Predicting Factors for Metabolic Non-Response to a Complex Lifestyle Intervention—A Replication Analysis to a Randomized-Controlled Trial
Abstract
1. Introduction
2. Research Design and Methods
Statistical Analyses
3. Results
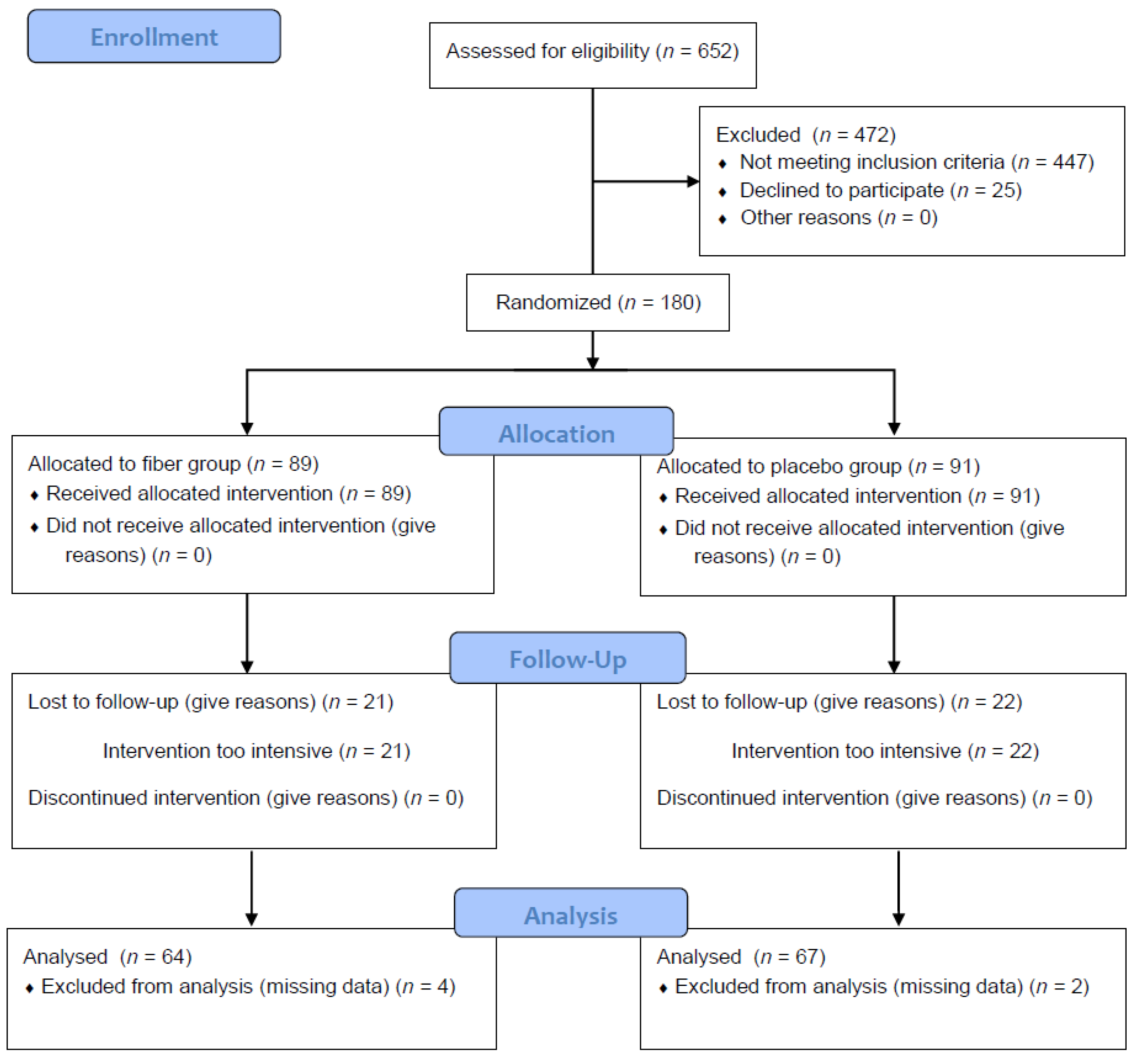
3.1. Cohort Structure
3.2. Intervention Effects and Factors Predicting Achievement of NGR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| BIA | bioelectric impedance analysis |
| FLI | fatty liver index |
| HIC | hepatic insulin clearance |
| HOMAIR | homeostasis model assessment insulin resistance index |
| HOMA-beta | homeostasis model assessment insulin for beta cell function |
| IFG | impaired fasting glucose |
| IGT | impaired glucose tolerance |
| ISIffa | insulin sensitivity index of blood free fatty acids |
| NAFLD | nonalcoholic fatty liver disease |
| NFG | normal fasting glucose |
| NGR | normal glucose regulation |
| NGT | normal glucose tolerance |
| OptiFiT | Optimal Fiber Trial for diabetes prevention |
| OGTT | oral glucose tolerance test |
| T2DM | type 2 diabetes mellitus |
Appendix A
| Characteristics of Participants at Study Entry | Placebo Arm | Fiber Arm | Full Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NGT (n = 23) | No NGT (n = 44) | p-Value | NGT (n = 23) | No NGT (n = 41) | p-Value | NGT (n = 46) | No NGT (n = 85) | p-Value | |
| Cohort Structure | |||||||||
| Age | 61 ± 10 | 60 ± 9 | n.s. | 56 ± 10 | 62 ± 8 | 0.018 | 58 ± 10 | 61 ± 9 | n.s. |
| Sex | 57% | 50% | n.s. | 78% | 68% | n.s. | 67% | 59% | n.s. |
| Anthropometry | |||||||||
| Weight (kg) | 92.2 ± 21.7 | 94.5 ± 20.9 | n.s. | 91.1 ± 13,1 | 86.1 ± 16.9 | n.s. | 91.7 ± 17.7 | 90.4 ± 19.4 | n.s. |
| BMI (kg/m2) | 33.7 ± 7.7 | 33.2 ± 6.0 | n.s. | 33.0 ± 4.7 | 31.5 ± 5.3 | n.s. | 33.3 ± 6.3 | 32.3 ± 5.7 | n.s. |
| Waist circumference (cm) | 106.8 ± 18.2 | 106.5 ± 12.8 | n.s. | 105.8 ± 12.0 | 100.8 ± 13.0 | n.s. | 106.3 ± 15.2 | 103.8 ± 13.1 | n.s. |
| Hip circumference (cm) | 115.1 ± 15.9 | 114.0 ± 12.7 | n.s. | 114.6 ± 11.3 | 109.8 ± 12.8 | n.s. | 114.8 ± 13.6 | 112.0 ± 12.8 | n.s. |
| WHR | 0.93 ± 0.08 | 0.94 ± 0.09 | n.s. | 0.92 ± 0.07 | 0.92 ± 0.09 | n.s. | 0.93 ± 0.07 | 0.93 ± 0.09 | n.s. |
| BIA—Body fat (%) | 36.8 ± 10.2 | 35.4 ± 7.4 | n.s. | 37.5 ± 8.7 | 36.7 ± 8.0 | n.s. | 37.1 ± 9.4 | 36.0 ± 7.7 | n.s. |
| Glycemia | |||||||||
| Fasting glucose (mg/dL) | 87.7 ± 9.2 | 93.1 ± 10.2 | n.s. (p = 0.053) | 91.7 ± 9.2 | 89.3 ± 11.3 | n.s. | 89.7 ± 9.3 | 91.3 ± 10.8 | n.s. |
| 1 h glucose (mg/dL) | 189.8 ± 34.8 | 205.4 ± 29.0 | n.s. (p = 0.069) | 201.7 ± 28.6 | 189.4 ± 35.8 | n.s. | 195.7 ± 32.1 | 202.0 ± 32.5 | n.s. |
| 2 h glucose (mg/dL) | 149.4 ± 13.2 | 166.6 ± 18.5 | <0.001 | 153.0 ± 12.9 | 160.4 ± 17.3 | n.s. (p = 0.078) | 151.2 ± 13.0 | 163.6 ± 18.1 | <0.001 |
| Glucose AUC (mg/dL*min) | 19,777.6 ± 2261.9 | 21,257.9 ± 2225.2 | 0.018 | 20,288.8 ± 2430.9 | 20,752.8 ± 2721.0 | n.s. | 20,039.2 ± 2336.1 | 21,017.7 ± 2470.9 | 0.027 |
| Insulin Resistance and Beta-Cell Function | |||||||||
| Fasting insulin (mU/L) | 8.7 ± 4.0 | 10.7 ± 6.3 | n.s. | 8.7 ± 3.8 | 9.0 ± 4.7 | n.s. | 8.7 ± 3,9 | 9.9 ± 5.6 | n.s. |
| Fasting c-Peptide (µg/L) | 1.6 ± 0.6 | 1.7 ± 0.7 | n.s. | 1.7 ± 0.8 | 1.6 ± 0.7 | n.s. | 1.7 ± 0.7 | 1.6 ± 0.7 | n.s. |
| Cederholm | 40.8 ± 10.7 | 35.0 ± 12.9 | 0.027 | 38.6 ± 12.1 | 36.7 ± 11.6 | n.s. | 39.7 ± 11.4 | 35.8 ± 12.2 | 0.034 |
| HOMA-beta | 79.1 ± 39.9 | 83.9 ± 40.3 | n.s. | 73.1 ± 30.8 | 76.9 ± 37.4 | n.s. (p = 0.093) | 76.1 ± 35.4 | 80.5 ± 38.9 | n.s. |
| NAFLD | |||||||||
| Fatty liver index | 70 ± 32 | 72 ± 24 | n.s. | 73 ± 24 | 65 ± 27 | n.s. | 72 ± 28 | 69 ± 26 | n.s. |
References
- Meigs, J.B.; Cupples, L.A.; Wilson, P.W. Parental transmission of type 2 diabetes: The Framingham Offspring Study. Diabetes 2000, 49, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Sigal, R.J.; Rich-Edwards, J.W.; Colditz, G.A.; Solomon, C.G.; Willett, W.C.; Speizer, F.E.; Manson, J.E. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: A prospective study. JAMA 1999, 282, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V.; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Tuomilehto, J.; Lindstrom, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Weickert, M.O. Nutritional modulation of insulin resistance. Scientifica 2012, 2012, 424780. [Google Scholar] [CrossRef][Green Version]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Kulzer, B.; Hermanns, N.; Gorges, D.; Schwarz, P.; Haak, T. Prevention of diabetes self-management program (PREDIAS): Effects on weight, metabolic risk factors, and behavioral outcomes. Diabetes Care 2009, 32, 1143–1146. [Google Scholar] [CrossRef][Green Version]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Stidsen, J.V.; Henriksen, J.E.; Olsen, M.H.; Thomsen, R.W.; Nielsen, J.S.; Rungby, J.; Ulrichsen, S.P.; Berencsi, K.; Kahlert, J.A.; Friborg, S.G.; et al. Pathophysiology-based phenotyping in type 2 diabetes: A clinical classification tool. Diabetes Metab. Res. Rev. 2018, 34, e3005. [Google Scholar] [CrossRef] [PubMed]
- Rückert, I.M.; Heier, M.; Rathmann, W.; Baumeister, S.E.; Döring, A.; Meisinger, C. Association between markers of fatty liver disease and impaired glucose regulation in men and women from the general population: The KORA-F4-study. PLoS ONE 2011, 6, e22932. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; Amini, M. Comparison of fasting glucose with post-load glucose values and glycated hemoglobin for prediction of type 2 diabetes: The Isfahan diabetes prevention study. Rev. Diabet. Stud. 2009, 6, 117–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pallardo, L.F.; Herranz, L.; Martin-Vaquero, P.; Garcia-Ingelmo, T.; Grande, C.; Jañez, M. Impaired fasting glucose and impaired glucose tolerance in women with prior gestational diabetes are associated with a different cardiovascular profile. Diabetes Care 2003, 26, 2318–2322. [Google Scholar] [CrossRef] [PubMed]
- Ghachem, A.; Brochu, M.; Dionne, I.J. Differential clusters of modifiable risk factors for impaired fasting glucose versus impaired glucose tolerance in adults 50 years of age and older. Ther. Adv. Chronic Dis. 2019, 10, 2040622319854239. [Google Scholar] [CrossRef]
- Kowall, B.; Rathmann, W.; Bongaerts, B.; Kuss, O.; Stang, A.; Roden, M.; Herder, C.; Koenig, W.; Huth, C.; Heier, M.; et al. Incidence Rates of Type 2 Diabetes in People with Impaired Fasting Glucose (ADA vs. WHO Criteria) and Impaired Glucose Tolerance: Results from an Older Population (KORA S4/F4/FF4 Study). Diabetes Care 2019, 42, e18–e20. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: An updated meta-analysis of 501,022 adult individuals. Gut 2021, 70, 962–969. [Google Scholar] [CrossRef]
- Jäger, S.; Jacobs, S.; Kröger, J.; Stefan, N.; Fritsche, A.; Weikert, C.; Boeing, H.; Schulze, M.B. Association between the Fatty Liver Index and Risk of Type 2 Diabetes in the EPIC-Potsdam Study. PLoS ONE 2015, 10, e0124749. [Google Scholar] [CrossRef]
- García-Escobar, E.; Valdés, S.; Soriguer, F.; Vendrell, J.; Urrutia-Etxebarria, I.M.; Maldonado-Araque, C.; Ortega, E.; Ocón, P.; Montanya, E.; Menéndez, E.; et al. Fatty liver index as a predictor for type 2 diabetes in subjects with normoglycemia in a nationwide cohort study. Sci. Rep. 2021, 11, 16453. [Google Scholar] [CrossRef]
- Chung, G.E.; Cho, E.J.; Yoon, J.W.; Yoo, J.-J.; Chang, Y.; Cho, Y.; Park, S.-H.; Han, K.; Shin, D.W.; Yu, S.J. Nonalcoholic fatty liver disease increases the risk of diabetes in young adults: A nationwide population-based study in Korea. Metabolism 2021, 123, 154866. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Koskinen, J.; Brown, E.; Magnussen, C.G.; Hutri-Kähönen, N.; Sabin, M.; Tossavainen, P.; Jokinen, E.; Laitinen, T.; Viikari, J.; et al. Fatty liver index predicts incident risk of prediabetes, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). Ann. Med. 2021, 53, 1256–1264. [Google Scholar] [CrossRef]
- Hulman, A.; Gujral, U.P.; Narayan, K.M.V.; Pradeepa, R.; Mohan, D.; Anjana, R.M.; Mohan, V.; Færch, K.; Witte, D.R. Glucose patterns during the OGTT and risk of future diabetes in an urban Indian population: The CARRS study. Diabetes Res. Clin. Pract. 2017, 126, 192–197. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Long-Term Effects of Metformin on Diabetes Prevention: Identification of Subgroups That Benefited Most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2019, 42, 601–608. [Google Scholar] [CrossRef]
- DREAM (Diabetes REduction Assessment with Ramipril and Rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [CrossRef]
- Hjorth, M.F.; Zohar, Y.; Hill, J.O.; Astrup, A. Personalized Dietary Management of Overweight and Obesity Based on Measures of Insulin and Glucose. Annu. Rev. Nutr. 2018, 38, 245–272. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Bray, G.A.; Zohar, Y.; Urban, L.; Miketinas, D.C.; Williamson, D.A.; Ryan, D.H.; Rood, J.; Champagne, C.M.; Sacks, F.M.; et al. Pretreatment Fasting Glucose and Insulin as Determinants of Weight Loss on Diets Varying in Macronutrients and Dietary Fibers—The POUNDS LOST Study. Nutrients 2019, 11, 586. [Google Scholar] [CrossRef]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Hjorth, M.F.; et al. Fasting Glucose State Determines Metabolic Response to Supplementation with Insoluble Cereal Fibre: A Secondary Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2385. [Google Scholar] [CrossRef]
- Saaristo, T.; Moilanen, L.; Korpi-Hyövälti, E.; Vanhala, M.; Saltevo, J.; Niskanen, L.; Jokelainen, J.; Peltonen, M.; Oksa, H.; Tuomilehto, J.; et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: One-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010, 33, 2146–2151. [Google Scholar] [CrossRef]
- Sakane, N.; Kotani, K.; Suganuma, A.; Takahashi, K.; Sato, J.; Suzuki, S.; Izumi, K.; Kato, M.; Noda, M.; Nirengi, S.; et al. Effects of obesity, metabolic syndrome, and non-alcoholic or alcoholic elevated liver enzymes on incidence of diabetes following lifestyle intervention: A subanalysis of the J-DOIT1. J. Occup. Health 2020, 62, e12109. [Google Scholar] [CrossRef]
- Stefan, N.; Staiger, H.; Wagner, R.; Machann, J.; Schick, F.; Häring, H.-U.; Fritsche, A. A high-risk phenotype associates with reduced improvement in glycaemia during a lifestyle intervention in prediabetes. Diabetologia 2015, 58, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Honsek, C.; Kabisch, S.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: The randomised controlled Optimal Fibre Trial (OptiFiT). Diabetologia 2018, 61, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Lindstrom, J.; Neumann, A.; Sheppard, K.E.; Gilis-Januszewska, A.; Greaves, C.J.; Handke, U.; Pajunen, P.; Puhl, S.; Pölönen, A.; Rissanen, A.; et al. Take action to prevent diabetes—The IMAGE toolkit for the prevention of type 2 diabetes in Europe. Horm. Metab. Res. 2010, 42 (Suppl. S1), S37–S55. [Google Scholar] [CrossRef] [PubMed]
- Wareham, N.J.; Jakes, R.W.; Rennie, K.L.; Mitchell, J.; Hennings, S.; Day, N.E. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int. J. Epidemiol. 2002, 31, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.M.; Vasquez-Caicedo, A.L.; Bell, S.; Krems, C.; Brombach, C. The German nutrient database: Basis for analysis of the nutritional status of the German Population. J. Food Comp. Anal. 2008, 21, 115–118. [Google Scholar] [CrossRef]
- Machann, J.; Thamer, C.; Schnoedt, B.; Stefan, N.; Haring, H.-U.; Claussen, C.D.; Fritsche, A.; Schick, F. Hepatic lipid accumulation in healthy subjects: A comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn. Reson. Med. 2006, 55, 913–917. [Google Scholar] [CrossRef]
- Retnakaran, R.; Shen, S.; Hanley, A.J.; Vuksan, V.; Hamilton, J.K.; Zinman, B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity 2008, 16, 1901–1907. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Kabisch, S.; Honsek, C.; Kemper, M.; Gerbracht, C.; Meyer, N.T.M.; Arafat, A.M.; Birkenfeld, A.L.; Machann, J.; Dambeck, U.; Osterhoff, M.A.; et al. Effects of Insoluble Cereal Fibre on Body Fat Distribution in the Optimal Fibre Trial (OptiFiT). Mol. Nutr. Food Res. 2021, 65, 2000991. [Google Scholar] [CrossRef]
- Cederholm, J.; Wibell, L. Insulin release and peripheral sensitivity at the oral glucose tolerance test. Diabetes Res. Clin. Pract. 1990, 10, 167–175. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Weickert, M.O.; et al. Obesity Does Not Modulate the Glycometabolic Benefit of Insoluble Cereal Fibre in Subjects with Prediabetes—A Stratified Post Hoc Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2726. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; Kahn, S.E.; Christophi, C.A.; Knowler, W.C.; Hamman, R.F.; Diabetes Prevention Program Research Group. Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009, 32, 1583–1588. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Temprosa, M.; Knowler, W.C.; Kahn, S.E.; Fowler, S.E.; Haffner, S.M.; Andres, R.; Saudek, C.; Edelstein, S.L.; Arakaki, R. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: Effects of lifestyle intervention and metformin. Diabetes 2005, 54, 2404–2414. [Google Scholar]
- Kanat, M.; Norton, L.; Winnier, D.; Jenkinson, C.; DeFronzo, R.A.; Abdul-Ghani, M.A. Impaired early—But not late-phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetol. 2011, 48, 209–217. [Google Scholar] [CrossRef]
- Sathananthan, A.; Man, C.D.; Zinsmeister, A.R.; Camilleri, M.; Rodeheffer, R.J.; Toffolo, G.; Cobelli, C.; Rizza, R.A.; Vella, A. A concerted decline in insulin secretion and action occurs across the spectrum of fasting and postchallenge glucose concentrations. Clin. Endocrinol. 2012, 76, 212–219. [Google Scholar] [CrossRef]
- Bae, J.C.; Rhee, E.J.; Lee, W.Y.; Park, S.E.; Park, C.Y.; Oh, K.W.; Park, S.W.; Kim, S.W. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: A 4-year retrospective longitudinal study. Diabetes Care 2011, 34, 727–729. [Google Scholar] [CrossRef]
- Li, G.; Hu, Y.; Yang, W.; Jiang, Y.; Wang, J.; Xiao, J.; Hu, Z.; Pan, X.; Howard, B.V.; Bennett, P.H. Effects of insulin resistance and insulin secretion on the efficacy of interventions to retard development of type 2 diabetes mellitus: The DA Qing IGT and Diabetes Study. Diabetes Res. Clin. Pract. 2002, 58, 193–200. [Google Scholar] [CrossRef]
- Wang, Q.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Keinänen-Kiukaanniemi, S.; Järvelin, M.-R.; Kettunen, J.; Mäkinen, V.-P.; Ala-Korpela, M. Insulin resistance and systemic metabolic changes in oral glucose tolerance test in 5340 individuals: An interventional study. BMC Med. 2019, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Machann, J.; Schick, F.; Fritsche, A.; Häring, H.U.; Stefan, N. The impact of liver fat vs. visceral fat in determining categories of prediabetes. Diabetologia 2010, 53, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, T.; Schipf, S.; Meisinger, C.; Schunk, M.; Maier, W.; Herder, C.; Roden, M.; Nauck, M.; Peters, A.; Völzke, H.; et al. Regional differences of undiagnosed type 2 diabetes and prediabetes prevalence are not explained by known risk factors. PLoS ONE 2014, 9, e113154. [Google Scholar]
- Sinn, D.H.; Kang, D.; Cho, S.J.; Paik, S.W.; Guallar, E.; Cho, J.; Gwak, G.-Y. Lean non-alcoholic fatty liver disease and development of diabetes: A cohort study. Eur. J. Endocrinol. 2019, 181, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-D.; Fu, K.-F.; Li, G.-M.; Lian, Y.-S.; Ren, A.-M.; Chen, Y.-J.; Xia, J.-R. Comparison of effects of obesity and non-alcoholic fatty liver disease on incidence of type 2 diabetes mellitus. World J. Gastroenterol. 2015, 21, 9607–9613. [Google Scholar] [CrossRef]
- Bergman, M.; Jagannathan, R.; Buysschaert, M.; Pareek, M.; Olsen, M.H.; Nilsson, P.M.; Medina, J.L.; Roth, J.; Chetrit, A.; Groop, L.; et al. Lessons learned from the 1-hour post-load glucose level during OGTT: Current screening recommendations for dysglycaemia should be revised. Diabetes Metab. Res. Rev. 2018, 34, e2992. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Lokken, R.P.; Hu, F.B.; van Dam, R.M. Physical activity of moderate intensity and risk of type 2 diabetes: A systematic review. Diabetes Care 2007, 30, 744–752. [Google Scholar] [CrossRef]
- Lindstrom, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Cockcroft, E.J.; Williams, C.A.; Jackman, S.R.; Armstrong, N.; Barker, A.R. Agreement and Reliability of Fasted and Oral Glucose Tolerance Test-Derived Indices of Insulin Sensitivity and Beta Cell Function in Boys. Int. J. Sports Med. 2017, 38, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Zethelius, B.; Cederholm, J. Comparison between indexes of insulin resistance for risk prediction of cardiovascular diseases or development of diabetes. Diabetes Res. Clin. Pract. 2015, 110, 183–192. [Google Scholar] [CrossRef]
- Haeckel, R.; Raber, R.; Wosniok, W. Comparability of indices for insulin resistance and insulin secretion determined during oral glucose tolerance tests. Clin. Chem. Lab. Med. 2006, 44, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Insulin secretory function in type 2 diabetes: Does it matter how you measure it? J. Diabetes 2009, 1, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, O.P.; Strassburger, K.; Strom, A.; Bönhof, G.J.; Karusheva, Y.; Antoniou, S.; Bódis, K.; Markgraf, D.F.; Burkart, V.; Müssig, K.; et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: A 5-year follow-up study. Lancet Diabetes Endocrinol. 2019, 7, 684–694. [Google Scholar] [CrossRef]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef]
- Ballestri, S.; Mantovani, A.; Byrne, C.D.; Lonardo, A.; Targher, G. Diagnostic accuracy of ultrasonography for the detection of hepatic steatosis: An updated meta-analysis of observational studies. Metab. Target Organ Damage 2021, 1, 7. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Bedogni, G.; Bellentani, S.; Tiribelli, C. The Fatty liver Index (FLI) 15 years later: A reappraisal. Metab. Target Organ Damage 2021, 1, 10. [Google Scholar] [CrossRef]
- Kabisch, S.; Bäther, S.; Dambeck, U.; Kemper, M.; Gerbracht, C.; Honsek, C.; Sachno, A.; Pfeiffer, A.F.H. Liver Fat Scores Moderately Reflect Interventional Changes in Liver Fat Content by a Low-Fat Diet but Not by a Low-Carb Diet. Nutrients 2018, 10, 157. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
| Characteristics of Participants at Study Entry | Placebo Arm | Fiber Arm | Full Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NGR (n = 19) | No NGR (n = 48) | p-Value | NGR (n = 14) | No NGR (n = 50) | p-Value | NGR (n = 33) | No NGR (n = 98) | p-Value | |
| Cohort Structure | |||||||||
| Age | 61 ± 10 | 60 ± 9 | n.s. | 58 ± 10 | 60 ± 9 | n.s. | 59 ± 10 | 60 ± 9 | n.s. |
| Sex | 63% | 48% | n.s. | 64% | 74% | n.s. | 64% | 61% | n.s. |
| Anthropometry | |||||||||
| Weight (kg) | 91.8 ± 22.3 | 94.5 ± 20.7 | n.s. | 90.0 ± 12.5 | 87.3 ± 16.6 | n.s. | 91.0 ± 18.5 | 90.8 ± 19.0 | n.s. |
| BMI (kg/m2) | 34.3 ± 8.1 | 33.0 ± 6.0 | n.s. | 32.0 ± 4.9 | 32.0 ± 5.2 | n.s. | 33.3 ± 6.9 | 32.5 ± 5.6 | n.s. |
| Waist circumference (cm) | 106.6 ± 18.1 | 106.6 ± 13.4 | n.s. | 105.3 ± 12.0 | 101.8 ± 13.0 | n.s. | 106.1 ± 15.6 | 104.2 ± 13.3 | n.s. |
| Hip circumference (cm) | 115.5 ± 15.9 | 114.0 ± 13.0 | n.s. | 111.7 ± 10.6 | 111.5 ± 12.9 | n.s. | 113.8 ± 13.9 | 112.7 ± 12.9 | n.s. |
| WHR | 0.92 ± 0.09 | 0.94 ± 0.08 | n.s. | 0.94 ± 0.07 | 0.92 ± 0.08 | n.s. | 0.93 ± 0.08 | 0.93 ± 0.08 | n.s. |
| BIA—Body fat (%) | 37.2 ± 10.3 | 35.3 ± 7.7 | n.s. | 32.6 ± 9.4 | 37.7 ± 7.7 | n.s. | 35.7 ± 1.01 | 36.6 ± 7.7 | n.s. |
| Glycemia | |||||||||
| Fasting glucose (mg/dL) | 87.6 ± 9.0 | 92.7 ± 10.3 | n.s. | 91.6 ± 10.4 | 89.8 ± 10.7 | n.s. | 89.3 ± 9.7 | 91.2 ± 10.5 | n.s. |
| 1-h glucose (mg/dL) | 183.5 ± 32.3 | 206.6 ± 29.4 | 0.011 | 207.0 ± 29.8 | 197.5 ± 34.1 | n.s. | 193.5 ± 33.0 | 202.0 ± 32.0 | n.s. |
| 2-h glucose (mg/dL) | 150.3 ± 14.2 | 164.8 ± 18.8 | 0.002 | 151.9 ± 12.1 | 159.4 ± 16.8 | n.s. (p = 0.102) | 151.0 ± 13.2 | 162.0 ± 17.9 | 0.001 |
| Glucose AUC (mg/dL*min) | 19,391.6 ± 2045.4 | 21,271.5 ± 2236.8 | 0.004 | 20,399.0 ± 2799.3 | 20,635.9 ± 2584.1 | n.s. | 19,828.2 ± 2410.1 | 20,950.4 ± 2426.8 | 0.020 |
| Insulin Resistance and Beta-Cell Function | |||||||||
| Fasting insulin (mU/L) | 9.0 ± 3.8 | 10.4 ± 6.3 | n.s. | 7.8 ± 3.6 | 9.2 ± 4.6 | n.s. | 8.5 ± 3.7 | 9.8 ± 5.5 | n.s. |
| Fasting c-Peptide (µg/L) | 1.7 ± 0.5 | 1.7 ± 0.8 | n.s. | 1.6 ± 0.8 | 1.6 ± 0.7 | n.s. | 1.6 ± 0.6 | 1.7 ± 0.7 | n.s. |
| Cederholm | 41.1 ± 10.3 | 35.4 ± 12.9 | 0.032 | 39.8 ± 15.1 | 36.7 ± 10.6 | n.s. | 40.5 ± 12.4 | 36.0 ± 11.7 | n.s. (p = 0.052) |
| HOMA-beta | 85.7 ± 39.6 | 80.9 ± 40.4 | n.s. | 62.0 ± 29.7 | 79.3 ± 35.7 | n.s. | 75.7 ± 37.2 | 8.1 ± 37.9 | n.s. |
| NAFLD | |||||||||
| Fatty liver index | 70 ± 32 | 72 ± 25 | n.s. | 71 ± 26 | 67 ± 26 | n.s. | 71 ± 29 | 70 ± 25 | n.s. |
| Lifestyle Changes over One Year | Full Cohort | ||
|---|---|---|---|
| NGR (n = 33) | No NGR (n = 98) | p-Value | |
| Eating Behavior | |||
| Change in body weight (kg) | −3.6 ± 5.1 | −3.3 ± 5.7 | n.s. |
| Change in energy intake (kcal) | −275 ± 547 | −273 ± 474 | n.s. |
| Change in fat intake (%) | −4.1 ± 7.4 | −1.8 ± 6.4 | n.s. |
| Change in dietary fiber intake (g) | 0 ± 8 | 0 ± 8 | n.s. |
| Change in supplemented fiber intake (g) | 6 ± 9 | 7 ± 10 | n.s. |
| Physical Activity | |||
| Change in steps per day (n) | −278 ± 2692 | 629 ± 2865 | n.s. |
| (A) | ||||||
|---|---|---|---|---|---|---|
| Variable | Change in Body fatBIA | Change in Waist Circumference | Change in FLI | |||
| ϱ | p | ϱ | p | ϱ | p | |
| Change in fasting glucose | −0.081 | 0.559 | 0.224 | 0.068 | 0.104 | 0.405 |
| Change in 2 h glucose | 0.034 | 0.807 | 0.075 | 0.545 | 0.217 | 0.080 |
| Change in Cederholm index | −0.171 | 0.221 | −0.149 | 0.232 | −0.320 | 0.009 ** |
| Change in HOMA-beta | 0.077 | 0.586 | 0.028 | 0.824 | 0.141 | 0.258 |
| (B) | ||||||
| Variable | Change in Body fatBIA | Change in Waist Circumference | Change in FLI | |||
| ϱ | p | ϱ | p | ϱ | p | |
| Change in fasting glucose | 0.159 | 0.265 | 0.223 | 0.077 | 0.089 | 0.487 |
| Change in 2 h glucose | 0.030 | 0.836 | 0.049 | 0.699 | 0.176 | 0.164 |
| Change in Cederholm index | −0.150 | 0.293 | −0.300 | 0.017 * | −0.489 | <0.001 *** |
| Change in HOMA-beta | 0.155 | 0.276 | −0.185 | 0.143 | −0.049 | 0.699 |
| (C) | ||||||
| Variable | Change in Body fatBIA | Change in Waist Circumference | Change in FLI | |||
| ϱ | p | ϱ | p | ϱ | p | |
| Change in fasting glucose | 0.038 | 0.700 | 0.233 | 0.007 ** | 0.104 | 0.241 |
| Change in 2 h glucose | 0.020 | 0.837 | 0.052 | 0.555 | 0.201 | 0.022 * |
| Change in Cederholm index | −0.144 | 0.145 | −0.216 | 0.014 * | −0.403 | <0.001 *** |
| Change in HOMA-beta | 0.098 | 0.320 | −0.065 | 0.465 | 0.057 | 0.523 |
| Placebo Group | Fiber Group | Total Cohort | ||||
|---|---|---|---|---|---|---|
| Variable | Likelihood Ratio χ2 | p Value | Likelihood Ratio χ2 | p Value | Likelihood Ratio χ2 | p Value |
| Sex | 0.062 | 0.803 | 0.100 | 0.751 | 0.133 | 0.715 |
| Age | 0.204 | 0.652 | 0.474 | 0.491 | 0.000 | 0.984 |
| BMI | 0.510 | 0.475 | 2.628 | 0.105 | 0.020 | 0.886 |
| Waist circumference | 0.014 | 0.907 | 2.545 | 0.111 | 0.327 | 0.567 |
| Fasting glucose levels | 0.782 | 0.377 | 0.233 | 0.629 | 0.033 | 0.856 |
| 2 h glucose levels | 3.360 | 0.067 | 2.282 | 0.131 | 5.588 | 0.018 * |
| HOMA-beta | 0.402 | 0.526 | 2.842 | 0.092 | 0.040 | 0.842 |
| Cederholm index | 0.089 | 0.765 | 0.474 | 0.491 | 0.073 | 0.787 |
| Fatty liver index (FLI) | 0.101 | 0.751 | 0.314 | 0.575 | 0.001 | 0.971 |
| Cederholm × FLI | 0.794 | 0.373 | 0.371 | 0.543 | 0.103 | 0.748 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Predicting Factors for Metabolic Non-Response to a Complex Lifestyle Intervention—A Replication Analysis to a Randomized-Controlled Trial. Nutrients 2022, 14, 4721. https://doi.org/10.3390/nu14224721
Kabisch S, Meyer NMT, Honsek C, Kemper M, Gerbracht C, Arafat AM, Dambeck U, Osterhoff MA, Weickert MO, Pfeiffer AFH. Predicting Factors for Metabolic Non-Response to a Complex Lifestyle Intervention—A Replication Analysis to a Randomized-Controlled Trial. Nutrients. 2022; 14(22):4721. https://doi.org/10.3390/nu14224721
Chicago/Turabian StyleKabisch, Stefan, Nina M. T. Meyer, Caroline Honsek, Margrit Kemper, Christiana Gerbracht, Ayman M. Arafat, Ulrike Dambeck, Martin A. Osterhoff, Martin O. Weickert, and Andreas F. H. Pfeiffer. 2022. "Predicting Factors for Metabolic Non-Response to a Complex Lifestyle Intervention—A Replication Analysis to a Randomized-Controlled Trial" Nutrients 14, no. 22: 4721. https://doi.org/10.3390/nu14224721
APA StyleKabisch, S., Meyer, N. M. T., Honsek, C., Kemper, M., Gerbracht, C., Arafat, A. M., Dambeck, U., Osterhoff, M. A., Weickert, M. O., & Pfeiffer, A. F. H. (2022). Predicting Factors for Metabolic Non-Response to a Complex Lifestyle Intervention—A Replication Analysis to a Randomized-Controlled Trial. Nutrients, 14(22), 4721. https://doi.org/10.3390/nu14224721







