Diet and Disease Activity in Patients with Axial Spondyloarthritis: SpondyloArthritis and NUTrition Study (SANUT)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.4. Food-Intake Survey and Calculation of the Indices for Fiber, Refined Sugars, Omega-3 PUFAs, Ultra-Processed Foods, and Vitamin C Consumption
2.5. Study Objectives and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
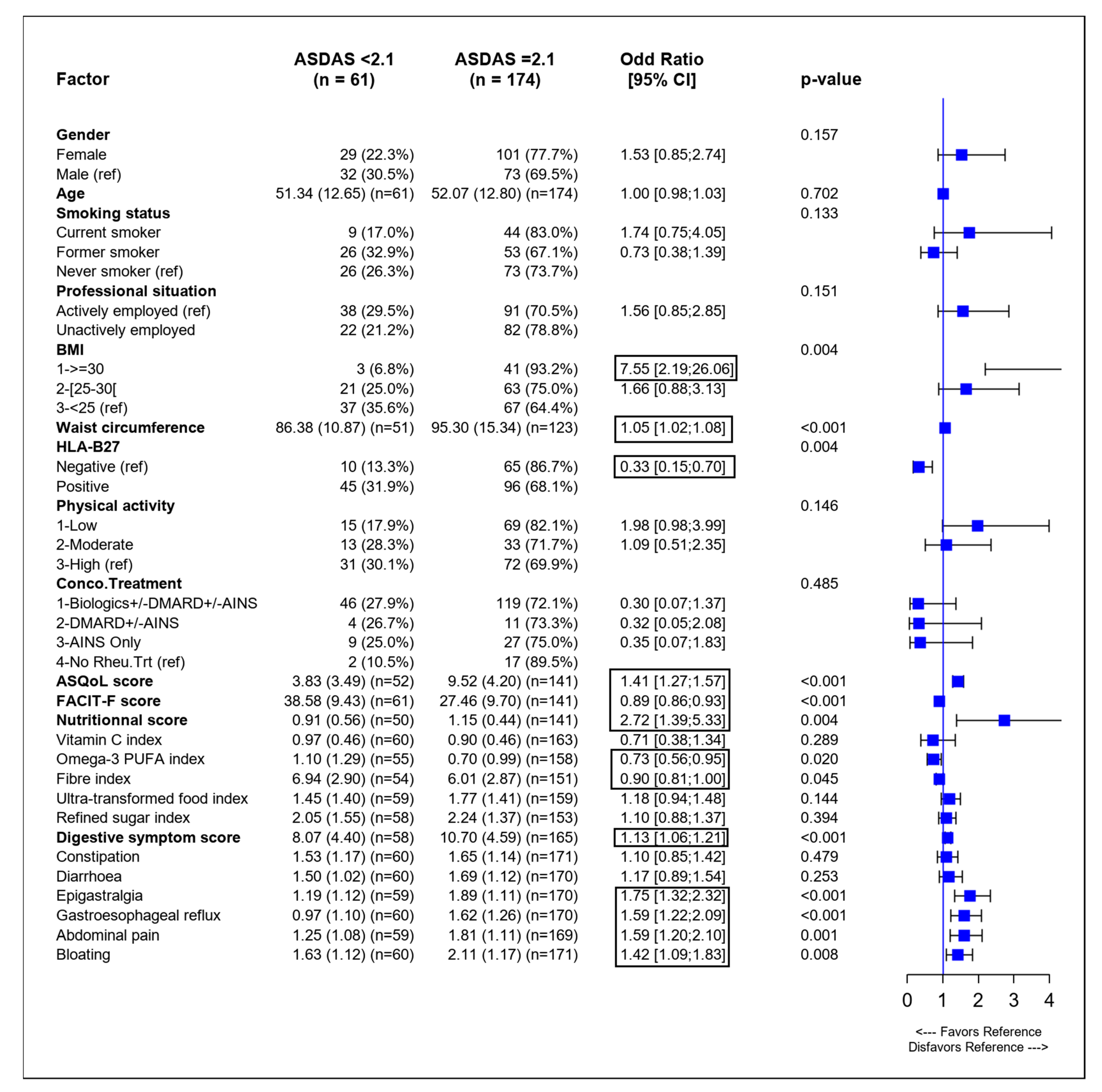
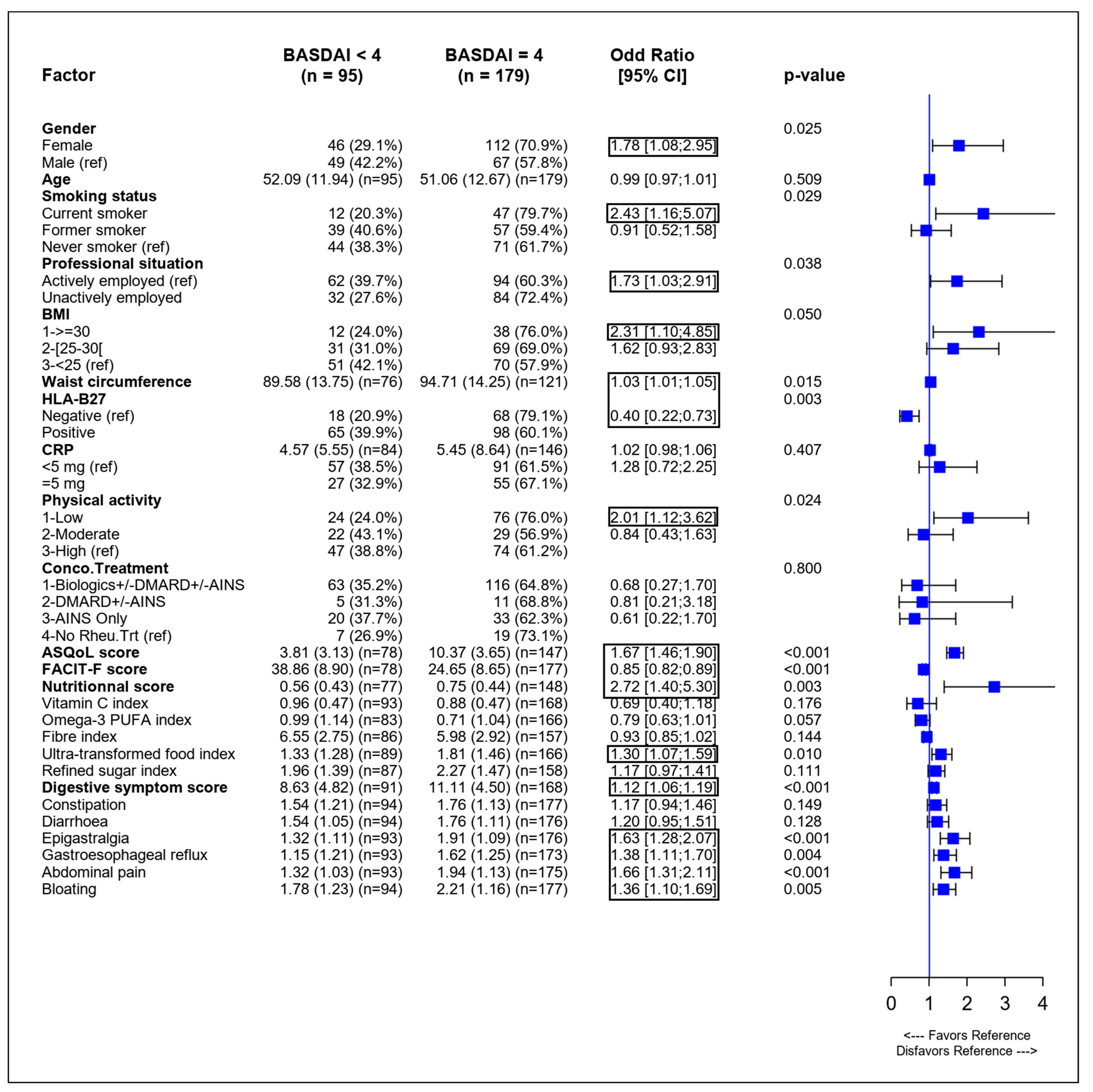
3.2. Identification of Variables Associated with SpA Activity in Univariate Analysis
3.3. Identification of Variables Associated with SpA Activity in Multivariate Analysis
3.4. Analysis of the Nutritional Factors Associated with Quality of Life
3.5. Post-Hoc Analyses to Define a Nutritional Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Proft, F.; Poddubnyy, D. Ankylosing spondylitis and axial spondyloarthritis: Recent insights and impact of new classification criteria. Ther. Adv. Musculoskelet. Dis. 2018, 10, 129–139. [Google Scholar] [CrossRef]
- Wendling, D. The gut in spondyloarthritis. Jt. Bone Spine 2016, 83, 401–405. [Google Scholar] [CrossRef]
- De Vos, M.; Mielants, H.; Cuvelier, C.; Elewaut, A.; Veys, E. Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology 1996, 110, 1696–1703. [Google Scholar] [CrossRef]
- Van Praet, L.; Van den Bosch, F.E.; Jacques, P.; Carron, P.; Jans, L.; Colman, R.; Glorieus, E.; Peeters, H.; Mielants, H.; De Vos, M.; et al. Microscopic gut inflammation in axial spondyloarthritis: A multiparametric predictive model. Ann. Rheum. Dis. 2013, 72, 414–417. [Google Scholar] [CrossRef]
- Dignass, A.U. Mechanisms and modulation of intestinal epithelial repair. Inflamm. Bowel Dis. 2001, 7, 68–77. [Google Scholar] [CrossRef]
- Amoroso, C.; Perillo, F.; Strati, F.; Fantini, M.C.; Caprioli, F.; Facciotti, F. The Role of Gut Microbiota Biomodulators on Mucosal Immunity and Intestinal Inflammation. Cells 2020, 9, 1234. [Google Scholar] [CrossRef]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.L. Gut microbes, immunity, and spondyloarthritis. Clin. Immunol. 2015, 159, 134–142. [Google Scholar] [CrossRef]
- Breban, M.; Beaufrere, M.; Glatigny, S. The microbiome in spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101495. [Google Scholar] [CrossRef]
- So, J.; Tam, L.S. Gut Microbiome and Its Interaction with Immune System in Spondyloarthritis. Microorganisms 2020, 8, 1727. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Xu, Y.; Li, P.; Ma, H.; Li, X.; Li, M. The correlation between intestinal dysbiosis and the development of ankylosing spondylitis. Microb. Pathog. 2019, 132, 188–192. [Google Scholar] [CrossRef]
- Raychaudhuri, S.K.; Saxena, A.; Raychaudhuri, S.P. Role of IL-17 in the pathogenesis of psoriatic arthritis and axial spondyloarthritis. Clin. Rheumatol. 2015, 34, 1019–1023. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic Potential of omega-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Marton, L.T.; Goulart, R.A.; Carvalho, A.C.A.; Barbalho, S.M. Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. Int. J. Mol. Sci. 2019, 20, 4851. [Google Scholar] [CrossRef]
- Rudwaleit, M.; van der Heijde, D.; Landewe, R.; Akkoc, N.; Brandt, J.; Chou, C.T.; Dougados, M.; Huang, F.; Gu, J.; Kirazli, Y.; et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 2011, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar]
- Machado, P.M.; Landewe, R.B.; van der Heijde, D.M. Endorsement of definitions of disease activity states and improvement scores for the Ankylosing Spondylitis Disease Activity Score: Results from OMERACT 10. J. Rheumatol. 2011, 38, 1502–1506. [Google Scholar] [CrossRef]
- Pham, T.; van der Heijde, D.M.; Pouchot, J.; Guillemin, F. Development and validation of the French ASQoL questionnaire. Clin. Exp. Rheumatol. 2010, 28, 379–385. [Google Scholar]
- Tinsley, A.; Macklin, E.A.; Korzenik, J.R.; Sands, B.E. Validation of the functional assessment of chronic illness therapy-fatigue (FACIT-F) in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2011, 34, 1328–1336. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Barrat, E.; Aubineau, N.; Maillot, M.; Derbord, E.; Barthes, P.; Lescuyer, J.F.; Boisseau, N.; Peltier, S.L. Repeatability and relative validity of a quantitative food-frequency questionnaire among French adults. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef][Green Version]
- Patterson, A.C.; Hogg, R.C.; Kishi, D.M.; Stark, K.D. Biomarker and dietary validation of a Canadian food frequency questionnaire to measure eicosapentaenoic and docosahexaenoic acid intakes from whole food, functional food, and nutraceutical sources. J. Acad. Nutr. Diet. 2012, 112, 1005–1014. [Google Scholar] [CrossRef]
- L’Agence nationale de sécurité sanitaire de l’alimentation, d.l.e.e.d.t.A. Table Ciqual. 2016. Available online: https://www.anses.fr/fr/content/mise-à-jour-majeure-de-la-table-ciqual-outil-de-référence-sur-la-composition-nutritionnelle. (accessed on 9 June 2017).
- Sundstrom, B.; Wallberg-Jonsson, S.; Johansson, G. Diet, disease activity, and gastrointestinal symptoms in patients with ankylosing spondylitis. Clin. Rheumatol. 2011, 30, 71–76. [Google Scholar] [CrossRef]
- Soleimanifar, N.; Nicknam, M.H.; Bidad, K.; Jamshidi, A.R.; Mahmoudi, M.; Mostafaei, S.; Hosseini-Khah, Z.; Nikbin, B. Effect of food intake and ambient air pollution exposure on ankylosing spondylitis disease activity. Adv. Rheumatol. 2019, 59, 9. [Google Scholar] [CrossRef]
- Durkin, L.A.; Childs, C.E.; Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and the Intestinal Epithelium-A Review. Foods 2021, 10, 199. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Omega-3 polyunsaturated fatty acids focusing on eicosapentaenoic acid and docosahexaenoic acid in the prevention of cardiovascular diseases: A review of the state-of-the-art. Expert Rev. Clin. Pharmacol. 2021, 14, 79–93. [Google Scholar] [CrossRef]
- Sigaux, J.; Mathieu, S.; Nguyen, Y.; Sanchez, P.; Letarouilly, J.G.; Soubrier, M.; Czernichow, S.; Flipo, R.M.; Sellam, J.; Daien, C. Impact of type and dose of oral polyunsaturated fatty acid supplementation on disease activity in inflammatory rheumatic diseases: A systematic literature review and meta-analysis. Arthritis Res. Ther. 2022, 24, 100. [Google Scholar] [CrossRef]
- Kaliannan, K.; Wang, B.; Li, X.Y.; Kim, K.J.; Kang, J.X. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci. Rep. 2015, 5, 11276. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]
- Shores, D.R.; Binion, D.G.; Freeman, B.A.; Baker, P.R. New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 2192–2204. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Armstrong, H.; Mander, I.; Zhang, Z.; Armstrong, D.; Wine, E. Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front. Pediatr. 2020, 8, 620189. [Google Scholar] [CrossRef]
- Vadell, A.K.E.; Barebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)-a randomized, controlled crossover trial indicating effects on disease activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Ometto, F.; Ortolan, A.; Farber, D.; Lorenzin, M.; Dellamaria, G.; Cozzi, G.; Favero, M.; Valentini, R.; Doria, A.; Ramonda, R. Mediterranean diet in axial spondyloarthritis: An observational study in an Italian monocentric cohort. Arthritis Res. Ther. 2021, 23, 219. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E. Perspective: Reductionist Nutrition Research Has Meaning Only within the Framework of Holistic and Ethical Thinking. Adv. Nutr. 2018, 9, 655–670. [Google Scholar] [CrossRef]
- Elizabeth, L.; Machado, P.; Zinocker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef]
- Schnabel, L.; Buscail, C.; Sabate, J.M.; Bouchoucha, M.; Kesse-Guyot, E.; Alles, B.; Touvier, M.; Monteiro, C.A.; Hercberg, S.; Benamouzig, R.; et al. Association Between Ultra-Processed Food Consumption and Functional Gastrointestinal Disorders: Results From the French NutriNet-Sante Cohort. Am. J. Gastroenterol. 2018, 113, 1217–1228. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Processed Food Additive Microbial Transglutaminase and Its Cross-Linked Gliadin Complexes Are Potential Public Health Concerns in Celiac Disease. Int. J. Mol. Sci. 2020, 21, 1127. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755. [Google Scholar] [CrossRef]
- Ortolan, A.; Lorenzin, M.; Felicetti, M.; Ramonda, R. Do Obesity and Overweight Influence Disease Activity Measures in Axial Spondyloarthritis? A Systematic Review and Meta-Analysis. Arthritis Care Res. 2021, 73, 1815–1825. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. omega-3 and omega-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
| Patients with Data, n | Details | ||
|---|---|---|---|
| Age (years), mean (SD) | 278 | 51.7 (12.6) | |
| Sex (female), n (%) | 278 | 150 (57.6) | |
| Height (cm), mean (SD) | 277 | 167.4 (9.2) | |
| Weight (kg), mean (SD) | 275 | 74.1 (16.1) | |
| BMI (kg/m2), mean (SD) | 274 | 26.4 (5.3) | |
| Waist circumference (cm), mean (SD) | 200 | 92.9 (14.4) | |
| Disease duration (years), mean (SD) | 263 | 13.9 (10.6) | |
| Radiographic axSpA, n (%) | 278 | 132 (47.5) | |
| Nonradiographic axSpA, n (%) | 278 | 146 (52.5) | |
| Positive for HLA-B27, n (%) | 252 | 165 (65.5) | |
| CRP (mg/L), mean (SD) | 233 | 5.2 (7.6) | |
| CRP < 5 mg, n (%) | 149 (63.9) | ||
| CRP ≥ 5 mg, n (%) | 84 (36.1) | ||
| ASDAS (using CRP), mean (SD) | 235 | 2.6 (0.8) | |
| ASDAS < 2.1, n (%) | 56 (23.8) | ||
| ASDAS ≥ 2.1, n (%) | 179 (76.2) | ||
| BASDAI score, mean (SD) | 274 | 4.6 (1.9) | |
| BASDAI < 4, n (%) | 95 (34.7) | ||
| BASDAI ≥ 4, n (%) | 179 (65.3) | ||
| ASQoL, mean (SD) | 226 | 8.1 (4.7) | |
| FACIT-F, mean (SD) | 276 | 29.4 (10.9) | |
| Digestive symptom score, mean (SD) | 261 | 10.2 (4.8) | |
| Professional activity, n (%) | 276 | ||
| Actively employed | 157 (56.9) | ||
| Not actively employed | 119 (43.1) | ||
| Smoking status, n (%) | 273 | ||
| Former smoker | 97 (35.5) | ||
| Current smoker | 59 (21.6) | ||
| Never smoker | 117 (42.9) | ||
| Treatments, n (%) | 278 | ||
| NSAIDs | 129 (46.4) | ||
| sDMARDs | 30 (10.8) | ||
| Anti-TNF | 150 (54.2) | ||
| Other biotherapies | 33 (11.9) | ||
| Steroids | 16 (5.8) | ||
| Anticholesterolemic | 33 (11.9) | ||
| Antidiabetic | 11 (4.0) | ||
| IPAQ, mean (SD) | 276 | 2789 (4113) | |
| Low physical activity, n (%) | 102 (37.0) | ||
| Moderate physical activity, n (%) | 52 (18.8) | ||
| High physical activity, n (%) | 122 (44.2) | ||
| Patients with Data, n | Details | ||
|---|---|---|---|
| Vitamin D supplementation, n (%) | 145 | ||
| None | 53 (36.6) | ||
| Annually | 28 (19.3) | ||
| Every 3 months | 29 (20.0) | ||
| Monthly | 13 (9.0) | ||
| Twice monthly | 9 (6.2) | ||
| Daily | 13 (9.0) | ||
| Specific diets, n (%) | 275 | ||
| None | 214 (77.8) | ||
| Diabetic diet | 6 (2.2) | ||
| Reduced-fat diet | 12 (4.4) | ||
| Gluten-free diet | 18 (6.5) | ||
| Other diets | 25 (9.1) | ||
| Nutritional supplement intake, n (%) | 275 | 52 (18.9) | |
| Consumption indices | |||
| Vitamin C index | 264 | ||
| Mean (SD) | 0.9 (0.5) | ||
| Median (IQR) | 0.8 (0.6, 1.2) | ||
| Omega-3 PUFA index | 251 | ||
| Mean (SD) | 0.8 (1.1) | ||
| Median (IQR) | 0.4 (0.0, 1.3) | ||
| Fiber index | 243 | ||
| Mean (SD) | 6.2 (2.9) | ||
| Median (IQR) | 6.0 (4.0, 7.5) | ||
| Ultra-transformed foods index | 257 | ||
| Mean (SD) | 1.6 (1.4) | ||
| Median (IQR) | 1.1 (0.6, 2.5) | ||
| Refined sugar index | 246 | ||
| Mean (SD) | 2.2 (1.4) | ||
| Median (IQR) | 2.0 (1.0, 3.0) | ||
| ASDAS ≥ 2.1 (n = 214) | BASDAI ≥ 4 (n = 220) | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Smoking status | Not significant in the multivariate model. | Not significant in the multivariate model. | |||
| Sex | Not significant in the multivariate model. | Not significant in the multivariate model. | |||
| BMI | 0.005 | Not significant in the multivariate model. | |||
| <25 (ref) | |||||
| (25;30) | 1.9 (0.9, 3.8) | ||||
| ≥30 | 7.1 (2.0, 25.0) | ||||
| Physical activity | Not significant in the multivariate model. | Not significant in the multivariate model | |||
| Digestive symptom score | Not significant in the multivariate model. | 1.14 (1.07, 1.23) | 0.0001 | ||
| Professional situation | Not significant in the multivariate model. | 0.003 | |||
| Actively employed (ref) | |||||
| Not actively employed | 2.7 (1.4, 5.1) | ||||
| HLA-B27 | 0.004 0.3 (0.1, 0.7) | 0.008 | |||
| Negative (ref) | 0.4 (0.2, 0.8) | ||||
| Positive | |||||
| Omega-3 PUFA index | Not significant in the multivariate model | Not significant in the multivariate model | |||
| Fiber index | Not significant in the multivariate model | Not significant in the multivariate model | |||
| Ultra-transformed foods index | Not significant in the multivariate model | 1.4 (1.1, 1.7) | 0.01 | ||
| ASDAS ≥ 2.1 (n = 168) | BASDAI ≥ 4 (n = 192) | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Smoking Status | Not significant in the multivariate model. | Not significant in the multivariate model. | |||
| Sex | Not significant in the multivariate model. | Not significant in the multivariate model. | |||
| BMI | Not significant in the multivariate model. | Not significant in the multivariate model. | |||
| Physical activity | Not significant in the multivariate model. | Not significant in the multivariate model | |||
| Digestive symptom score | 1.1 (1.01, 1.2) | 0.03 | 1.1 (1.06, 1.2) | 0.007 | |
| Professional situation | 0.03 | 0.008 | |||
| Actively employed (ref) | |||||
| Not actively employed | 2.5 (1.1, 5.7) | 3.7 (1.7, 7.8) | |||
| HLA-B27 | 0.03 0.4 (0.2;0.9) | 0.02 | |||
| Negative (ref) | 0.4 (0.2, 0.9) | ||||
| Positive | |||||
| Nutritional score * | 3.1 (1.4, 6.8) | 0.006 | 3.1 (1.5, 6.6) | 0.003 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergne-Salle, P.; Salle, L.; Fressinaud-Marie, A.C.; Descamps-Deplas, A.; Montestruc, F.; Bonnet, C.; Bertin, P. Diet and Disease Activity in Patients with Axial Spondyloarthritis: SpondyloArthritis and NUTrition Study (SANUT). Nutrients 2022, 14, 4730. https://doi.org/10.3390/nu14224730
Vergne-Salle P, Salle L, Fressinaud-Marie AC, Descamps-Deplas A, Montestruc F, Bonnet C, Bertin P. Diet and Disease Activity in Patients with Axial Spondyloarthritis: SpondyloArthritis and NUTrition Study (SANUT). Nutrients. 2022; 14(22):4730. https://doi.org/10.3390/nu14224730
Chicago/Turabian StyleVergne-Salle, Pascale, Laurence Salle, Anne Catherine Fressinaud-Marie, Adeline Descamps-Deplas, François Montestruc, Christine Bonnet, and Philippe Bertin. 2022. "Diet and Disease Activity in Patients with Axial Spondyloarthritis: SpondyloArthritis and NUTrition Study (SANUT)" Nutrients 14, no. 22: 4730. https://doi.org/10.3390/nu14224730
APA StyleVergne-Salle, P., Salle, L., Fressinaud-Marie, A. C., Descamps-Deplas, A., Montestruc, F., Bonnet, C., & Bertin, P. (2022). Diet and Disease Activity in Patients with Axial Spondyloarthritis: SpondyloArthritis and NUTrition Study (SANUT). Nutrients, 14(22), 4730. https://doi.org/10.3390/nu14224730






