Association between the Intake of Different Protein Sources and Obesity Coexisting with Low Handgrip Strength in Persons near Retirement Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Data Collection and Variables
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
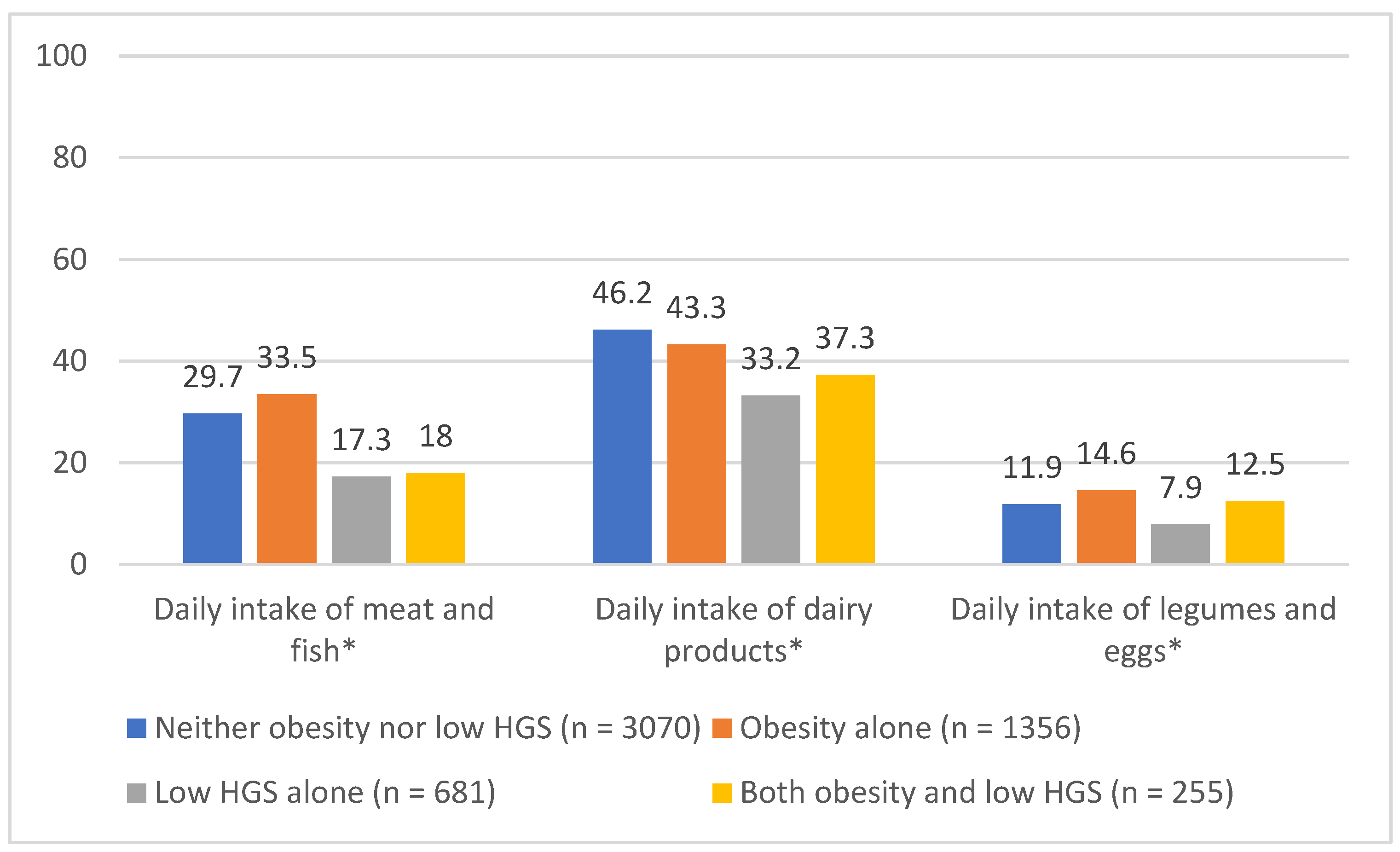
3.2. Daily Intake of Different Protein Sources
3.3. Association between Protein Intake and Other Factors and Obesity Coexisting with Low Handgrip Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.; Faith, M.S.; Pietrobelli, A.; Heymsfield, S.B. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. Am. J. Clin. Nutr. 2012, 95, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Santanasto, A.J.; Goodpaster, B.H.; Kritchevsky, S.B.; Miljkovic, I.; Satterfield, S.; Schwartz, A.V.; Cummings, S.R.; Boudreau, R.M.; Harris, T.B.; Newman, A.B. Body Composition Remodeling and Mortality: The Health Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Morio, B.; Denis, J.B.; Mioche, L. Age-Related Changes in Segmental Body Composition by Ethnicity and History of Weight Change across the Adult Lifespan. Int. J. Environ. Res. Public Health 2016, 13, 821. [Google Scholar] [CrossRef]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef]
- Godard, M. Gaining weight through retirement? Results from the SHARE survey. J. Health Econ. 2016, 45, 27–46. [Google Scholar] [CrossRef]
- OECD. Pensions at a Glance 2019; OECD: Paris, French, 2019. [Google Scholar] [CrossRef]
- Schoufour, J.D.; Tieland, M.; Barazzoni, R.; Ben Allouch, S.; van der Bie, J.; Boirie, Y.; Cruz-Jentoft, A.J.; Eglseer, D.; Topinková, E.; Visser, B.; et al. The Relevance of Diet, Physical Activity, Exercise, and Persuasive Technology in the Prevention and Treatment of Sarcopenic Obesity in Older Adults. Front. Nutr. 2021, 8, 661449. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef]
- Alexandre, T.D.S.; Scholes, S.; Ferreira Santos, J.L.; Duarte, Y.A.O.; de Oliveira, C. The combination of dynapenia and abdominal obesity as a risk factor for worse trajectories of IADL disability among older adults. Clin. Nutr. 2018, 37, 2045–2053. [Google Scholar] [CrossRef]
- Batsis, J.A.; Zbehlik, A.J.; Pidgeon, D.; Bartels, S.J. Dynapenic obesity and the effect on long-term physical function and quality of life: Data from the osteoarthritis initiative. BMC Geriatr. 2015, 15, 118. [Google Scholar] [CrossRef]
- Follis, S.; Cook, A.; Bea, J.W.; Going, S.B.; Laddu, D.; Cauley, J.A.; Shadyab, A.H.; Stefanick, M.L.; Chen, Z. Association between Sarcopenic Obesity and Falls in a Multiethnic Cohort of Postmenopausal Women. J. Am. Geriatr. Soc. 2018, 66, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Lauwers-Cances, V.; Cristini, C.; Abellan van Kan, G.; Janssen, I.; Morley, J.E.; Vellas, B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: The EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am. J. Clin. Nutr. 2009, 89, 1895–1900. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, J.J.; Park, Y.S. Relationship between sarcopenic obesity and cardiovascular disease risk as estimated by the Framingham risk score. J. Korean Med. Sci. 2015, 30, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Mei, F.; Shang, Y.; Hu, K.; Chen, F.; Zhao, L.; Ma, B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 4633–4641. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; Wolfe, R.R. The Link between Dietary Protein Intake, Skeletal Muscle Function and Health in Older Adults. Healthcare 2015, 3, 529–543. [Google Scholar] [CrossRef]
- Coker, R.H.; Miller, S.; Schutzler, S.; Deutz, N.; Wolfe, R.R. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr. J. 2012, 11, 105. [Google Scholar] [CrossRef]
- Deer, R.R.; Volpi, E. Protein intake and muscle function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 248–253. [Google Scholar] [CrossRef]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2021, 119, 428–442. [Google Scholar] [CrossRef]
- Xu, Z.R.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Muscariello, E.; Nasti, G.; Siervo, M.; Di Maro, M.; Lapi, D.; D’Addio, G.; Colantuoni, A. Dietary protein intake in sarcopenic obese older women. Clin. Interv. Aging 2016, 11, 133–140. [Google Scholar] [CrossRef]
- Fulgoni, V.L., 3rd. Current protein intake in America: Analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008, 87, 1554s–1557s. [Google Scholar] [CrossRef]
- SHARE Wave 8 Methodology: Collecting Cross-National Survey Data in Times of COVID-19; Bergmann, M., Börsch-Supan, A., Eds.; MEA, Max Planck Institute for Social Law and Social Policy: Munich, Germany, 2021. [Google Scholar]
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S. Data Resource Profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). Int. J. Epidemiol. 2013, 42, 992–1001. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Yoo, J.I.; Choi, H.; Ha, Y.C. Mean Hand Grip Strength and Cut-off Value for Sarcopenia in Korean Adults Using KNHANES VI. J. Korean Med. Sci. 2017, 32, 868–872. [Google Scholar] [CrossRef]
- Hair, J.H.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M. A Primer on Partial Least Squares Structural Equation Modeling (PLS-SEM), 2nd ed.; SAGE Publications: California, CA, USA, 2016. [Google Scholar]
- Aubertin-Leheudre, M.; Adlercreutz, H. Relationship between animal protein intake and muscle mass index in healthy women. Br. J. Nutr. 2009, 102, 1803–1810. [Google Scholar] [CrossRef]
- Lord, C.; Chaput, J.P.; Aubertin-Leheudre, M.; Labonté, M.; Dionne, I.J. Dietary animal protein intake: Association with muscle mass index in older women. J. Nutr. Health Aging 2007, 11, 383–387. [Google Scholar]
- Campbell, W.W.; Barton, M.L., Jr.; Cyr-Campbell, D.; Davey, S.L.; Beard, J.L.; Parise, G.; Evans, W.J. Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. Am. J. Clin. Nutr. 1999, 70, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; O’Connell, S.L.; Mundell, N.L.; Grimes, C.A.; Dunstan, D.W.; Nowson, C.A. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: A cluster randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Haub, M.D.; Wells, A.M.; Tarnopolsky, M.A.; Campbell, W.W. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am. J. Clin. Nutr. 2002, 76, 511–517. [Google Scholar] [CrossRef]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef]
- Abete, I.; Romaguera, D.; Vieira, A.R.; Lopez de Munain, A.; Norat, T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Rigon, C.; Perna, S.; Gasparri, C.; Iannello, G.; Akber, R.; Alalwan, T.A.; Freije, A.M. Novel Insights on Intake of Fish and Prevention of Sarcopenia: All Reasons for an Adequate Consumption. Nutrients 2020, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, A.R.; Kwon, Y.J. Association between dairy protein and body composition in middle-aged and older women: A community-based, 12-year, prospective cohort study. Clin. Nutr. 2022, 41, 460–467. [Google Scholar] [CrossRef]
- Devries, M.C.; McGlory, C.; Bolster, D.R.; Kamil, A.; Rahn, M.; Harkness, L.; Baker, S.K.; Phillips, S.M. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: A randomized, controlled trial. Am. J. Clin. Nutr. 2018, 107, 217–226. [Google Scholar] [CrossRef]
- Pei, R.; Martin, D.A.; DiMarco, D.M.; Bolling, B.W. Evidence for the effects of yogurt on gut health and obesity. Crit. Rev. Food Sci. Nutr. 2017, 57, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Putra, C.; Konow, N.; Gage, M.; York, C.G.; Mangano, K.M. Protein Source and Muscle Health in Older Adults: A Literature Review. Nutrients 2021, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
| All Participants (n = 5362) | Participants with Obesity Coexisting with Low Handgrip Strength (n = 255) | Participants without Obesity Coexisting with Low Handgrip Strength (n = 5107) | p-Value * | |
|---|---|---|---|---|
| Female, n (%) | 3054 (57.0) | 153 (60.0) | 2901 (56.8) | 0.315 |
| Age (y), median (range) | 62 (50–70) | 65 (52–70) | 62 (50–70) | <0.001 |
| Retired, n (%) | 2.363 (44.1) | 148 (58.0) | 2215 (43.4) | <0.001 |
| BMI, median (range) | 27.5 (16–57) | 33.5 (30–57) | 27.3 (16–54) | <0.001 |
| Total number of chronic diseases, median (range) | 1 (0–10) | 2 (0–10) | 1 (0–10) | <0.001 |
| Most common diseases, n (%) | ||||
| Hypertension | 2114 (39.4) | 150 (58.8) | 1964 (38.5) | <0.001 |
| Hypercholesterolemia | 1085 (20.2) | 64 (25.1) | 1021 (20.0) | 0.048 |
| Osteoarthritis/other rheumatism | 773 (14.4) | 60 (23.5) | 713 (14.0) | <0.001 |
| Diabetes/high blood sugar | 591 (11.0) | 70 (27.5) | 521 (10.2) | <0.001 |
| Rheumatoid arthritis | 351 (6.5) | 24 (9.4) | 327 (6.4) | 0.058 |
| Chronic lung disease | 215 (4.0) | 17 (6.7) | 198 (3.9) | 0.027 |
| Chronic kidney disease | 116 (2.2) | 10 (3.9) | 106 (2.1) | 0.048 |
| More than one limitation in instrumental activities of daily living, n (%) | 422 (7.9) | 58 (22.7) | 364 (7.1) | <0.001 |
| Handgrip strength, mean kg (SD) | 34.1 (11.2) | 21.8 (6.5) | 34.7 (11.0) | <0.001 |
| Moderate activity > 1×/week, n (%) | 3448 (64.3) | 106 (41.6) | 3342 (65.4) | <0.001 |
| Daily intake of protein sources, n (%) | ||||
| Dairy products | 2325 (43.4) | 95 (37.3) | 2230 (43.7) | 0.044 |
| Meat and fish | 1529 (28.5) | 46 (18.0) | 1483 (29.0) | <0.001 |
| Eggs and legumes | 649 (12.1) | 32 (12.5) | 617 (12.1) | 0.823 |
| Univariate Logistic Regression | Multivariate Logistic Regression | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age | 1.10 (1.07–1.1) | 0.000 | 1.07 (1.03–1.11) | 0.000 |
| Female | 1.14 (0.88–1.47) | 0.315 | ||
| Being retired | 1.80 (1.40–2.33) | 0.000 | 0.98 (0.70–1.36) | 0.878 |
| Having 2+ chronic diseases | 2.64 (2.03–3.43) | 0.000 | 2.22 (1.69–2.93) | 0.000 |
| Having 1+ IADL limitations | 3.83 (2.81–5.23) | 0.000 | 2.23 (1.60–3.11) | 0.000 |
| Moderate activity > 1×/wk | 0.38 (0.29–0.49) | 0.000 | 0.44 (0.33–0.57) | 0.000 |
| Daily intake of meat or fish | 0.54 (0.39–0.74) | 0.000 | 0.56 (0.40–0.79) | 0.001 |
| Daily intake of dairy products | 0.77 (0.59–0.99) | 0.044 | 0.79 (0.60–1.03) | 0.085 |
| Daily intake of eggs or legumes | 1.04 (0.71–1.53) | 0.823 | 1.50 (1.00–2.25) | 0.052 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eglseer, D.; Traxler, M.; Bauer, S. Association between the Intake of Different Protein Sources and Obesity Coexisting with Low Handgrip Strength in Persons near Retirement Age. Nutrients 2022, 14, 4684. https://doi.org/10.3390/nu14214684
Eglseer D, Traxler M, Bauer S. Association between the Intake of Different Protein Sources and Obesity Coexisting with Low Handgrip Strength in Persons near Retirement Age. Nutrients. 2022; 14(21):4684. https://doi.org/10.3390/nu14214684
Chicago/Turabian StyleEglseer, Doris, Mariella Traxler, and Silvia Bauer. 2022. "Association between the Intake of Different Protein Sources and Obesity Coexisting with Low Handgrip Strength in Persons near Retirement Age" Nutrients 14, no. 21: 4684. https://doi.org/10.3390/nu14214684
APA StyleEglseer, D., Traxler, M., & Bauer, S. (2022). Association between the Intake of Different Protein Sources and Obesity Coexisting with Low Handgrip Strength in Persons near Retirement Age. Nutrients, 14(21), 4684. https://doi.org/10.3390/nu14214684








