Combination of High Energy Intake and Intensive Rehabilitation Is Associated with the Most Favorable Functional Recovery in Acute Stroke Patients with Sarcopenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Setting
2.2. Diagnosis of Sarcopenia
2.3. Rehabilitation during Hospitalization
2.4. Nutritional Management during Hospitalization
2.5. Data Collection
2.6. Outcomes
2.7. Sample Size Calculation
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; van Vliet, S.; Stokes, T.; Mittendorfer, B.; Phillips, S.M. The impact of exercise and nutrition on the regulation of skeletal muscle mass. J. Physiol. 2019, 597, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gonzalez-Buonomo, J.; Ghuman, J.; Huang, X.; Malik, A.; Yozbatiran, N.; Magat, E.; Francisco, G.E.; Wu, H.; Frontera, W.R. Aging after stroke: How to define post-stroke sarcopenia and what are its risk factors? Eur. J. Phys. Rehabil. Med. 2022, 58, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, M.; Kanai, M.; Kubo, H.; Yamamoto, M.; Shimada, S.; Mase, K. Prestroke sarcopenia and functional outcomes in elderly patients who have had an acute stroke: A prospective cohort study. Nutrition 2019, 66, 44–47. [Google Scholar] [CrossRef]
- Abe, T.; Iwata, K.; Yoshimura, Y.; Shinoda, T.; Inagaki, Y.; Ohya, S.; Yamada, K.; Oyanagi, K.; Maekawa, Y.; Honda, A.; et al. Low Muscle Mass is Associated with Walking Function in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105259. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition 2019, 61, 111–118. [Google Scholar] [CrossRef]
- Kido, Y.; Yoshimura, Y.; Wakabayashi, H.; Momosaki, R.; Nagano, F.; Bise, T.; Shimazu, S.; Shiraishi, A. Sarcopenia is associated with incontinence and recovery of independence in urination and defecation in post-acute rehabilitation patients. Nutrition 2021, 91–92, 111397. [Google Scholar] [CrossRef]
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 16–23. [Google Scholar] [CrossRef]
- Kim, H.; Suzuki, T.; Saito, K.; Yoshida, H.; Kojima, N.; Kim, M.; Sudo, M.; Yamashiro, Y.; Tokimitsu, I. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. Geriatr. Gerontol. Int. 2013, 13, 458–465. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef]
- Kim, H.; Suzuki, T.; Saito, K.; Kojima, N.; Hosoi, E.; Yoshida, H. Long-term effects of exercise and amino acid supplementation on muscle mass, physical function and falls in community-dwelling elderly Japanese sarcopenic women: A 4-year follow-up study. Geriatr. Gerontol. Int. 2016, 16, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for Treating Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Am. Med. Dir. Assoc. 2017, 18, e551–e553. [Google Scholar] [CrossRef]
- Rabadi, M.H.; Coar, P.L.; Lukin, M.; Lesser, M.; Blass, J.P. Intensive nutritional supplements can improve outcomes in stroke rehabilitation. Neurology 2008, 71, 1856–1861. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Bise, T.; Shimazu, S.; Shiraishi, A.; Kido, Y.; Matsumoto, A. Chair-Stand Exercise Improves Sarcopenia in Rehabilitation Patients after Stroke. Nutrients 2022, 14, 461. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef]
- Momosaki, R.; Abo, M.; Urashima, M. Vitamin D Supplementation and Post-Stroke Rehabilitation: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, K.; Nakano, H.; Watanabe, Y. The eye response test alone is sufficient to predict stroke outcome--reintroduction of Japan Coma Scale: A cohort study. BMJ Open. 2013, 3, e002736. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshimura, Y.; Abe, T. Nutrition in the First Week after Stroke Is Associated with Discharge to Home. Nutrients 2021, 13, 943. [Google Scholar] [CrossRef]
- Oyanagi, K.; Kitai, T.; Yoshimura, Y.; Yokoi, Y.; Ohara, N.; Kohara, N.; Sakai, N.; Honda, A.; Onishi, H.; Iwata, K. Effect of early intensive rehabilitation on the clinical outcomes of patients with acute stroke. Geriatr. Gerontol. Int. 2021, 21, 623–628. [Google Scholar] [CrossRef]
- Miyamoto, S.; Ogasawara, K.; Kuroda, S.; Itabashi, R.; Toyoda, K.; Itoh, Y.; Iguchi, Y.; Shiokawa, Y.; Takagi, Y.; Ohtsuki, T.; et al. Japan Stroke Society Guideline 2021 for the Treatment of Stroke. Int. J. Stroke 2022, 17, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ohno, T.; Nomoto, A.; Kayashita, J.; Mori, N.; The Japanese Working Group On Sarcopenic, D. Nutritional Management Enhances the Recovery of Swallowing Ability in Older Patients with Sarcopenic Dysphagia. Nutrients 2021, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Kokura, Y.; Kato, M.; Taniguchi, Y.; Kimoto, K.; Okada, Y. Energy intake during the acute phase and changes in femoral muscle thickness in older hemiplegic inpatients with stroke. Nutrition 2020, 70, 110582. [Google Scholar] [CrossRef] [PubMed]
- Lyden, P.; Brott, T.; Tilley, B.; Welch, K.M.; Mascha, E.J.; Levine, S.; Haley, E.C.; Grotta, J.; Marler, J. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994, 25, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Crary, M.A.; Mann, G.D.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, K.J.; Hsu, Y.; Granger, C.V.; Fiedler, R.C. The reliability of the functional independence measure: A quantitative review. Arch. Phys. Med. Rehabil. 1996, 77, 1226–1232. [Google Scholar] [CrossRef]
- Kokura, Y.; Wakabayashi, H.; Nishioka, S.; Maeda, K. Nutritional intake is associated with activities of daily living and complications in older inpatients with stroke. Geriatr. Gerontol. Int. 2018, 18, 1334–1339. [Google Scholar] [CrossRef]
- Nagano, F.; Yoshimura, Y.; Bise, T.; Shimazu, S.; Shiraishi, A. Muscle mass gain is positively associated with functional recovery in patients with sarcopenia after stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105017. [Google Scholar] [CrossRef]
- Abe, T.; Yoshimura, Y.; Imai, R.; Yoneoka, Y.; Tsubaki, A.; Sato, Y. Impact of Phase Angle on Physical Function in Patients with Acute Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105941. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Freburger, J.K.; Chou, A.; Euloth, T.; Matcho, B.; Bilderback, A. Association Between Use of Rehabilitation in the Acute Care Hospital and Hospital Readmission or Mortality in Patients With Stroke. Arch. Phys. Med. Rehabil. 2021, 102, 1700–1707.e1704. [Google Scholar] [CrossRef]
- Bernhardt, J.; Churilov, L.; Ellery, F.; Collier, J.; Chamberlain, J.; Langhorne, P.; Lindley, R.I.; Moodie, M.; Dewey, H.; Thrift, A.G.; et al. Prespecified dose-response analysis for A Very Early Rehabilitation Trial (AVERT). Neurology 2016, 86, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Nip, W.F.; Perry, L.; McLaren, S.; Mackenzie, A. Dietary intake, nutritional status and rehabilitation outcomes of stroke patients in hospital. J. Hum. Nutr. Diet. 2011, 24, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Ido, Y.; Yoshimura, Y.; Mutai, H. Relationship of Malnutrition During Hospitalization With Functional Recovery and Postdischarge Destination in Elderly Stroke Patients. J. Stroke Cerebrovasc. Dis. 2019, 28, 1866–1872. [Google Scholar] [CrossRef]
- Hirano, Y.; Maeshima, S.; Osawa, A.; Nishio, D.; Takeda, K.; Baba, M.; Kigawa, H. The effect of voluntary training with family participation on early home discharge in patients with severe stroke at a convalescent rehabilitation ward. Eur. Neurol. 2012, 68, 221–228. [Google Scholar] [CrossRef]
- O’Connor, M.; Moriarty, H.; Schneider, A.; Dowdell, E.B.; Bowles, K.H. Patients’ and caregivers’ perspectives in determining discharge readiness from home health. Geriatr. Nurs. 2021, 42, 151–158. [Google Scholar] [CrossRef]
- Tanwir, S.; Montgomery, K.; Chari, V.; Nesathurai, S. Stroke rehabilitation: Availability of a family member as caregiver and discharge destination. Eur. J. Phys. Rehabil. Med. 2014, 50, 355–362. [Google Scholar]
- Wakabayashi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Uchida, K.; Jeong, S.; Yamaga, M. Effects of Nutritional Supplements on Muscle Mass and Activities of Daily Living in Elderly Rehabilitation Patients with Decreased Muscle Mass: A Randomized Controlled Trial. J. Nutr. Health Aging 2016, 20, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Aragane, H.; Suzuki, N.; Yoshimura, Y.; Fujiwara, D.; Mori, T.; Kanehisa, Y.; Iida, Y.; Higashi, K.; Yoshimura-Yokoi, Y.; et al. Clinical practice guidelines for rehabilitation nutrition in cerebrovascular disease, hip fracture, cancer, and acute illness: 2020 update. Clin. Nutr. ESPEN 2021, 43, 90–103. [Google Scholar] [CrossRef] [PubMed]


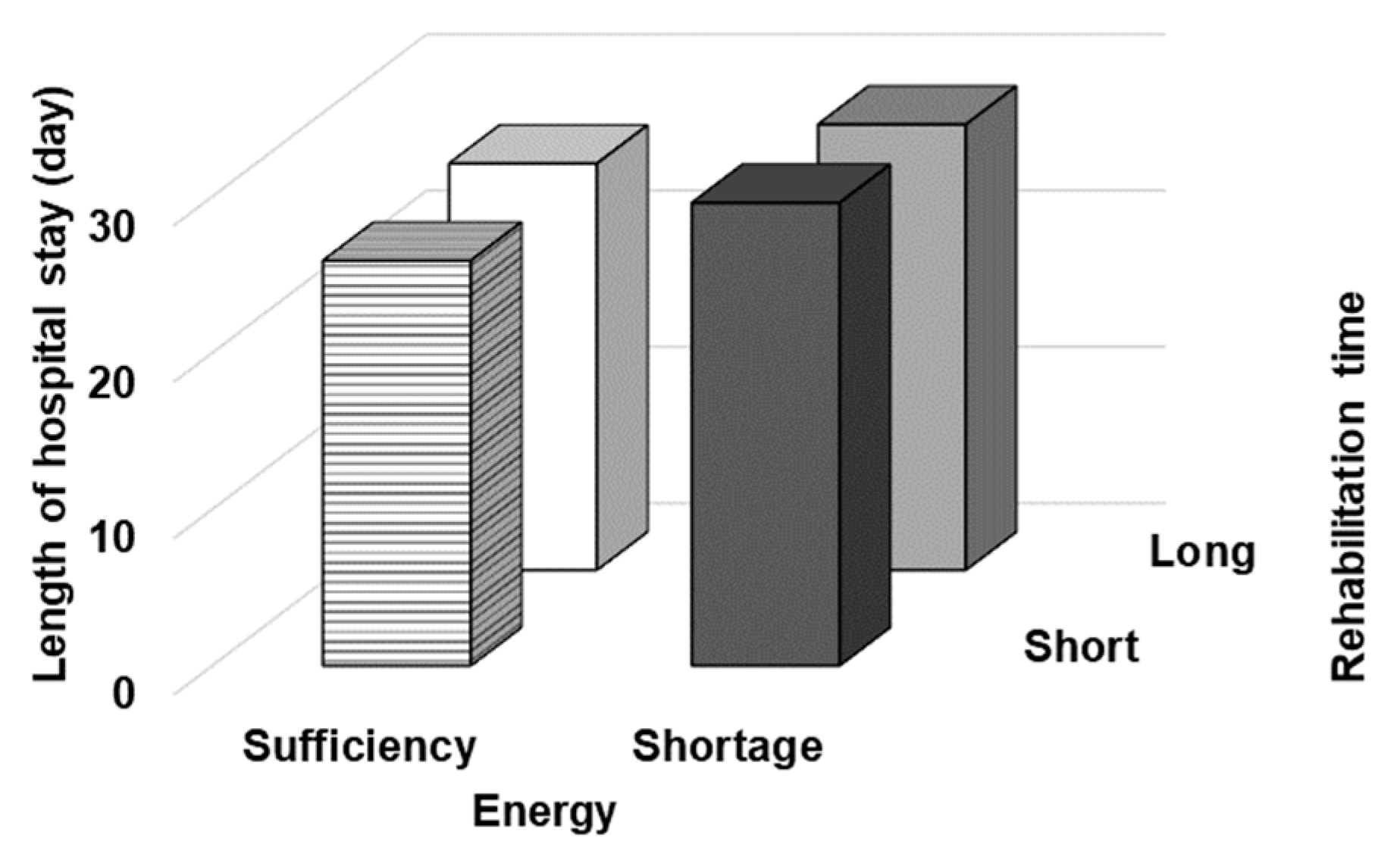
| Variables | Total (n = 140) | Men (n = 67) | Women (n = 73) |
|---|---|---|---|
| Age (y) | 82.6 (9.6) | 78.6 (8.6) | 86.4 (9.1) |
| Body mass index (kg/m2) | 21.1 (3.6) | 21.4 (3.9) | 20.9 (3.2) |
| NIHSS score | 6 [4–10] | 5 [3–9] | 7 [5–12] |
| Days from onset to rehabilitation (days) | 1 [1–1] | 1 [1–1] | 1 [1–1] |
| Stroke type (infarct/hemorrhage) | 107/33 | 52/15 | 55/18 |
| Thrombolysis or Surgery (n) | 7 | 3 | 4 |
| Comorbidity (n) | |||
| -Hypertension | 91 | 40 | 51 |
| -Diabetes | 41 | 24 | 17 |
| -Previous stroke | 35 | 18 | 17 |
| -Dyslipidemia | 19 | 11 | 8 |
| -Atrial fibrillation | 41 | 20 | 21 |
| Laboratory data | |||
| -Albumin (g/dL) | 3.9 (0.5) | 3.9 (0.5) | 3.8 (0.5) |
| -Hemoglobin (g/dL) | 12.8 (1.9) | 13.4 (2.0) | 12.3 (1.6) |
| GNRI | 98.1 (9.9) | 99.8 (9.5) | 96.5 (10.0) |
| Length of hospital stay (day) | 27.5 (14.1) | 27.9 (15.8) | 26.2 (12.4) |
| FOIS at admission | 4 [1–6] | 5 [2–6] | 4 [1–5] |
| Handgrip strength at admission (kg) | 9.1 (8.2) | 12.6 (9.1) | 7.9 (5.6) |
| SMI at admission (kg/m2) | 5.5 (1.0) | 6.3 (0.5) | 4.7 (0.7) |
| Energy intake during the first week of hospitalization (kcal/ABW/day) | 20.2 (7.9) | 20.3 (7.5) | 20.1 (8.2) |
| -Oral intake | 15.5 (10.8) | 17.5 (10.1) | 13.6 (11.2) |
| -Parenteral nutrition | 3.0 (4.1) | 1.9 (3.1) | 4.1 (4.6) |
| -Enteral nutrition | 1.7 (5.3) | 0.9 (3.5) | 2.5 (6.5) |
| FIM at admission | |||
| -motor | 30 [15–44] | 33 [16–46] | 29 [14–39] |
| -cognitive | 17 [7–25] | 16 [7–27] | 18 [7–29] |
| -total | 45 [24–67] | 48 [25–75] | 42 [20–66] |
| Rehabilitation time (min/day) | 54.2 [41.2–69.8] | 55.7 [42.9–75.4] | 52.8 [40.3–64.2] |
| Means (SD) or median [25−75%tile] | |||
| NIHSS: National Institute of Health Stroke Scale, GNRI: Geriatric Nutritional Risk Index, FOIS: Functional Oral Intake Scale, | |||
| SMI: Skeletal Muscle Index, ABW: Actual Body Weight, FIM: Functional Independence Measure | |||
| Short × Shortage (n = 35) | Long × Shortage (n = 33) | Short × Sufficiency (n = 35) | Long × Sufficiency (n = 37) | p Value | |
|---|---|---|---|---|---|
| Age (y) | 82.9 (10.4) | 81.3 (11.0) | 83.8 (6.4) | 79.6 (9.1) | 0.111 |
| Men (n) | 14 | 17 | 15 | 21 | 0.463 |
| Body mass index (kg/m2) | 21.3 (3.2) | 22.0 (4.7) | 20.7 (3.6) | 20.5 (2.7) | 0.308 |
| NIHSS score | 7 [6–10] | 8 [5–13] | 6 [4–9] | 7 [4–10] | 0.121 |
| GNRI | 97.1 (11.8) | 102.1 (6.4) | 95.5 (11.2) | 97.8 (8.4) | 0.092 |
| FOIS at admission | 4 [1,5] | 5 [1,6] | 5 [1,6] | 5 [1,6] | 0.332 |
| Handgrip strength at admission (kg) | |||||
| -Men | 12.6 (5.7) | 10.8 (9.6) | 13.5 (6.7) | 12.6 (9.1) | 0.099 |
| -Women | 7.5 (5.7) | 7.4 (5.4) | 7.9 (4.8) | 8.2 (5.6) | 0.105 |
| SMI at admission (kg/m2) | |||||
| -Men | 6.3 (0.5) | 6.4 (0.4) | 6.3 (0.4) | 6.3 (0.5) | 0.183 |
| -Women | 4.9 (0.4) | 4.6 (0.6) | 4.4 (0.8) | 5.0 (0.4) | 0.133 |
| FIM at admission | |||||
| -motor | 30 [15–44] | 29 [15–40] | 33 [16–45] | 32 [15–46] | 0.094 |
| -cognitive | 16 [7–27] | 15 [7–24] | 16 [7–29] | 17 [8–26] | 0.176 |
| -total | 45 [21–67] | 43 [21–62] | 45 [22–73] | 47 [21–75] | 0.123 |
| Home discharge (n) | 10 | 10 | 16 | 15 | 0.385 |
| Mean (SD) or Median [25–75%tile] | |||||
| Short: Short rehabilitation time, Long: Long rehabilitation time, Shortage: Energy shortage, Sufficiency: Energy sufficiency | |||||
| NIHSS: National Institute of Health Stroke Scale, GNRI: Geriatric Nutritional Risk Index, FOIS: Functional Oral Intake Scale, | |||||
| SMI: Skeletal Muscle Index, FIM: Functional Independence Measure | |||||
| FIM-Motor Gain | Length of Hospital Stay | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | |
| Short × Shortage | −0.225 | 0.009 | −0.002 | 0.985 | ||||||||||||
| Long × Shortage | −0.104 | 0.211 | −0.002 | 0.981 | ||||||||||||
| Short × Sufficiency | −0.073 | 0.388 | −0.066 | 0.413 | ||||||||||||
| Long × Sufficiency | 0.391 | <0.001 | 0.069 | 0.397 | ||||||||||||
| Short: Short rehabilitation time, Long: Long rehabilitation time, Shortage: Energy shortage, Sufficiency: Energy sufficiency | ||||||||||||||||
| β: Standardized partial regression coefficient Adjusted for age, sex, body mass index, National Institutes of Health Stroke Scale, Geriatric Nutritional Risk Index, Skeletal muscle mass index, handgrip strength, Functional Independence Measure-total, Functional Oral Intake Scale at admission | ||||||||||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| Short × Shortage | 0.753 (0.186–3.056) | 0.455 | ||||||
| Long × Shortage | 0.511 (0.119–2.198) | 0.367 | ||||||
| Short × Sufficiency | 1.065 (0.920–1.232) | 0.400 | ||||||
| Long × Sufficiency | 0.620 (0.177–2.169) | 0.455 | ||||||
| Home = “1”, Other (rehabilitation hospital or nursing facility) = “0” Short: Short rehabilitation time, Long: Long rehabilitation time, Shortage: Energy shortage, Sufficiency: Energy sufficiency | ||||||||
| Adjusted for age, sex, body mass index, National Institutes of Health Stroke Scale, Geriatric Nutritional Risk Index, | ||||||||
| Skeletal muscle mass index, handgrip strength, Functional Independence Measure-total, Functional Oral Intake Scale at admission | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Yoshimura, Y.; Abe, T.; Nagano, F.; Matsumoto, A.; Kokura, Y.; Momosaki, R. Combination of High Energy Intake and Intensive Rehabilitation Is Associated with the Most Favorable Functional Recovery in Acute Stroke Patients with Sarcopenia. Nutrients 2022, 14, 4740. https://doi.org/10.3390/nu14224740
Sato Y, Yoshimura Y, Abe T, Nagano F, Matsumoto A, Kokura Y, Momosaki R. Combination of High Energy Intake and Intensive Rehabilitation Is Associated with the Most Favorable Functional Recovery in Acute Stroke Patients with Sarcopenia. Nutrients. 2022; 14(22):4740. https://doi.org/10.3390/nu14224740
Chicago/Turabian StyleSato, Yoichi, Yoshihiro Yoshimura, Takafumi Abe, Fumihiko Nagano, Ayaka Matsumoto, Yoji Kokura, and Ryo Momosaki. 2022. "Combination of High Energy Intake and Intensive Rehabilitation Is Associated with the Most Favorable Functional Recovery in Acute Stroke Patients with Sarcopenia" Nutrients 14, no. 22: 4740. https://doi.org/10.3390/nu14224740
APA StyleSato, Y., Yoshimura, Y., Abe, T., Nagano, F., Matsumoto, A., Kokura, Y., & Momosaki, R. (2022). Combination of High Energy Intake and Intensive Rehabilitation Is Associated with the Most Favorable Functional Recovery in Acute Stroke Patients with Sarcopenia. Nutrients, 14(22), 4740. https://doi.org/10.3390/nu14224740









