Threshold Ferritin Concentrations Reflecting Early Iron Deficiency Based on Hepcidin and Soluble Transferrin Receptor Serum Levels in Patients with Absolute Iron Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Analyses and Iron Absorption Determination
2.3. Statistical Analysis
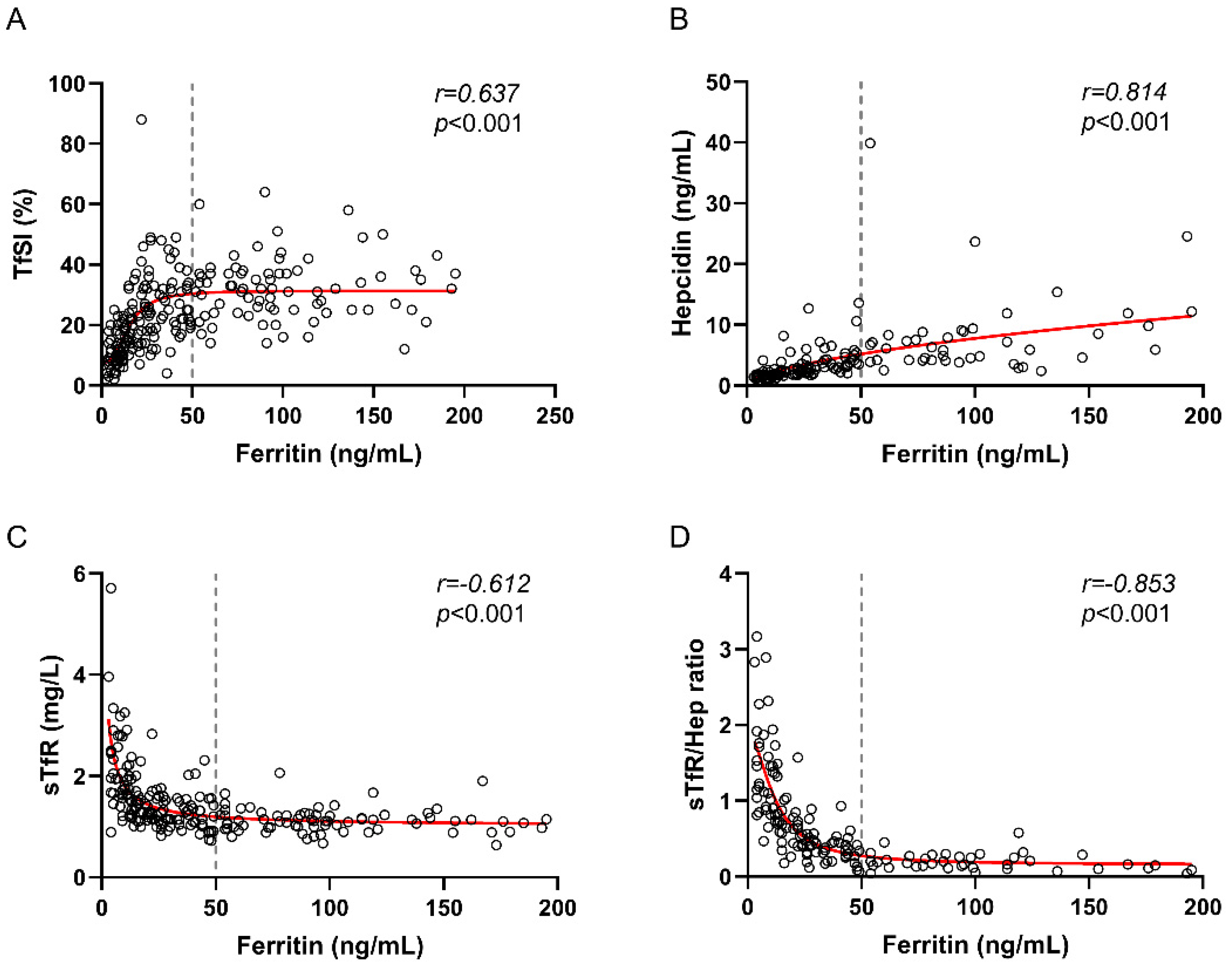
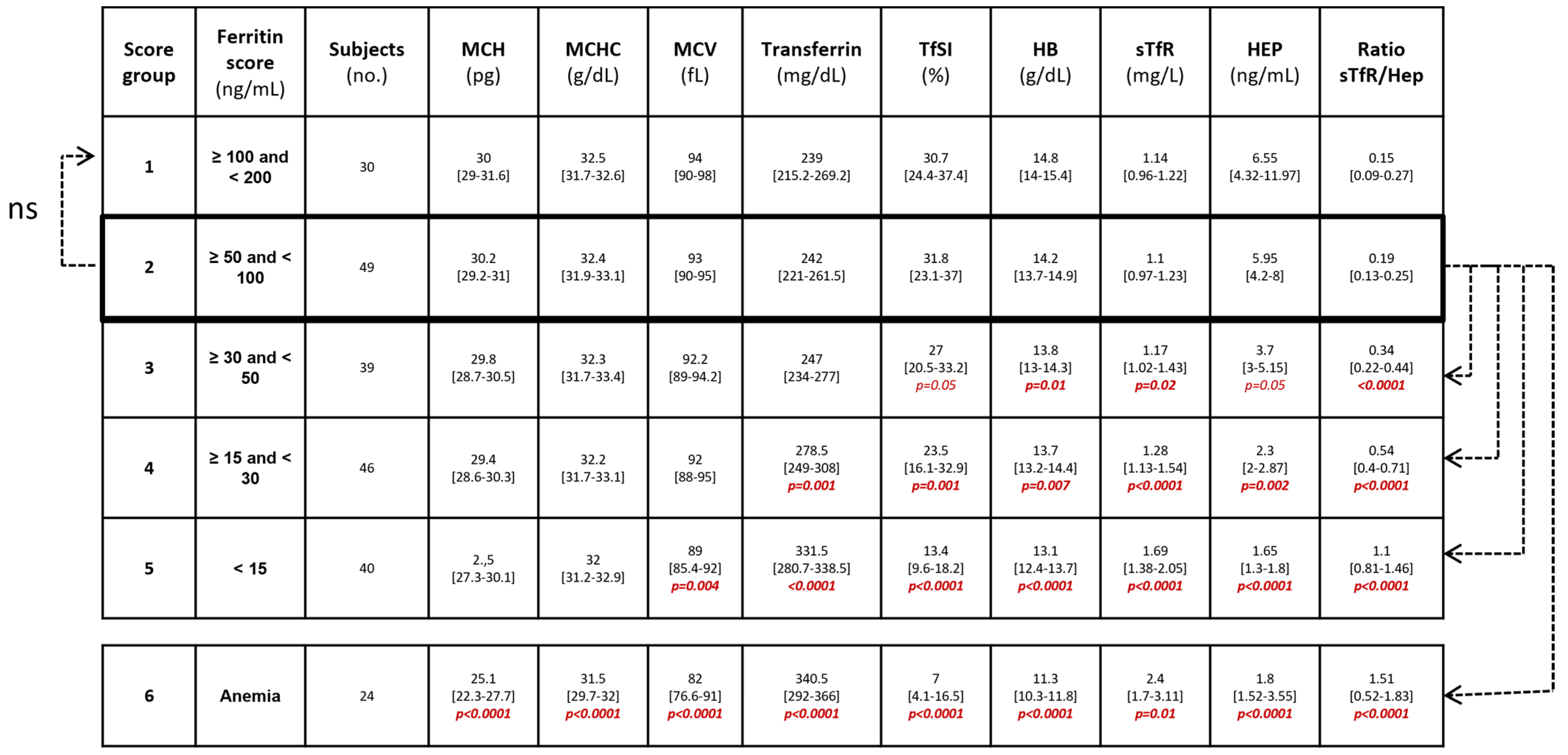
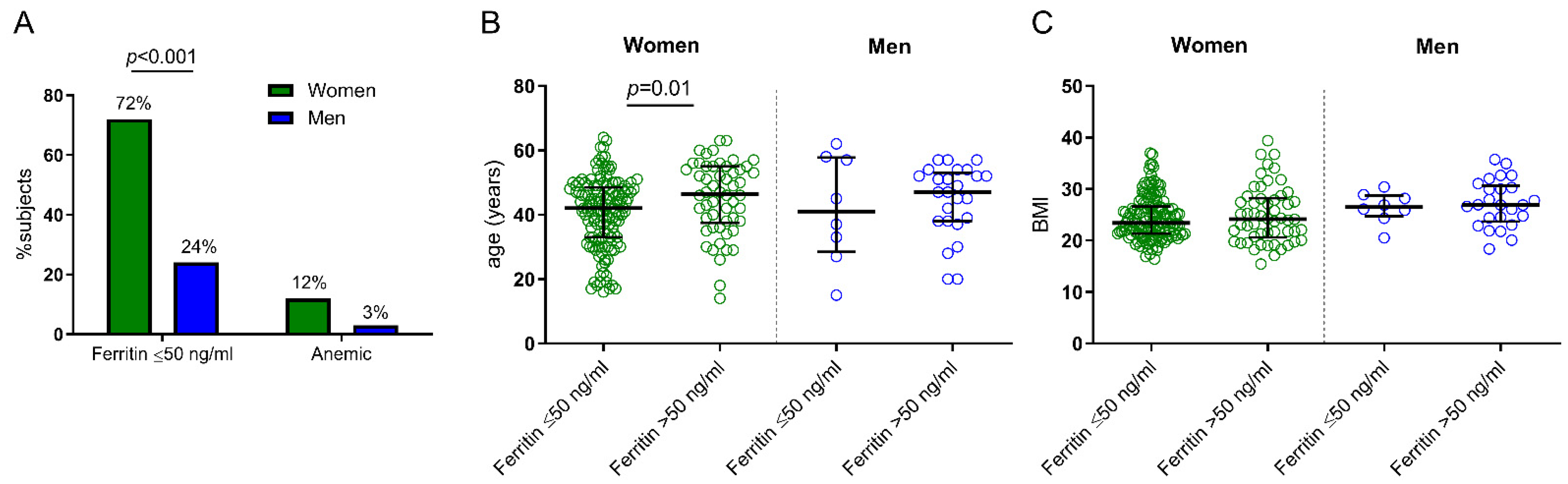
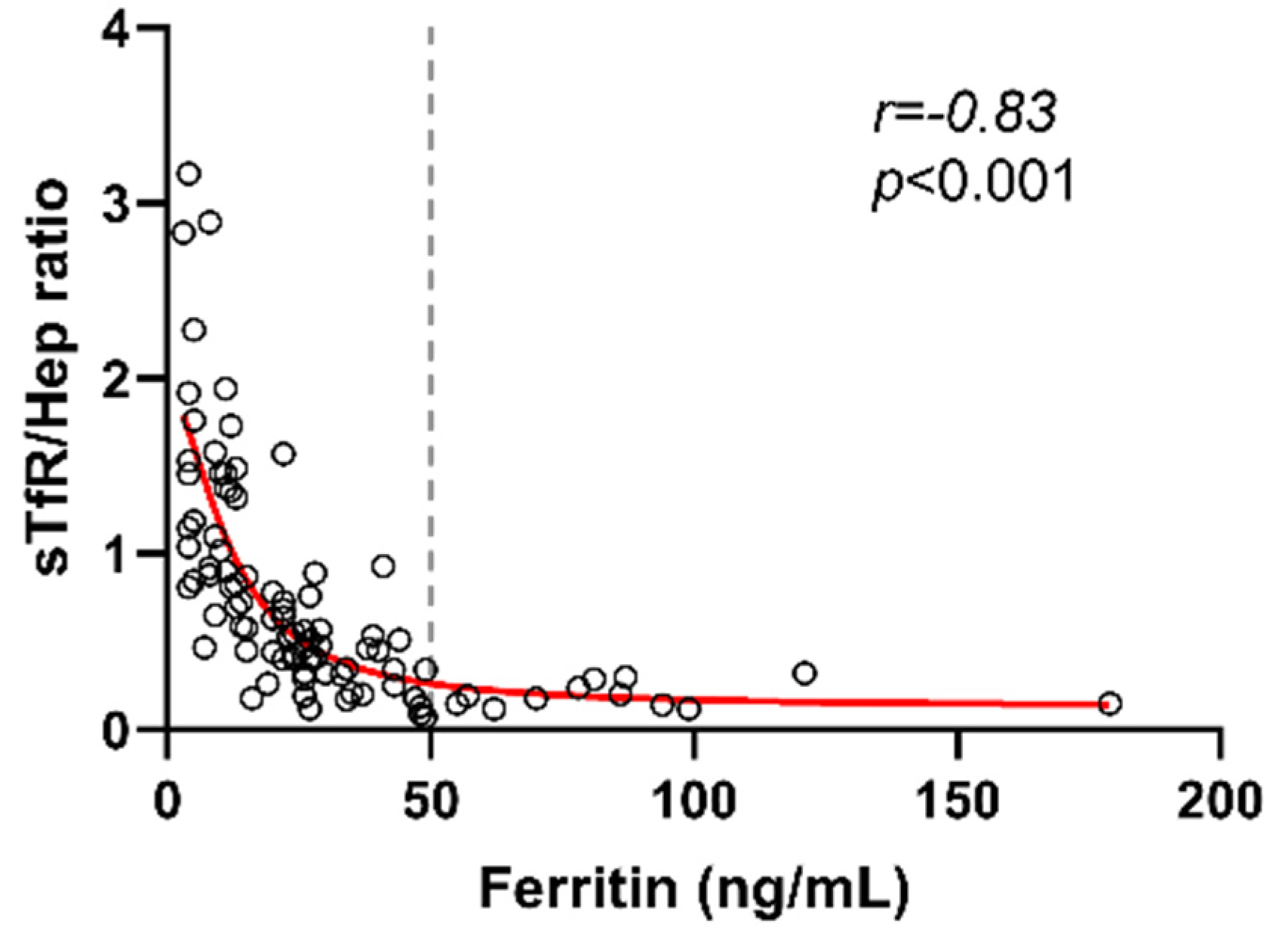
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A Systematic Analysis of Global Anemia Burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Gómez-Ramírez, S.; Besser, M.; Pavía, J.; Gomollón, F.; Liumbruno, G.M.; Bhandari, S.; Cladellas, M.; Shander, A.; Auerbach, M. Current Misconceptions in Diagnosis and Management of Iron Deficiency. Blood Transfus. 2017, 15, 422–437. [Google Scholar] [PubMed]
- AlRajeh, L.; Zaher, A.; Alghamdi, A.; Alsheikh, R.; AlSultan, O. Effects of Iron Deficiency and Its Indicators on Lymphocyte Subsets: A Study at King Fahd Hospital of the University, Saudi Arabia. JBM 2022, 13, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Weyh, C.; Krüger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef]
- Nairz, M.; Weiss, G. Iron in Infection and Immunity. Mol. Asp. Med. 2020, 75, 100864. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Uyoga, M.A.; Mutuku, F.M.; Frost, J.N.; Mwasi, E.; Paganini, D.; van der Klis, F.R.M.; Malhotra, I.J.; LaBeaud, A.D.; Ricci, C.; et al. Iron Deficiency Anemia at Time of Vaccination Predicts Decreased Vaccine Response and Iron Supplementation at Time of Vaccination Increases Humoral Vaccine Response: A Birth Cohort Study and a Randomized Trial Follow-Up Study in Kenyan Infants. Front. Immunol. 2020, 11, 1313. [Google Scholar] [CrossRef]
- Drakesmith, H.; Pasricha, S.-R.; Cabantchik, I.; Hershko, C.; Weiss, G.; Girelli, D.; Stoffel, N.; Muckenthaler, M.U.; Nemeth, E.; Camaschella, C.; et al. Vaccine Efficacy and Iron Deficiency: An Intertwined Pair? Lancet Haematol. 2021, 8, e666–e669. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations; World Health Organization (WHO): Geneva, Switzerland, 2020. [Google Scholar]
- Snook, J.; Bhala, N.; Beales, I.L.P.; Cannings, D.; Kightley, C.; Logan, R.P.; Pritchard, D.M.; Sidhu, R.; Surgenor, S.; Thomas, W.; et al. British Society of Gastroenterology Guidelines for the Management of Iron Deficiency Anaemia in Adults. Gut 2021, 70, 2030–2051. [Google Scholar] [CrossRef]
- Galetti, V.; Stoffel, N.U.; Sieber, C.; Zeder, C.; Moretti, D.; Zimmermann, M.B. Threshold Ferritin and Hepcidin Concentrations Indicating Early Iron Deficiency in Young Women Based on Upregulation of Iron Absorption. EClinicalMedicine 2021, 39, 101052. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Williet, N.; Cacoub, P. Guidelines on the Diagnosis and Treatment of Iron Deficiency across Indications: A Systematic Review. Am. J. Clin. Nutr. 2015, 102, 1585–1594. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Sierpinski, R.; Josiak, K.; Suchocki, T.; Wojtas-Polc, K.; Mazur, G.; Butrym, A.; Rozentryt, P.; Meer, P.; Comin-Colet, J.; Haehling, S.; et al. High Soluble Transferrin Receptor in Patients with Heart Failure: A Measure of Iron Deficiency and a Strong Predictor of Mortality. Eur. J. Heart Fail. 2021, 23, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kwon, S.; Lee, W.; Kim, Y.; Bae, E.; Lee, J.; Park, J.Y.; Kim, Y.C.; Kim, E.Y.; Kim, D.K.; et al. Soluble Transferrin Receptor Can Predict All-Cause Mortality Regardless of Anaemia and Iron Storage Status. Sci. Rep. 2022, 12, 11911. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Wylie-Rosett, J.; Gunter, M.J.; Negassa, A.; Kabat, G.C.; Rohan, T.E.; Crandall, J. Diabetes Prevention Program (DPP) Research Group Biomarkers of Body Iron Stores and Risk of Developing Type 2 Diabetes. Diabetes Obes. Metab. 2009, 11, 472–479. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Yang, H.; Shao, M.; Xu, S.; Deng, J.; Gao, X.; Liu, H.; Shuai, Z.; Xu, S.; et al. Serum Levels of Hepcidin in Rheumatoid Arthritis and Its Correlation with Disease Activity and Anemia: A Meta-Analysis. Immunol. Investig. 2021, 50, 243–258. [Google Scholar] [CrossRef]
- Minchella, P.A.; Armitage, A.E.; Darboe, B.; Jallow, M.W.; Drakesmith, H.; Jaye, A.; Prentice, A.M.; McDermid, J.M. Elevated Hepcidin Is Part of a Complex Relation That Links Mortality with Iron Homeostasis and Anemia in Men and Women with HIV Infection. J. Nutr. 2015, 145, 1194–1201. [Google Scholar] [CrossRef]
- Nai, A.; Lorè, N.I.; Pagani, A.; De Lorenzo, R.; Di Modica, S.; Saliu, F.; Cirillo, D.M.; Rovere-Querini, P.; Manfredi, A.A.; Silvestri, L. Hepcidin Levels Predict COVID-19 Severity and Mortality in a Cohort of Hospitalized Italian Patients. Am. J. Hematol. 2021, 96, E32–E35. [Google Scholar] [CrossRef]
- Sasu, B.J.; Cooke, K.S.; Arvedson, T.L.; Plewa, C.; Ellison, A.R.; Sheng, J.; Winters, A.; Juan, T.; Li, H.; Begley, C.G.; et al. Antihepcidin Antibody Treatment Modulates Iron Metabolism and Is Effective in a Mouse Model of Inflammation-Induced Anemia. Blood 2010, 115, 3616–3624. [Google Scholar] [CrossRef]
- Lanser, L.; Fuchs, D.; Kurz, K.; Weiss, G. Physiology and Inflammation Driven Pathophysiology of Iron Homeostasis—Mechanistic Insights into Anemia of Inflammation and Its Treatment. Nutrients 2021, 13, 3732. [Google Scholar] [CrossRef]
- Wirth, J.P.; Woodruff, B.A.; Engle-Stone, R.; Namaste, S.M.; Temple, V.J.; Petry, N.; Macdonald, B.; Suchdev, P.S.; Rohner, F.; Aaron, G.J. Predictors of Anemia in Women of Reproductive Age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Am. J. Clin. Nutr. 2017, 106, 416S–427S. [Google Scholar]
- Milman, N.; Taylor, C.L.; Merkel, J.; Brannon, P.M. Iron Status in Pregnant Women and Women of Reproductive Age in Europe. Am. J. Clin. Nutr. 2017, 106, 1655S–1662S. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. IJMS 2020, 21, 5529. [Google Scholar] [CrossRef] [PubMed]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an Emerging Risk Factor for Iron Deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Zeder, C.; Muthayya, S.; Winichagoon, P.; Chaouki, N.; Aeberli, I.; Hurrell, R.F. Adiposity in Women and Children from Transition Countries Predicts Decreased Iron Absorption, Iron Deficiency and a Reduced Response to Iron Fortification. Int. J. Obes. 2008, 32, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Vila Zárate, C.; Martín González, C.; González Álvarez, R.J.; Soto Darias, I.; Díaz Pérez, B.; Abreu González, P.; Medina Arana, V.; Martínez Riera, A. Ferritin, Serum Iron and Hemoglobin as Acute Phase Reactants in Laparoscopic and Open Surgery of Cholecystectomy: An Observational Prospective Study. Pathophysiology 2022, 29, 583–594. [Google Scholar] [CrossRef]
| Number of Subjects | 228 |
|---|---|
| Female sex (%) | 195 (86) |
| Age (years) | 44 (35–51) |
| Body mass index (Kg/m2) | 24.3 (21.5–28) |
| Body mass index ≥ 30 (%) | 34 (15) |
| Hemoglobin (g/dL) | 13.8 (12.8–14.4) |
| Mean corpuscle volume (fL) | 91.4 (88–94.8) |
| Mean corpuscle hemoglobin concentration (pg) | 29.5 (28.3–30.5) |
| Subjects with anemia (%) | 24 (11) |
| Transferrin level (mg/dL) | 268.5 (238.3–306.3) |
| Saturation transferrin index | 23.1 (14.4–32.6) |
| Sideremia (µg/dL) | 77 (54–105) |
| Ferritin (ng/mL) | 34 (14–74) |
| Subjects with ferritin < 50 (ng/mL) | 149 (65) |
| Soluble transferrin receptor (mg/L) a | 1.31 (1.28–1.66) |
| Hepcidin (ng/mL) b | 2.9 (1.8–5.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarancon-Diez, L.; Genebat, M.; Roman-Enry, M.; Vázquez-Alejo, E.; Espinar-Buitrago, M.d.l.S.; Leal, M.; Muñoz-Fernandez, M.Á. Threshold Ferritin Concentrations Reflecting Early Iron Deficiency Based on Hepcidin and Soluble Transferrin Receptor Serum Levels in Patients with Absolute Iron Deficiency. Nutrients 2022, 14, 4739. https://doi.org/10.3390/nu14224739
Tarancon-Diez L, Genebat M, Roman-Enry M, Vázquez-Alejo E, Espinar-Buitrago MdlS, Leal M, Muñoz-Fernandez MÁ. Threshold Ferritin Concentrations Reflecting Early Iron Deficiency Based on Hepcidin and Soluble Transferrin Receptor Serum Levels in Patients with Absolute Iron Deficiency. Nutrients. 2022; 14(22):4739. https://doi.org/10.3390/nu14224739
Chicago/Turabian StyleTarancon-Diez, Laura, Miguel Genebat, Manuela Roman-Enry, Elena Vázquez-Alejo, Maria de la Sierra Espinar-Buitrago, Manuel Leal, and Mª Ángeles Muñoz-Fernandez. 2022. "Threshold Ferritin Concentrations Reflecting Early Iron Deficiency Based on Hepcidin and Soluble Transferrin Receptor Serum Levels in Patients with Absolute Iron Deficiency" Nutrients 14, no. 22: 4739. https://doi.org/10.3390/nu14224739
APA StyleTarancon-Diez, L., Genebat, M., Roman-Enry, M., Vázquez-Alejo, E., Espinar-Buitrago, M. d. l. S., Leal, M., & Muñoz-Fernandez, M. Á. (2022). Threshold Ferritin Concentrations Reflecting Early Iron Deficiency Based on Hepcidin and Soluble Transferrin Receptor Serum Levels in Patients with Absolute Iron Deficiency. Nutrients, 14(22), 4739. https://doi.org/10.3390/nu14224739







