model. PLoS ONE 2020, 15, e0233612. [Google Scholar] [CrossRef]
- Karin, M.; Lawrence, T.; Nizet, V. Innate Immunity Gone Awry: Linking Microbial Infections to Chronic Inflammation and Cancer. Cell 2006, 124, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Yamamoto, Y.; Wang, Q.M. The IKK NF-ΚB System: A Treasure Trove for Drug Development. Nat. Rev. Drug Discov. 2004, 3, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.; Kozlov, S. Inflammation and Cancer: When NF-κB Amalgamates the Perilous Partnership. Curr. Cancer Drug Targets 2005, 5, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF-B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Krishnaveni, M.; Jayachandran, S. Inhibition of MAP Kinases and down Regulation of TNF-α, IL-β and COX-2 Genes by the Crude Extracts from Marine Bacteria. Biomed. Pharmacother. 2009, 63, 469–476. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, X.; Wu, X.; Yang, X.; Han, C.; Wang, Z.; Du, Q.; Zhao, X.; Liu, S.-L.; Tong, D.; et al. Brucella Downregulates Tumor Necrosis Factor-α to Promote Intracellular Survival via Omp25 Regulation of Different MicroRNAs in Porcine and Murine Macrophages. Front. Immunol. 2018, 8, 2013. [Google Scholar] [CrossRef]
- Claud, E.C.; Keegan, K.P.; Brulc, J.M.; Lu, L.; Bartels, D.; Glass, E.; Chang, E.B.; Meyer, F.; Antonopoulos, D.A. Bacterial Community Structure and Functional Contributions to Emergence of Health or Necrotizing Enterocolitis in Preterm Infants. Microbiome 2013, 1, 20. [Google Scholar] [CrossRef]
- Hill, H.R.; Hunt, C.E.; Matsen, J.M. Nosocomial Colonization with Klebsiella, Type 26, in a Neonatal Intensive-Care Unit Associated with an Outbreak of Sepsis, Meningitis, and Necrotizing Enterocolitis. J. Pediatr. 1974, 85, 415–419. [Google Scholar] [CrossRef]
- Azzaroli, F.; Turco, L.; Ceroni, L.; Sartoni Galloni, S.; Buonfiglioi, F.; Calvanese, C.; Mazzella, G. Pneumatosis Cystoides Intestinalis. WJG 2011, 17, 4932. [Google Scholar] [CrossRef]
- Ling, F.; Guo, D.; Zhu, L. Pneumatosis Cystoides Intestinalis: A Case Report and Literature Review. BMC Gastroenterol. 2019, 19, 176. [Google Scholar] [CrossRef] [PubMed]
- Meini, S.; Zini, C.; Passaleva, M.T.; Frullini, A.; Fusco, F.; Carpi, R.; Piani, F. Pneumatosis Intestinalis in COVID-19. BMJ Open Gastroenterol. 2020, 7, e000434. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Anjum, F. Pneumatosis Intestinalis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA; St. Petersburg, Russia, 2021; pp. 1–9. [Google Scholar]
- Tarracchini, C.; Milani, C.; Longhi, G.; Fontana, F.; Mancabelli, L.; Pintus, R.; Lugli, G.A.; Alessandri, G.; Anzalone, R.; Viappiani, A.; et al. Unraveling the Microbiome of Necrotizing Enterocolitis: Insights in Novel Microbial and Metabolomic Biomarkers. Microbiol. Spectr. 2021, 9, e01176-21. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.J.; Bean, J.F. Cronobacter: An Emerging Opportunistic Pathogen Associated with Neonatal Meningitis, Sepsis and Necrotizing Enterocolitis. J. Perinatol. 2013, 33, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shan, G.; Sodergren, E.; Weinstock, G.; Walker, W.A.; Gregory, K.E. Longitudinal Analysis of the Premature Infant Intestinal Microbiome Prior to Necrotizing Enterocolitis: A Case-Control Study. PLoS ONE 2015, 10, e0118632. [Google Scholar] [CrossRef]
- Schönherr-Hellec, S.; Aires, J. Clostridia and Necrotizing Enterocolitis in Preterm Neonates. Anaerobe 2019, 58, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; Baptiste, E.; Levasseur, A.; La Scola, B. Molecular Epidemiology of Clostridium Neonatale and Its Relationship with the Occurrence of Necrotizing Enterocolitis in Preterm Neonates. New Microbes New Infect. 2019, 32, 100612. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, J.R.; Claud, E.C. Necrotizing Enterocolitis and the Preterm Infant Microbiome. In Probiotics and Child Gastrointestinal Health; Guandalini, S., Indrio, F., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1125, pp. 25–36. ISBN 978-3-030-14635-1. [Google Scholar]
- Neu, J.; Pammi, M. Necrotizing Enterocolitis: The Intestinal Microbiome, Metabolome and Inflammatory Mediators. Semin. Fetal Neonatal Med. 2018, 23, 400–405. [Google Scholar] [CrossRef]
- Garcia, M.I.; Ghiani, M.; Lefort, A.; Libert, F.; Strollo, S.; Vassart, G. LGR5 Deficiency Deregulates Wnt Signaling and Leads to Precocious Paneth Cell Differentiation in the Fetal Intestine. Dev. Biol. 2009, 331, 58–67. [Google Scholar] [CrossRef]
- Juber, B.A.; McElroy, S.J. The Paneth Cell and Its Role in the Development of NEC. In Necrotizing Enterocolitis, 1st ed.; Hackam, D.J., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 242–247. ISBN 978-0-429-28830-2. [Google Scholar]
- Liu, D.; Xu, Y.; Feng, J.; Yu, J.; Huang, J.; Li, Z. Mucins and Tight Junctions Are Severely Altered in Necrotizing Enterocolitis Neonates. Am. J. Perinatol. 2021, 38, 1174–1180. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut–Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Q.-Y.; Wang, W.-Z.; Chu, S.; Liu, X.-X.; Liu, Y.-J.; Tan, C.; Zhu, F.; Deng, S.-J.; Dong, Y.-L.; et al. Compound Sophorae Decoction Enhances Intestinal Barrier Function of Dextran Sodium Sulfate Induced Colitis via Regulating Notch Signaling Pathway in Mice. Biomed. Pharmacother. 2021, 133, 110937. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, L.; Li, Z.; Li, C.; Hong, Y.; Gu, Z. Butyrylated Starch Protects Mice from DSS-Induced Colitis: Combined Effects of Butyrate Release and Prebiotic Supply. Food Funct. 2021, 12, 11290–11302. [Google Scholar] [CrossRef] [PubMed]
- Gehart, H.; Clevers, H. Tales from the Crypt: New Insights into Intestinal Stem Cells. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Colman, M.J.; Schewe, M.; Meerlo, M.; Stigter, E.; Gerrits, J.; Pras-Raves, M.; Sacchetti, A.; Hornsveld, M.; Oost, K.C.; Snippert, H.J.; et al. Interplay between Metabolic Identities in the Intestinal Crypt Supports Stem Cell Function. Nature 2017, 543, 424–427. [Google Scholar] [CrossRef]
- Bel, S.; Pendse, M.; Wang, Y.; Li, Y.; Ruhn, K.A.; Hassell, B.; Leal, T.; Winter, S.E.; Xavier, R.J.; Hooper, L.V. Paneth Cells Secrete Lysozyme via Secretory Autophagy during Bacterial Infection of the Intestine. Science 2017, 357, 1047–1052. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N.H. Paneth Cells, Antimicrobial Peptides and Maintenance of Intestinal Homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef]
| Target Gene | Forward (5′-3′) | Reverse (3′-5′) | Amplicon Length (Base Pairs) | NCBI Accession | Ref. |
|---|---|---|---|---|---|
| Inflammatory Genes | |||||
| NF-κβ | CACAGCTGGAGGGAAGTAAAT | TTGAGTAAGGAAGTGAGGTTGAG | 100 | 2130627 | |
| TNF-α | GACAGCCTATGCCAACAAGTA | TTACAGGAAGGGCAACTCATC | 109 | 53854909 | |
| IL-1β | TCATCCATCCCAAGTTCATTCA | GACACACTTCTCTGCCATCTT | 105 | 395872 | |
| IL-6 | ACCTCATCCTCCGAGACTTTA | GCACTGAAACTCCTGGTCTT | 105 | 302315692 | |
| Brush Border Membrane (BBM) Functionality Genes | |||||
| OCLN | GTCTGTGGGTTCCTCATCGT | GTTCTTCACCCACTCCTCCA | 124 | 396026 | [74] |
| MUC2 | CCTGCTGCAAGGAAGTAGAA | GGAAGATCAGAGTGGTGCATAG | 272 | 423101 | |
| AP | CGTCAGCCAGTTTGACTATGTA | CTCTCAAAGAAGCTGAGGATGG | 138 | 45382360 | |
| SI | CCAGCAATGCCAGCATATTG | CGGTTTCTCCTTACCACTTCTT | 95 | 2246388 | |
| SGLT1 | GCATCCTTACTCTGTGGTACTG | TATCCGCACATCACACATCC | 106 | 8346783 | |
| 18S rRNA | GCAAGACGAACTAAAGCGAAAG | TCGGAACTACGACGGTATCT | 100 | 7262899 | |
| Target Gene | Forward (5′-3′) | Reverse (3′-5′) | Ref. |
|---|---|---|---|
| Lactobacillus spp. | CATCCAGTGCAAACCTAAGAG | GATCCGCTTGCCTTCGCA | [77] |
| Bifidobacterium spp. | GGGTGGTAATGCCGGATG | CCACCGTTACACCGGGAA | [77] |
| E. coli spp. | GACCTCGGTTTAGTTCACAGA | CACACGCTGACGCTGACCA | [77] |
| Clostridium spp. | AAAGGAAGATTAATACCGCATAA | ATCTTGCGACCGTACTCCCC | [77] |
| Klebsiella spp. | CGCGTACTATACGCCATGAACGTA | ACCGTTGATCACTTCGGTCAGG | [78,79] |
| 16S rRNA | CGTGCCAGCCGCGGTAATACG | GGGTTGCGCTCGTTGCGGGACTTAACCCAACAT | [77] |
| Group | Body Weight (g) | Cecum Weight (g) | Cecum: Body Weight |
|---|---|---|---|
| No Injection | 40.06 ± 4.06 b | 0.42 ± 0.06 b | 0.015 ± 0.005 a |
| H2O Injection | 47.49 ± 1.21 a | 0.47 ± 0.03 a,b | 0.010 ± 0.001 a |
| 0.1% DSS | 45.81 ± 1.23 a,b | 0.62 ± 0.08 a | 0.013 ± 0.002 a |
| 0.25% DSS | 45.25 ± 1.01 a,b | 0.49 ± 0.05 a,b | 0.011 ± 0.001 a |
| 0.50% DSS | 45.24 ± 1.11 a,b | 0.64 ± 0.08 a | 0.014 ± 0.002 a |
| 0.75% DSS | 45.78 ± 0.86 a,b | 0.60 ± 0.06 a,b | 0.010 ± 0.000 a |
| Group | Hb (g/dL) | Hepatic Glycogen (mg/mL) |
|---|---|---|
| No Injection | 10.48 ± 1.31 a | 0.002 ± 0.001 b |
| H2O Injection | 9.82 ± 0.77 a | 0.003 ± 0.001 b |
| 0.1% DSS | 10.70 ± 1.16 a | 0.003 ± 0.001 b |
| 0.25% DSS | 10.22 ± 1.56 a | 0.004 ± 0.001 b |
| 0.50% DSS | 10.13 ± 0.77 a | 0.004 ± 0.001 b |
| 0.75% DSS | 10.94 ± 3.24 a | 0.008 ± 0.002 a |
| Treatment | Villus Surface Area (µm2) | Crypt Depth (µm) | Villi Goblet Diameter (µm) | Crypt Goblet Diameter (µm) | Paneth Cell # | Paneth Cell Diameter (µM) |
|---|---|---|---|---|---|---|
| No Injection | 109.99 ± 3.06 d | 25.17 ± 0.93 a,b | 3.57 ± 0.05 d | 2.99 ± 0.05 b | 1.09 ± 0.02 c | 1.56 ± 0.03 b |
| H2O Injection | 205.15 ± 5.03 a | 26.35 ± 0.98 a,b | 4.04 ± 0.06 c | 3.16 ± 0.04 a | 1.03 ± 0.01 c | 1.47 ± 0.02 c |
| 0.1% DSS | 147.51 ± 3.28 b | 27.89 ± 1.08 a | 4.55 ± 0.07 a | 2.99 ± 0.04 b | 1.80 ± 0.05 b | 1.69 ± 0.03 a |
| 0.75% DSS | 130.35 ± 0.03 c | 24.32 ± 0.78 b | 4.25 ± 0.05 b | 2.73 ± 0.04 c | 1.93 ± 0.06 a | 1.67 ± 0.03 a |
| Treatment | Crypt Goblet Cell # | Crypt Goblet Cell Type Number | Villi Goblet Cell # | Villi Goblet Cell Type Number | ||||
|---|---|---|---|---|---|---|---|---|
| Acidic | Neutral | Mixed | Acidic | Neutral | Mixed | |||
| No Injection | 8.57 ± 0.32 c | 6.59 ± 0.26 c | 0.00 ± 0.0 | 1.97 ± 0.18 d | 15.78 ± 0.45 c | 13.7 ± 0.42 c | 0.00 ± 0.00 | 2.08 ± 0.13 c |
| H2O Injection | 7.96 ± 0.24 c | 7.42 ± 0.22 b | 0.00 ± 0.00 | 0.49 ± 0.06 c | 22.93 ± 0.6 b | 18.4 ± 0.53 b | 0.00 ± 0.00 | 4.53 ± 0.23 b |
| 0.1% DSS | 14.7 ± 0.41 a | 10.23 ± 0.31 a | 0.00 ± 0.00 | 4.47 ± 0.19 a | 30.37 ± 0.84 a | 23.91 ± 0.68 a | 0.00 ± 0.00 | 6.46 ± 0.36 a |
| 0.75% DSS | 13.48 ± 0.04 b | 9.76 ± 0.32 a | 0.00 ± 0.00 | 3.74 ± 0.19 b | 16.64 ± 0.56 c | 14.63 ± 0.49 c | 0.00 ± 0.00 | 2.09 ± 0.14 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolba, N.; Cheng, J.; Jackson, C.D.; Tako, E. Intra-Amniotic Administration—An Emerging Method to Investigate Necrotizing Enterocolitis, In Vivo (Gallus gallus). Nutrients 2022, 14, 4795. https://doi.org/10.3390/nu14224795
Kolba N, Cheng J, Jackson CD, Tako E. Intra-Amniotic Administration—An Emerging Method to Investigate Necrotizing Enterocolitis, In Vivo (Gallus gallus). Nutrients. 2022; 14(22):4795. https://doi.org/10.3390/nu14224795
Chicago/Turabian StyleKolba, Nikolai, Jacquelyn Cheng, Cydney D. Jackson, and Elad Tako. 2022. "Intra-Amniotic Administration—An Emerging Method to Investigate Necrotizing Enterocolitis, In Vivo (Gallus gallus)" Nutrients 14, no. 22: 4795. https://doi.org/10.3390/nu14224795
APA StyleKolba, N., Cheng, J., Jackson, C. D., & Tako, E. (2022). Intra-Amniotic Administration—An Emerging Method to Investigate Necrotizing Enterocolitis, In Vivo (Gallus gallus). Nutrients, 14(22), 4795. https://doi.org/10.3390/nu14224795








