Anti-Inflammatory Protein Isolated from Tamarind Promotes Better Histological Aspects in the Intestine Regardless of the Improvement of Intestinal Permeability in a Preclinical Study of Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
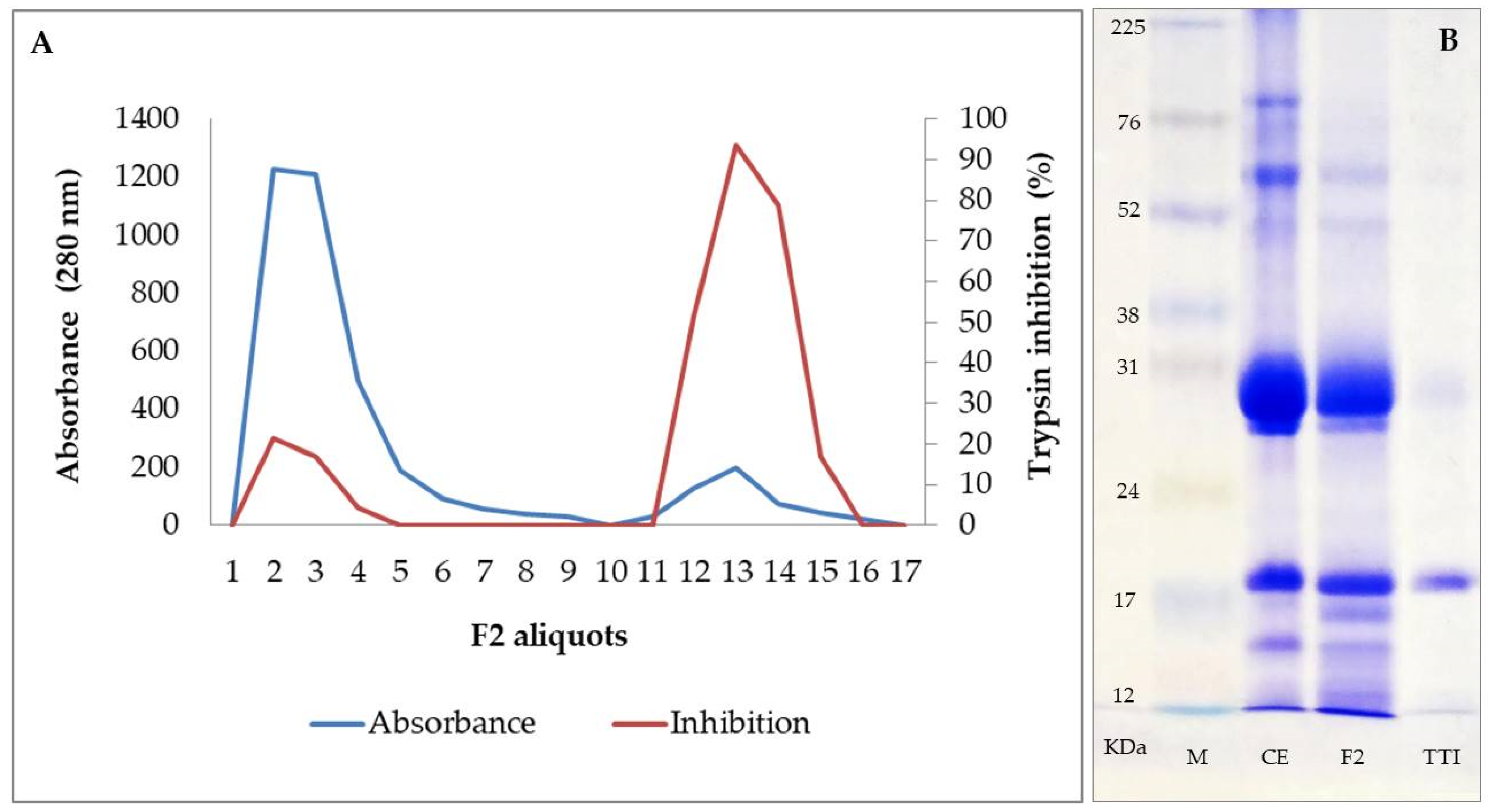
2.1. Obtaining the Trypsin Inhibitor Isolated from Tamarind Seeds (TTI)
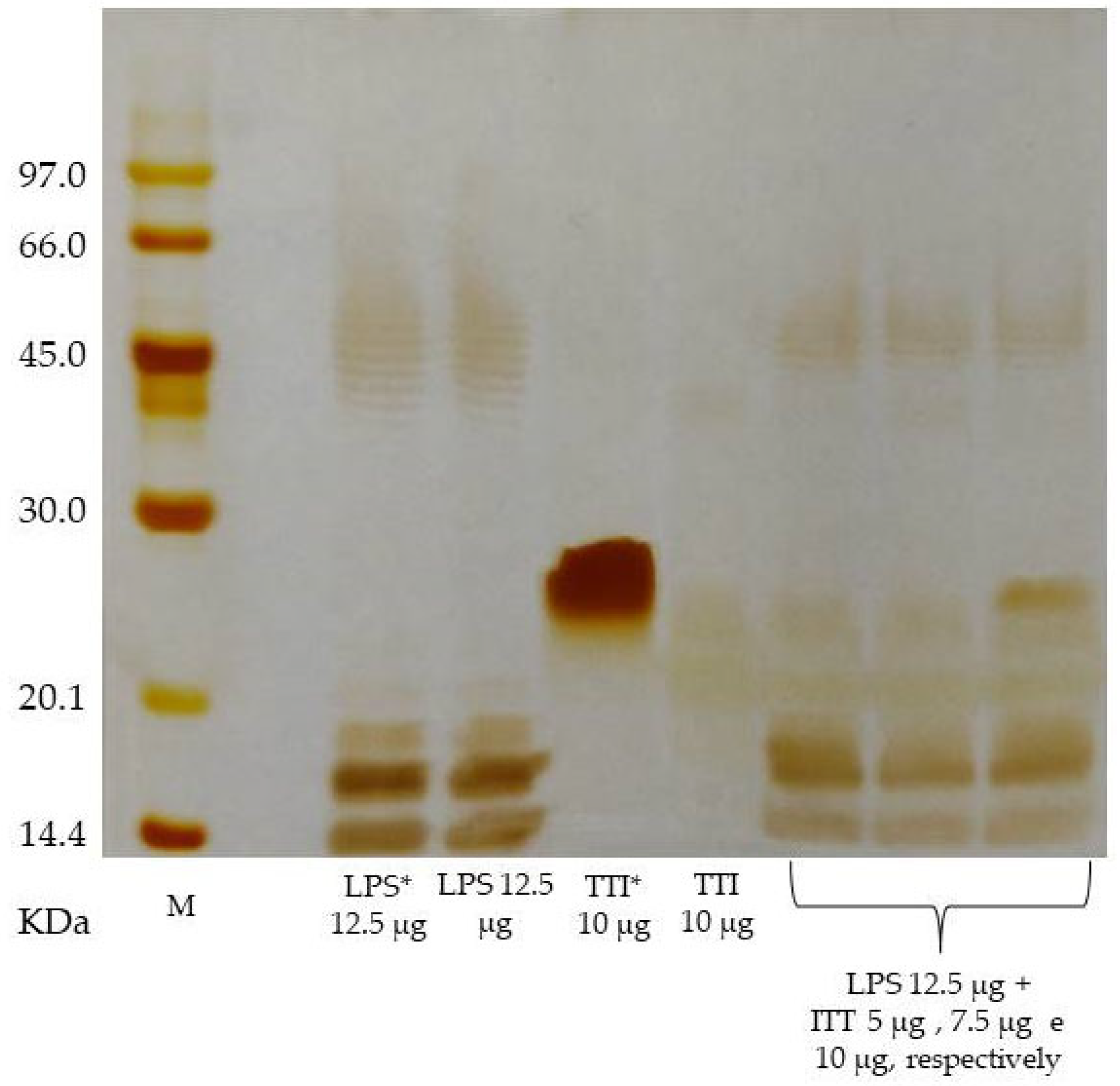
2.2. In Vitro Interaction of TTI with Bacterial LPS
2.3. Inhibitory Activity against Human Neutrophil Elastase (HNE)
2.4. In Vitro Cellular Assays
2.4.1. Cell Cultures
2.4.2. Caco-2
2.4.3. HT29-MTX
2.4.4. Semi-Confluent Cultures of Caco-2 Cells
2.4.5. Differentiated Monolayers of Caco-2:HT29-MTX Cells
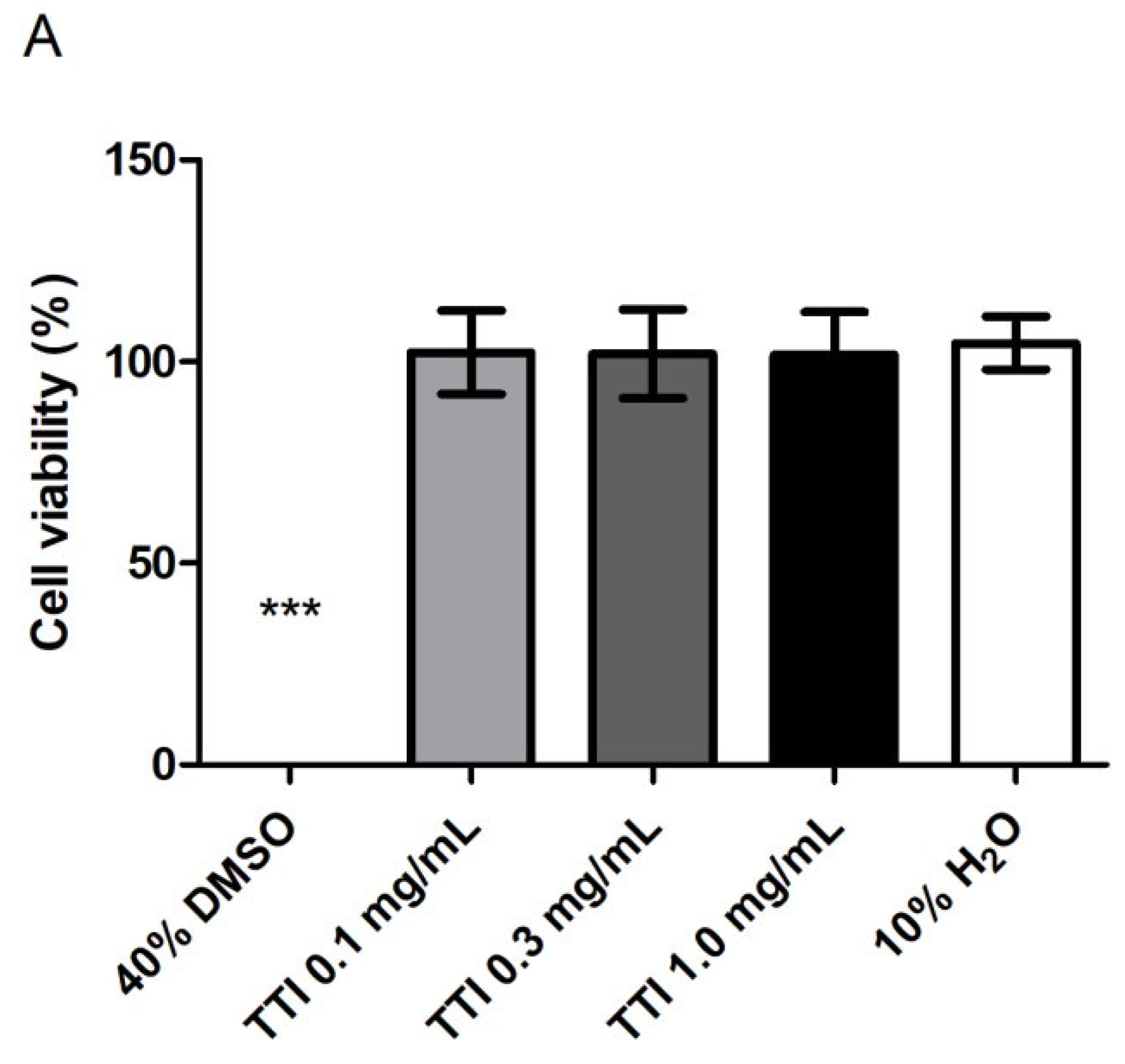
2.4.6. Cell Metabolic Activity
2.4.7. Intracellular Production of Reactive Oxygen Species (ROS)
2.4.8. Inflammation Induction
IL-8 Quantification
2.4.9. Effect of TTI on Barrier Integrity and Paracellular Permeability in Inflamed Co-Cultures
Transepithelial Electrical Resistance (TEER)
Lucifer Yellow (LY) Permeability
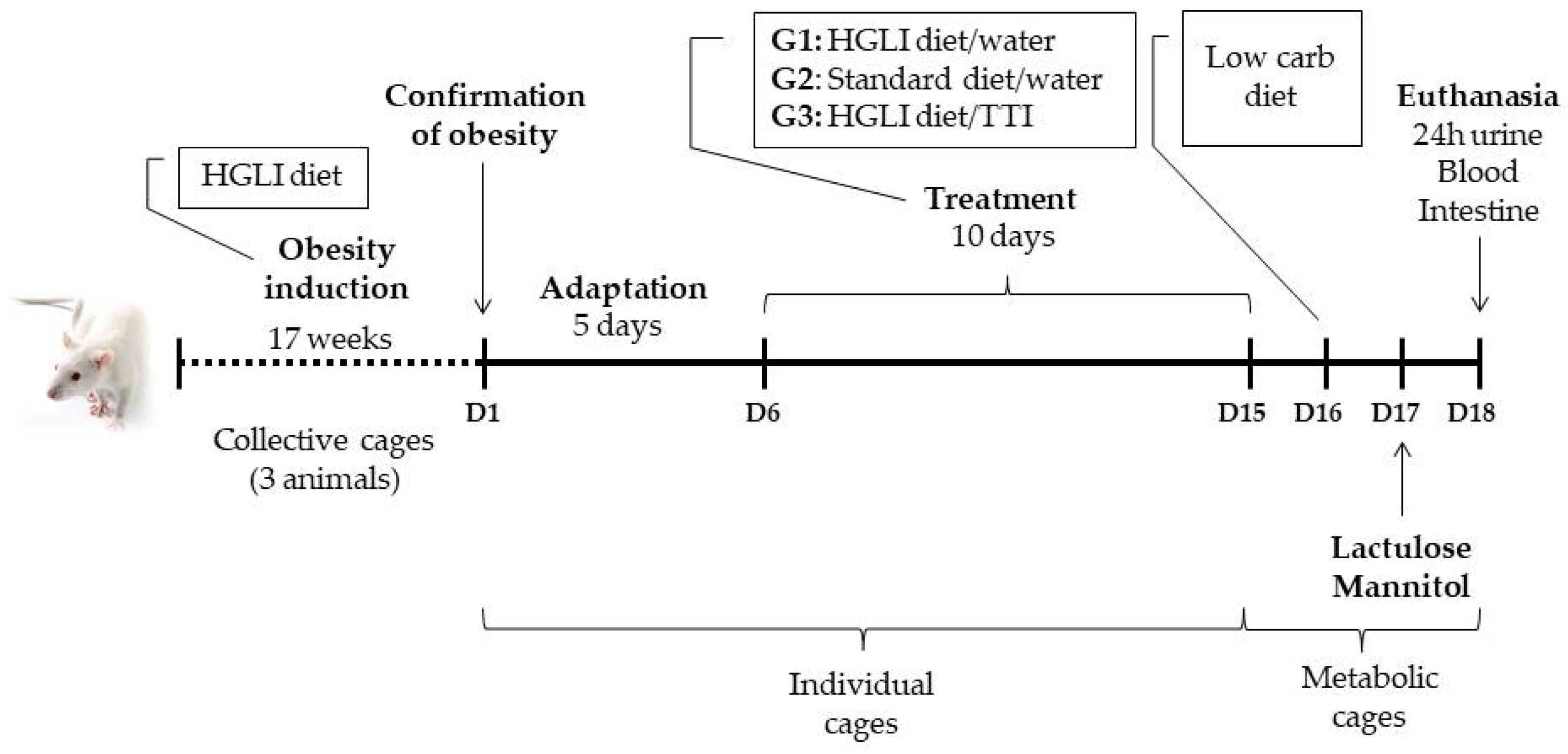
2.5. Preclinical Study
2.5.1. Animals and Study Ethics
2.5.2. Diets
- (A)
- Standard diet Labina®, considered nutritionally adequate for age, commercially obtained (Presence®, Paulínia, São Paulo, Brazil);
- (B)
- High glycemic index and high glycemic load (HGLI) diet, described by Luz et al. [40] as inducing obesity and epithelial damage in the intestinal barrier;
- (C)
- Low carbohydrate diet RH195172, commercially obtained (Rhoster®, São Paulo, Brazil).
2.5.3. Experimental Design
- (1)
- Group with HGLI diet and no treatment (n = 5), composed of animals with obesity induced by HGLI diet that, during the experimental period, continued to receive this diet and were not treated, receiving only 1 mL of water by gavage;
- (2)
- Group treated with a nutritionally adequate diet (n = 5), composed of animals with obesity induced by the HGLI diet that, during the experiment, received the standard Labina® diet + 1 mL of water by gavage;
- (3)
2.5.4. Evaluation of Hematological, Biochemical and Inflammatory Parameters
2.5.5. TNF-α and IL-6 in the Small Intestine
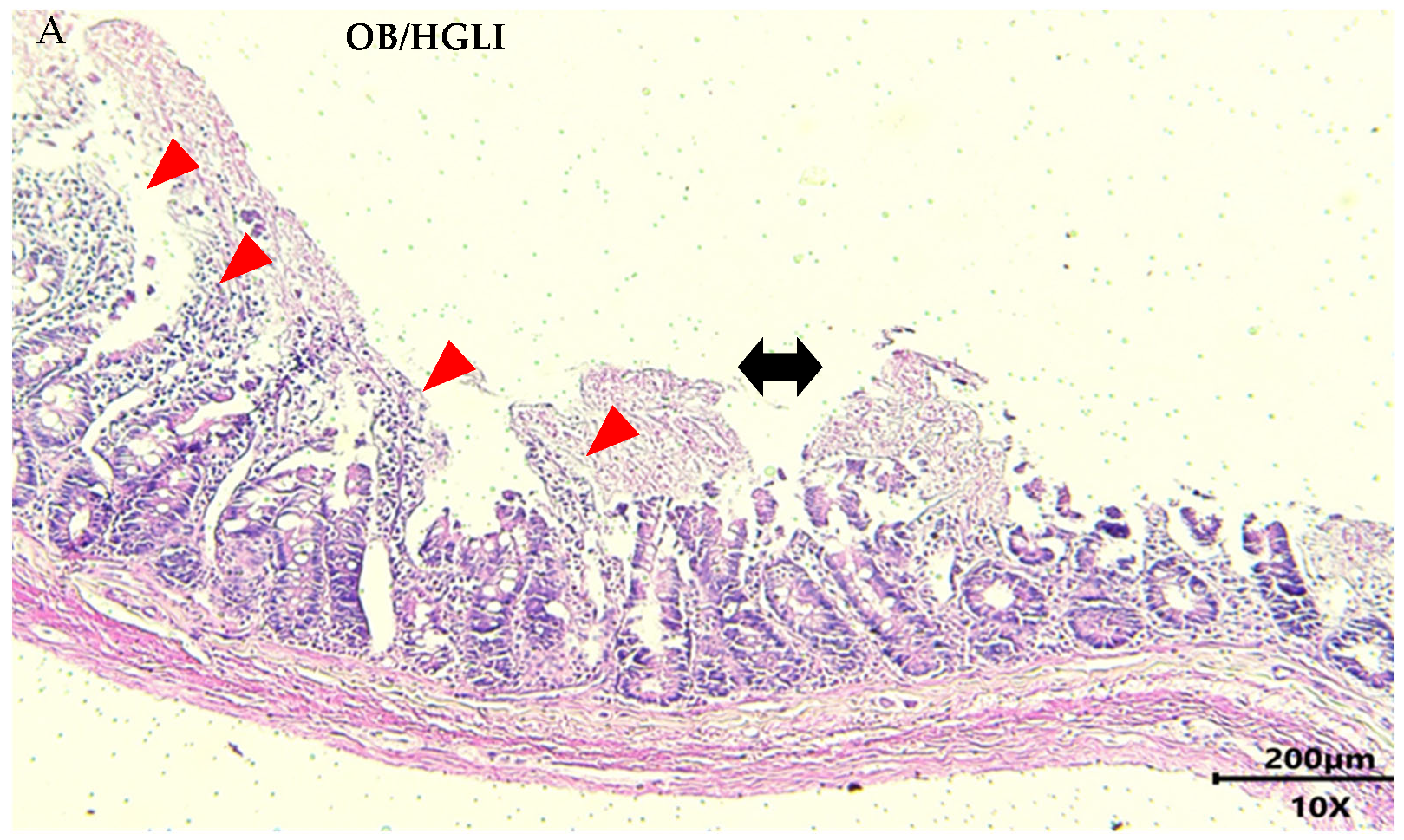
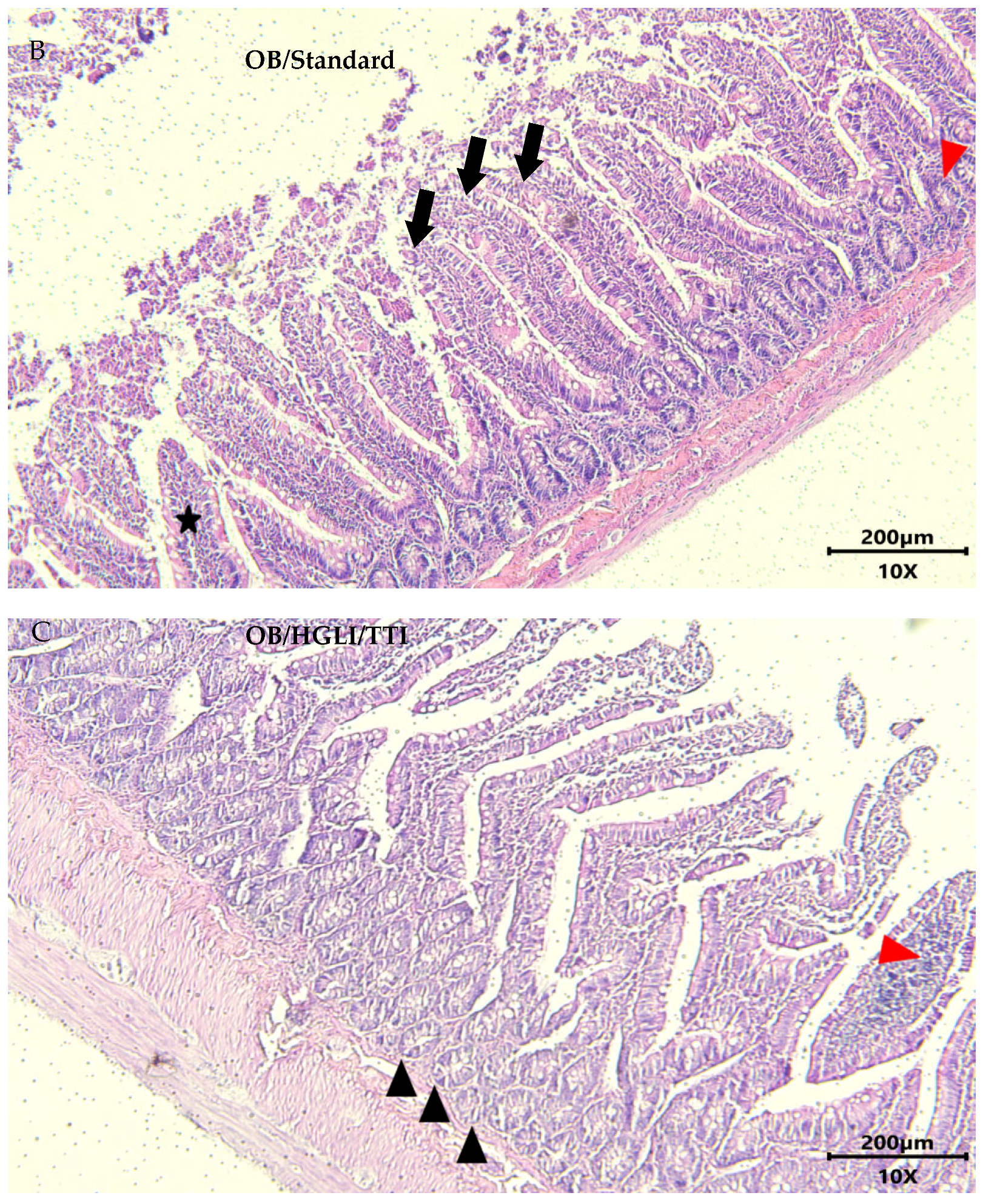
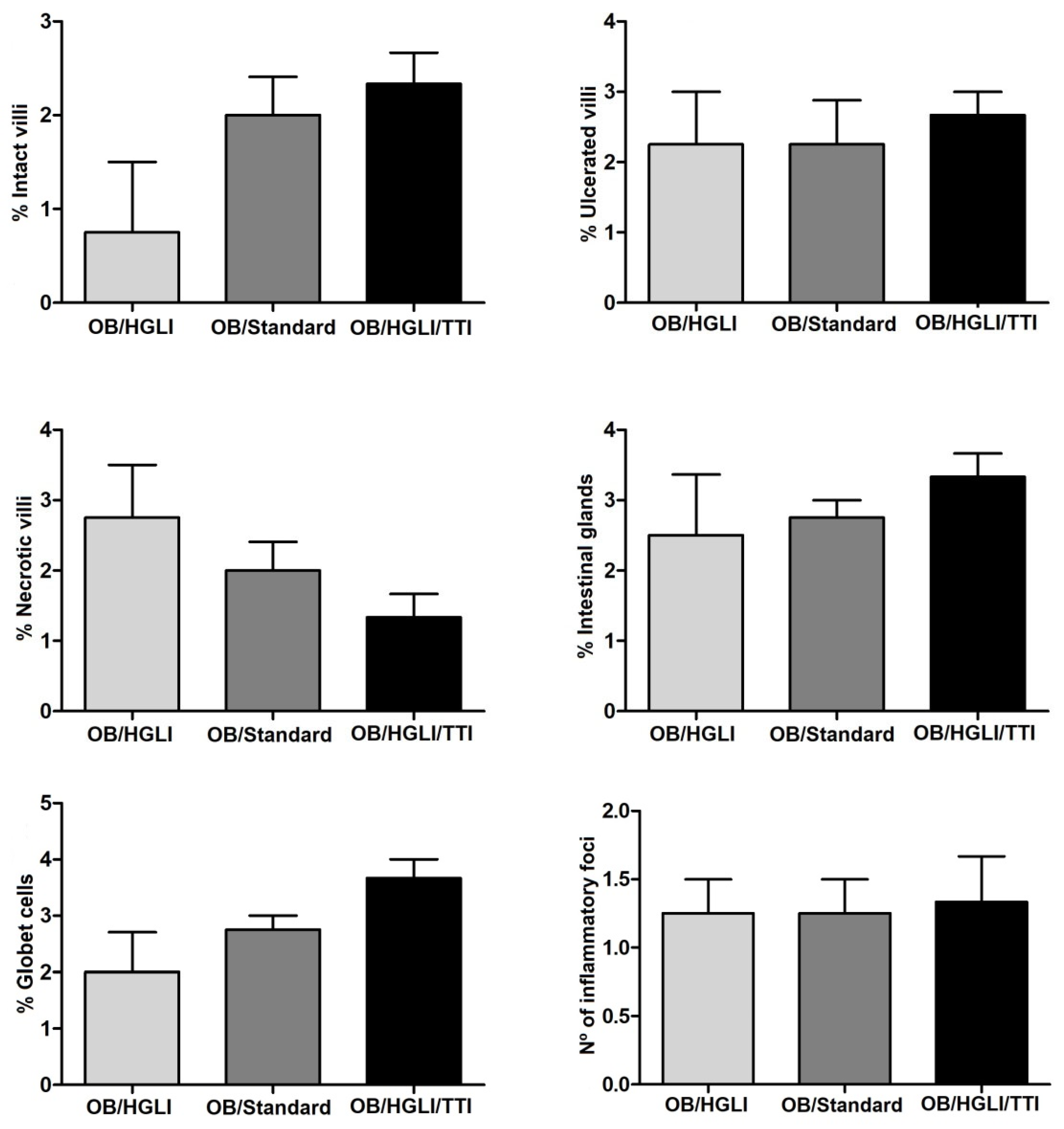
2.5.6. Histopathology and Histomorphometry of the Small Intestine
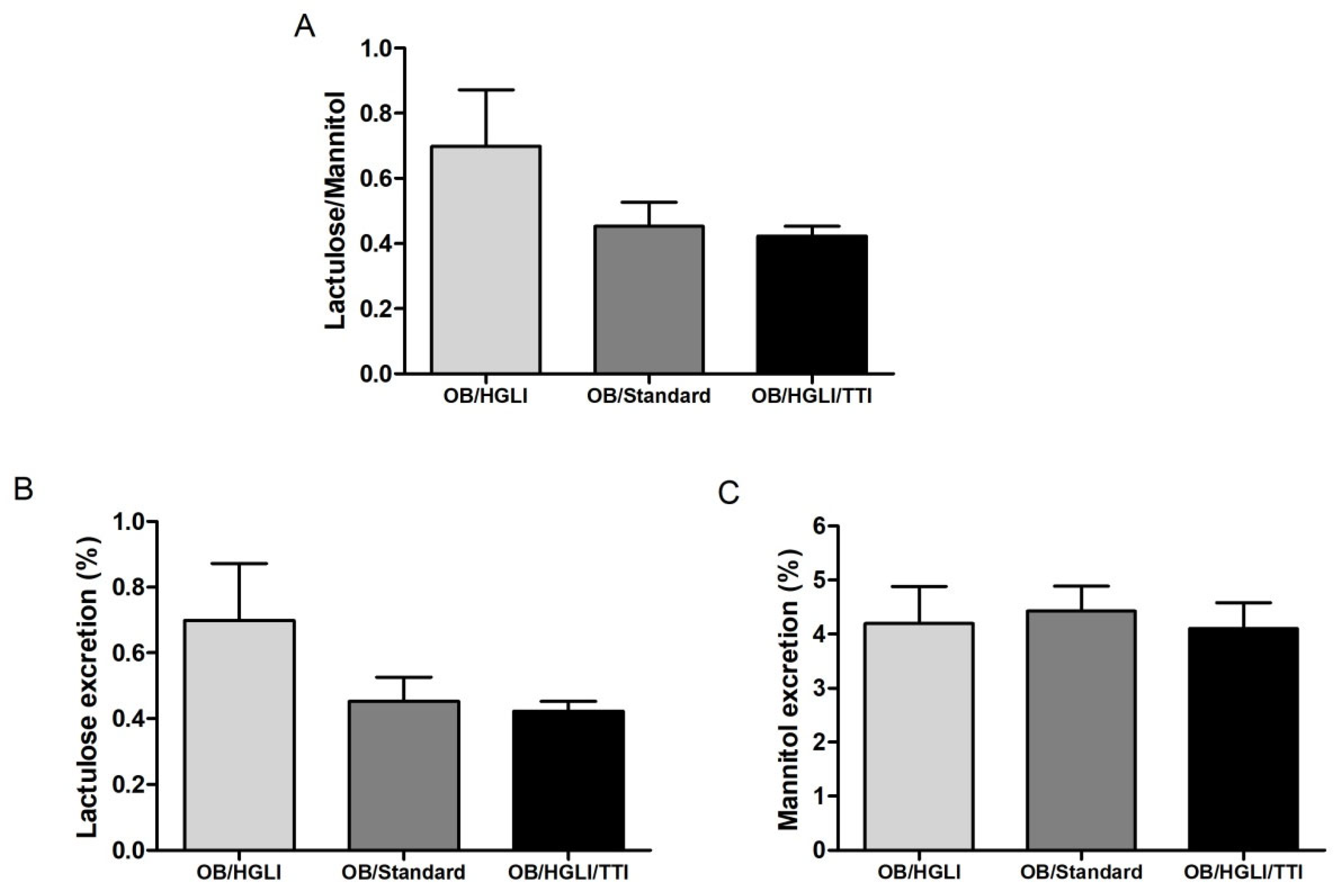
2.5.7. In Vivo Intestinal Permeability Test
2.6. Statistical Analysis
3. Results
3.1. TTI Isolation, Interaction with LPS, and Inhibitory Activity against HNE
3.2. In Vitro Studies
3.2.1. Cell Metabolic Activity
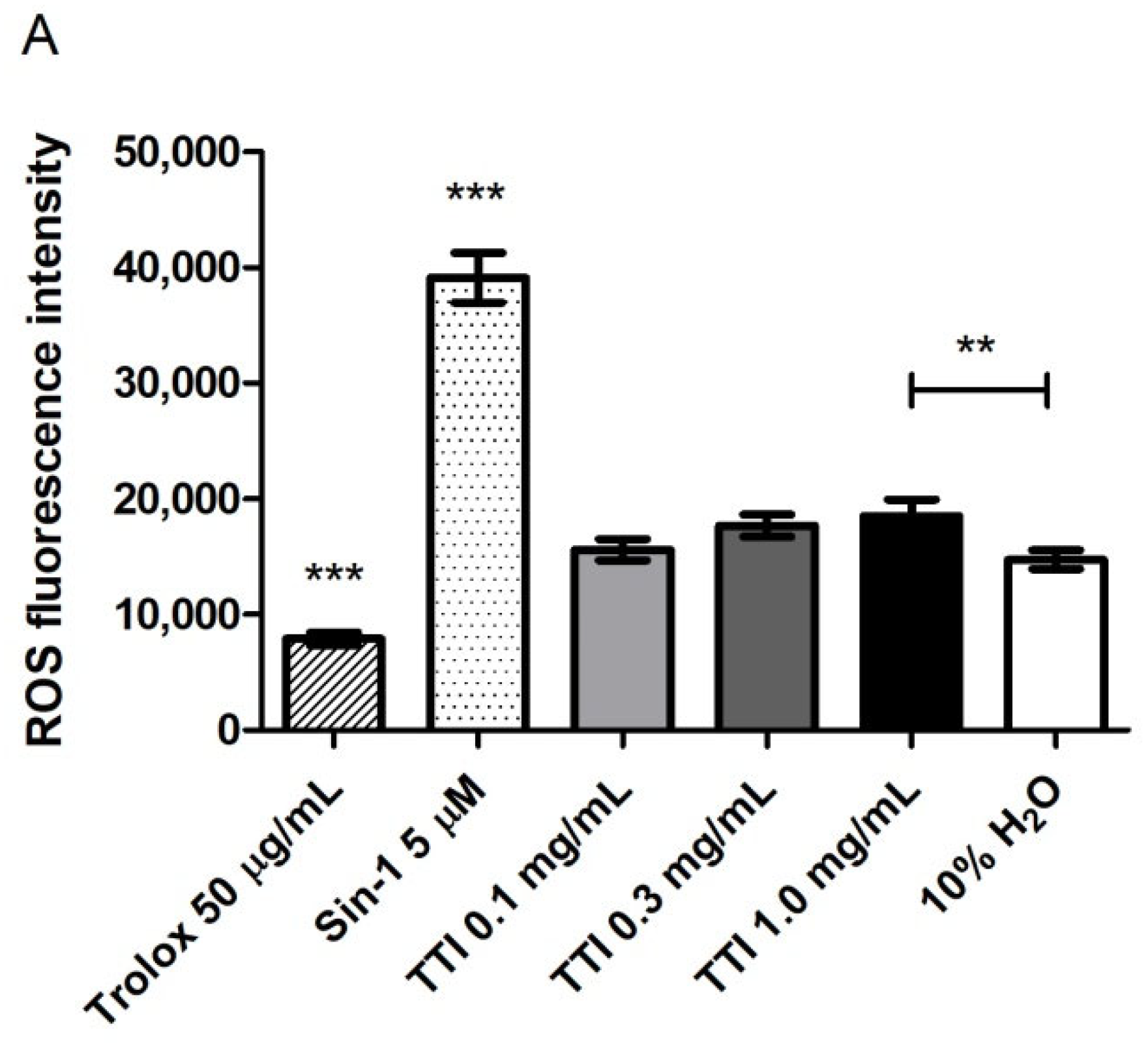
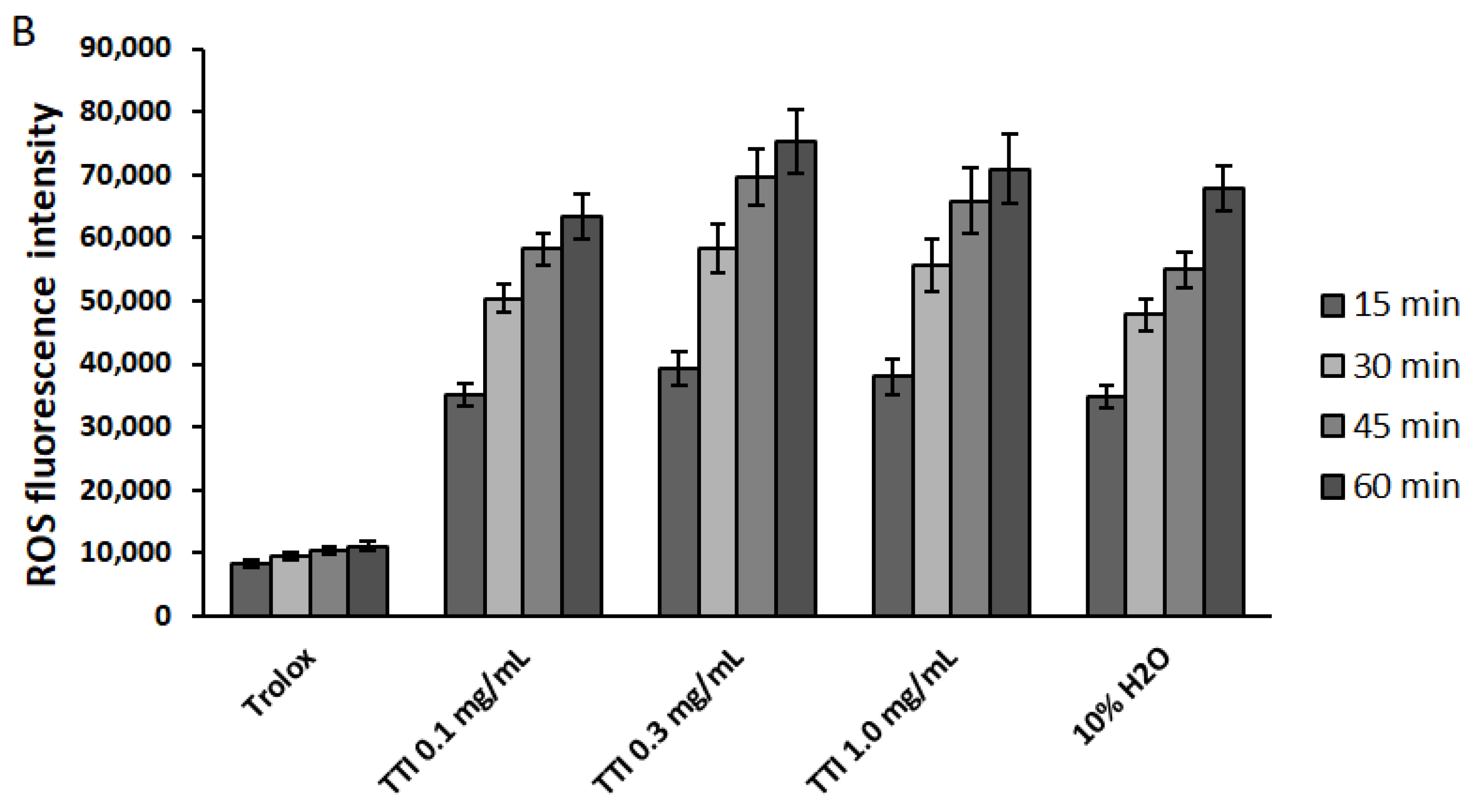
3.2.2. Intracellular Production of Reactive Oxygen Species (ROS)
3.2.3. Inflammation Induction in Cell Co-Cultures
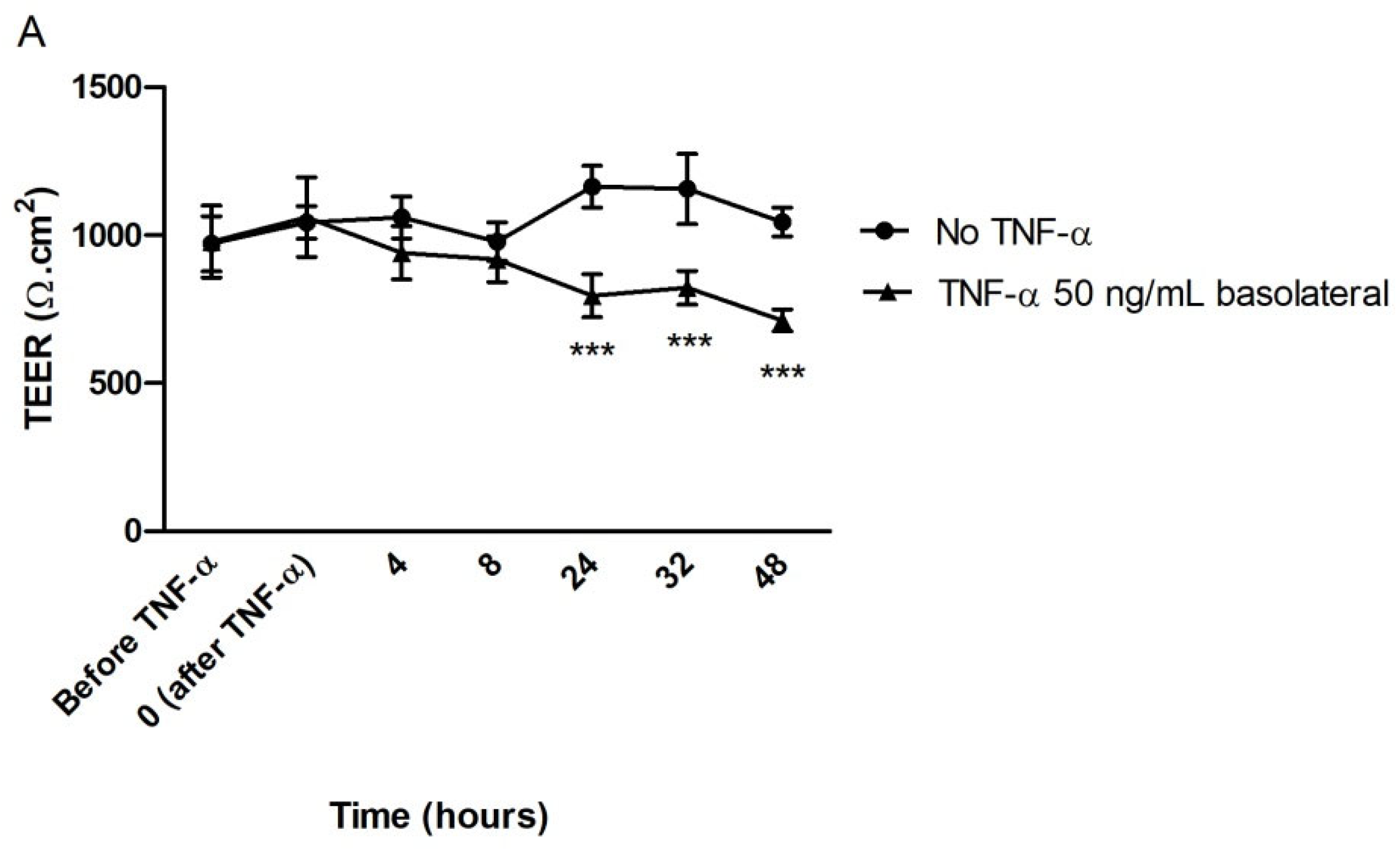
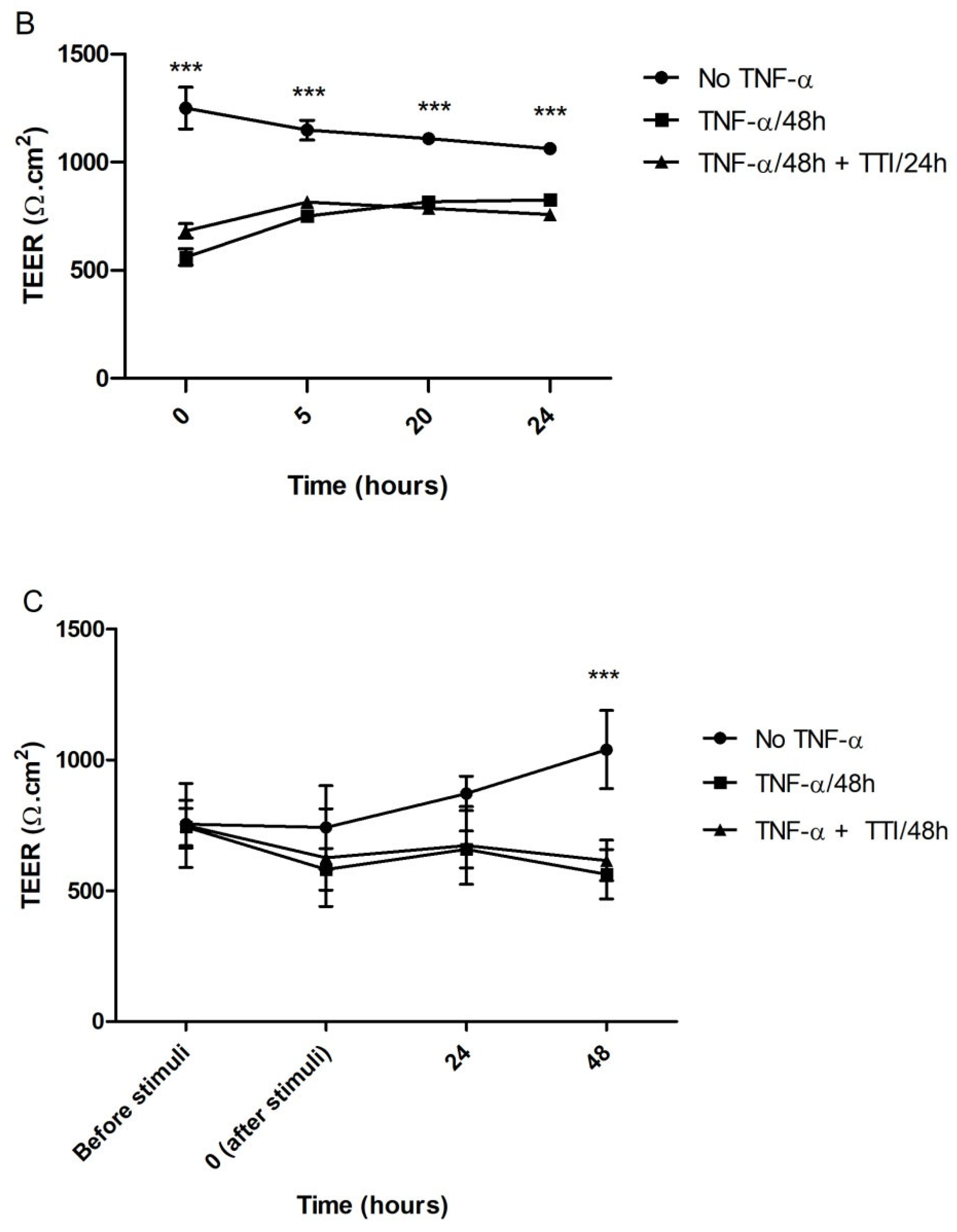
3.2.4. Effect of TTI on Transepithelial Electrical Resistance (TEER) in Inflamed Co-Cultures
3.2.5. Effect of TTI on Permeability in Inflamed Co-Cultures
3.3. Preclinical Study
3.3.1. Hematological and Biochemical Parameters
3.3.2. Plasma TNF-α and IL-6
3.3.3. TNF-α and IL-6 in the Small Intestine
3.3.4. Histopathology and Histomorphometry
3.3.5. Intestinal Permeability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Obesity Federation. World Obesity Atlas 2022; World Obesity Federation: London, UK, 2022; p. 288. [Google Scholar]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 6 July 2021).
- Thomas, D.; Apovian, C.M. Macrophage functions in lean and obese adipose tissue. Metabolism 2017, 72, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Mankertz, J.; Amasheh, M.; Krug, S.M.; Fromm, A.; Amasheh, S.; Hillenbrand, B.; Tavalali, S.; Fromm, M.; Schulzke, J.D. TNF-alpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009, 336, 67–77. [Google Scholar] [CrossRef]
- Watson, A.J.M.; Hughes, K.R. TNF-α–induced intestinal epithelial cell shedding: Implications for intestinal barrier function. Ann. N. Y. Acad. Sci. 2012, 1258, 1–8. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-α Modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 2013, 183, 1871–1884. [Google Scholar] [CrossRef]
- Cao, M.; Wang, P.; Sun, C.; He, W.; Wang, F. Amelioration of IFN-γ and TNF-α-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PLoS ONE 2013, 8, e61944. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- León-Rodríguez, M.C.C.P.; Guyot, J.-P.; Laurent-Babot, C. Intestinal in vitro cell culture models and their potential to study the effect of food components on intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2018, 59, 3648–3666. [Google Scholar] [CrossRef] [PubMed]
- Michielan, A.; D’Incà, R. Intestinal permeability in inflammatory bowel disease: Pathogenesis, clinical evaluation, and therapy of leaky gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.; Aguado, J.M. Risk of infection associated with anti-TNF-α therapy. Expert Rev. Anti Infect. Ther. 2018, 16, 939–956. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.S.R.; de Lima, V.C.O.; Piuvezam, G.; de Azevedo, K.P.M.; Maciel, B.L.L.; Morais, A.H.A. Mechanisms of action of anti-inflammatory proteins and peptides with anti-TNF-alpha activity and their effects on the intestinal barrier: A systematic review. PLoS ONE 2022, 17, e0270749. [Google Scholar] [CrossRef]
- Motta, J.P.; Rolland, C.; Edir, A.; Florence, A.C.; Sagnat, D.; Bonnart, C.; Rousset, P.; Guiraud, L.; Quaranta-Nicaise, M.; Mas, E.; et al. Epithelial production of elastase is increased in inflammatory bowel disease and causes mucosal inflammation. Mucosal Immunol. 2021, 14, 667–678. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.Y.; Kim, J.H.; Kang, J.E.; Park, M.H.; Kim, E.J.; et al. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef]
- Li, Y.; Smith, C.; Wu, H.; Teng, P.; Shi, Y.; Padhee, S.; Jones, T.; Nguyen, A.M.; Cao, C.; Yin, H.; et al. Short antimicrobial lipo-α/γ-AA hybrid peptides. ChemBioChem 2014, 15, 2275–2280. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Xu, D.; Lu, W. Defensins: A double-edged sword in host immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, L.; Zhang, R.; Koci, M.; Si, D.; Ahmad, B.; Cheng, J.; Wang, J.; Aihemaiti, M.; Zhang, M. A novel Cecropin-LL37 hybrid peptide protects mice against EHEC infection-mediated changes in gut microbiota, intestinal inflammation, and impairment of mucosal barrier functions. Front. Immunol. 2020, 11, 1361. [Google Scholar] [CrossRef]
- Lin, Q.; Su, G.; Wu, A.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Mao, X.; Zheng, P.; Yu, J.; et al. Bombyx mori gloverin A2 alleviates enterotoxigenic Escherichia coli-induced inflammation and intestinal mucosa disruption. Antimicrob. Resist. Infect. Control 2019, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Xie, H.; Su, G.; Chen, D.; Yu, B.; Mao, X.; Huang, Z.; Yu, J.; Luo, J.; Zheng, P.; et al. β-defensin 129 attenuates bacterial endotoxin-induced inflammation and intestinal epithelial cell apoptosis. Front. Immunol. 2019, 10, 2333. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.N.; Serquiz, A.C.; Silva, P.F.S.; Barbosa, P.B.B.M.; Sampaio, T.B.M.; Araújo Junior, R.F.; Oliveira, A.S.; Machado, R.J.A.; Maciel, B.L.L.; Uchôa, A.F.; et al. Trypsin inhibitor from tamarindus indica L. seeds reduces weight gain and food consumption and increases plasmatic cholecystokinin levels. Clinics 2015, 70, 136–143. [Google Scholar] [CrossRef]
- Carvalho, F.M.C.; Lima, V.C.O.; Costa, I.S.; Medeiros, A.F.; Serquiz, A.C.; Lima, M.C.J.S.; Serquiz, R.P.; Maciel, B.L.L.; Uchôa, A.F.; Santos, E.A.; et al. A trypsin inhibitor from tamarind reduces food intake and improves inflammatory status in rats with metabolic syndrome regardless of weight loss. Nutrients 2016, 8, 544. [Google Scholar] [CrossRef]
- Costa, I.S.; Carvalho, F.M.C.; Lima, V.C.O.; Medeiros, A.F.; Serqui, A.C.; Pinheiro, L.G.S.D.; Maciel, B.L.L.; Santos, E.A.; Morais, A.H.A. Proteínas bioativas das sementes de tamarindo reduzem leptina plasmática independente da perda de peso em ratos com obesidade. Saúde 2018, 44, 1–8. [Google Scholar] [CrossRef]
- Fook, J.M.; Macedo, L.L.; Moura, G.E.; Teixeira, F.M.; Oliveira, A.S.; Queiroz, A.F.; Sales, M.P. A serine proteinase inhibitor isolated from Tamarindus indica seeds and its effects on the release of human neutrophil elastase. Life Sci. 2005, 76, 2881–2891. [Google Scholar] [CrossRef]
- Lima, V.C.O.; Luz, A.B.S.; Amarante, M.D.S.M.; Lima, M.C.J.S.; Carvalho, F.M.C.; Figueredo, J.B.S.; Santos, P.P.A.; Camillo, C.S.; Ladd, F.V.L.; Maciel, B.L.L.; et al. Tamarind multifunctional protein: Safety and anti-inflammatory potential in intestinal mucosa and adipose tissue in a preclinical model of diet- induced obesity. Obes. Facts 2021, 14, 357–369. [Google Scholar] [CrossRef]
- Kakade, M.L.; Simons, N.; Liener, I.E. An evaluation of natural vs. synthetic substrates for measuring the antitryptic activity of soybean samples. Cereal Chem. 1969, 46, 518–526. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.R.; Kirsch, D.R.; Morris, N.R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal. Biochem. 1980, 105, 361–363. [Google Scholar] [CrossRef]
- Johansson, S.; Göransson, U.; Luijendijk, T.; Backlund, A.; Claeson, P.; Bohlin, L. A neutrophil multitarget functional bioassay to detect anti-inflammatory natural products. J. Nat. Prod. 2002, 65, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.S. Avaliação da Genotoxicidade do Inibidor de Tripsina Isolado de Semente de Tamarindo (Tamarindus indica L.) e do Potencial Antibacteriano In Vitro e In Silico de Seus Peptídeos Derivados. Master’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brasil, 2022. [Google Scholar]
- Kämpfer, A.A.M.; Urbán, P.; Gioria, S.; Kanase, N.; Stone, V.; Kinsner-Ovaskainen, A. Development of an in vitro co-culture model to mimic the human intestine in healthy and diseased state. Toxicol. Vitr. 2017, 45, 31–43. [Google Scholar] [CrossRef]
- Novelli, E.L.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.; Cicogna, A.C.; Novelli Filho, J.L. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Luz, A.B.S.; Figueredo, J.B.S.; Salviano, B.D.P.D.; Aguiar, A.J.F.C.; Pinheiro, L.G.S.D.; Krause, M.F.D.; Camillo, C.S.; Ladd, F.V.L.; Bortolin, R.H.; Silbiger, V.N.; et al. Adipocytes and intestinal epithelium dysfunctions linking obesity to inflammation induced by high glycemic index pellet-diet in Wistar rats. Biosci. Rep. 2018, 38, BSR20180304. [Google Scholar] [CrossRef]
- Costa, I.; Lima, M.; Medeiros, A.; Bezerra, L.; Santos, P.; Serquiz, A.; Lima, M.; Oliveira, G.; Santos, E.; Maciel, B.; et al. An Insulin Receptor-Binding Multifunctional Protein from Tamarindus indica L. Presents a Hypoglycemic Effect in a Diet-Induced Type 2 Diabetes—Preclinical Study. Foods 2022, 11, 2207. [Google Scholar] [CrossRef]
- Matias, L.L.R.; Costa, R.O.A.; Passos, T.S.; Queiroz, J.L.C.; Serquiz, A.C.; Maciel, B.L.L.; Santos, P.P.A.; Camillo, C.S.; Gonçalves, C.; Amado, I.R.; et al. Tamarind trypsin inhibitor in chitosan–whey protein nanoparticles reduces fasting blood glucose levels without compromising insulinemia: A preclinical study. Nutrients 2019, 11, 2770. [Google Scholar] [CrossRef]
- Costa, R.O.A.; Matias, L.L.R.; Passos, T.S.; Queiroz, J.L.C.; Carvalho, F.M.C.; Maciel, B.L.L.; Uchôa, A.F.; Amado, I.R.; Gonçalves, C.; Pastrana, L.; et al. Safety and potential functionality of nanoparticles loaded with a trypsin inhibitor isolated from tamarind seeds. Future Foods 2020, 1–2, 100001. [Google Scholar] [CrossRef]
- Silva, P.F.; Mcgurk, C.; Knudsen, D.L.; Adams, A.; Thompson, K.D.; Bron, J.E. Histological evaluation of soya bean-induced enteritis in Atlantic salmon (Salmo salar L.): Quantitative image analysis vs. semi-quantitative visual scoring. Aquaculture 2015, 445, 42–56. [Google Scholar] [CrossRef]
- Lee, G.O.; Kosek, P.; Lima, A.A.; Singh, R.; Yori, P.P.; Olortegui, M.P.; Lamsam, J.L.; Oliveira, D.B.; Guerrant, R.L.; Kosek, M. Lactulose: Mannitol diagnostic test by hplc and lc-msms platforms: Considerations for field studies of intestinal barrier function and environmental enteropathy. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.A.P.; Medeiros, P.H.Q.S.; Prata, M.M.G.; Lima, A.A.M. Fisiologia da barreira epitelial intestinal. In Sistema Digestório: Integração Básico-Clínica; Oriá, R.B., Brito, G.A.C., Eds.; Blucher: São Paulo, Brazil, 2016; pp. 441–477. [Google Scholar]
- Cochran, W.G. Sampling Techniques, 2nd ed.; John Wiley and Sons Inc.: New York, USA, 1963; p. 413. [Google Scholar]
- Barrea, L.; Somma, C.D.; Muscogiuri, G.; Tarantino, G.; Tenore, G.C.; Orio, F.; Colao, A.; Savastano, S. Nutrition, inflammation and liver-spleen axis. Crit. Rev. Food Sci. Nutr. 2019, 58, 3141–3158. [Google Scholar] [CrossRef]
- Lima, V.C.O.; Piuvezam, G.; Maciel, B.L.L.; Morais, A.H.A. Trypsin inhibitors: Promising candidate satietogenic proteins as complementary treatment for obesity and metabolic disorders? J. Enzyme Inhib. Med. Chem. 2019, 34, 405–419. [Google Scholar] [CrossRef]
- Carvalho, F.M.C.D.; Maciel, B.L.L.; Morais, A.H.D.A. Tamarind Enzymatic Inhibitors: Activities and Health Application Perspectives. Food Rev. Int. 2020, 1–14. [Google Scholar] [CrossRef]
- Krotova, K.; Khodayari, N.; Oshins, R.; Aslanidi, G.; Brantly, M.L. Neutrophil elastase promotes macrophage cell adhesion and cytokine production through the integrin-Src kinases pathway. Sci. Rep. 2020, 10, 15874. [Google Scholar] [CrossRef]
- Han, J.M.; Levings, M.K. Immune regulation in obesity-associated adipose inflammation. J. Immunol. 2013, 191, 527–532. [Google Scholar] [CrossRef]
- Rapa, S.F.; Waltenberger, B.; Di Paola, R.; Adesso, S.; Siracusa, R.; Peritore, A.F.; D’Amico, R.; Autore, G.; Cuzzocrea, S.; Stuppner, H.; et al. Plumericin prevents intestinal inflammation and oxidative stress in vitro and in vivo. FASEB J. 2020, 34, 1576–1590. [Google Scholar] [CrossRef]
- Arifa, R.D.N.; de Paula, T.P.; Lima, R.L.; Brito, C.B.; Andrade, M.E.R.; Cardoso, V.N.; Pinheiro, M.V.B.; Ladeira, L.O.; Krambrock, K.; Teixeira, M.M.; et al. Anti-inflammatory and antioxidant effects of the nanocomposite Fullerol decrease the severity of intestinal inflammation induced by gut ischemia and reperfusion. Eur. J. Pharmacol. 2021, 5, 173984. [Google Scholar] [CrossRef]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef]
- Weitgasser, R.; Ratzinger, M.; Hemetsberger, M.; Siostrzonek, P. LDL-cholesterol and cardiovascular events: The lower the better? Wien. Med. Wochenschr. 2018, 168, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Pintó, X. Colesterol LDL, cuanto más bajo mejor. Clin Investig. Arterioscler. 2019, 31, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Ibarrola-Jurado, N.; Bulló, M.; Martínez-González, M.A.; Wärnberg, J.; Salaverría, I.; Ortega-Calvo, M.; Estruch, R.; Serra-Majem, L.; Covas, M.I.; et al. White blood cell counts as risk markers of developing metabolic syndrome and its components in the PREDIMED study. PLoS ONE 2013, 8, e58354. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Hu, K.; Huang, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; et al. White blood cells count as an indicator to identify whether obesity leads to increased risk of type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 141, 140–147. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Morenga, L.T. Sugar and type 2 diabetes. Br. Med. Bull. 2016, 120, 43–53. [Google Scholar] [CrossRef]
- Aguiar, A.J.F.C.; de Queiroz, J.L.C.; Santos, P.P.A.; Camillo, C.S.; Serquiz, A.C.; Costa, I.S.; Oliveira, G.G.; Gomes, A.F.T.; Matias, L.L.R.; Costa, R.O.A.; et al. Benefical effects of tamarindo trypsin inhibitor in chitosan-whey protein nanoparticles on hepatic injury induced high glicemic index diet: A preclinical study. Int. J. Mol. Sci. 2019, 22, 9968. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R. Dietary sugars and endogenous formation of advanced glycation endproducts: Emerging mechanisms of disease. Nutrients 2017, 9, 385. [Google Scholar] [CrossRef]
- Carvalho, F.M.C.; Lima, V.C.O.; Costa, I.S.; Luz, A.B.S.; Ladd, F.V.L.; Serquiz, A.C.; Bortolin, R.H.; Silbiger, V.N.; Maciel, B.L.L.; Santos, E.A.; et al. Anti-TNF-α agent tamarind kunitz trypsin inhibitor improves lipid profile of wistar rats presenting dyslipidemia and diet-induced obesity regardless of PPAR-γ induction. Nutrients 2019, 11, 512. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Prykhodko, O.; Hållenius, F.F.; Nyman, M. Monobutyrin Reduces Liver Cholesterol and Improves Intestinal Barrier Function in Rats Fed High-Fat Diets. Nutrients 2019, 11, 308. [Google Scholar] [CrossRef]
- Bona, M.D.; Torres, C.H.M.; Lima, S.C.V.C.; Morais, A.H.A.; Lima, A.Â.M.; Maciel, B.L.L. Intestinal Barrier Permeability in Obese Individuals with or without Metabolic Syndrome: A Systematic Review. Nutrients 2022, 14, 3649. [Google Scholar] [CrossRef]





| Parameter (Unit) | Reference Value * [42,43] |
|---|---|
| Hemoglobin (g/dL) | 23.90 (15.70) |
| Hematocrit (%) | 39.80 (4.82) |
| Total leukocyte count (×103/µL) | 6.44 (0.66) |
| Platelets (×105/µL) | 3.41 (0.69) |
| Fasting blood glucose (mg/dL) | 88.80 (17.87) |
| Total cholesterol (mg/dL) | 112.00 (54.00) |
| HDL-c (mg/dL) | 23.40 (4.04) |
| LDL-c (mg/dL) | 22.76 (4.05) |
| VLDL-c (mg/dL) | 20.65 (5.59) |
| GOT (U/L) | 49.20 (11.90) |
| GPT (U/L) | 43.40 (6.11) |
| GGT (U/L) | 33.50 (3.38) |
| Alkaline phosphatase (U/L) | 64.50 (6.59) |
| Urea (mg/dL) | 32.20 (7.08) |
| Creatinine (mg/dL) | 0.80 (0.16) |
| Total proteins (mg/dL) | 6.46 (0.42) |
| Albumin (mg/dL) | 2.30 (0.26) |
| TNF-α (pg/mL) | 3.16 (0.54) |
| IL-6 (pg/mL) | 1.30 (0.16) |
| Parameters | OB/HGLI | OB/Standard | OB/HGLI/TTI | p Value * |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 13.4 (0.6) a | 14.3 (1.3) a.b | 12.8 (0.6) a.c | 0.029 |
| Hematocrit (%) | 39.0 (0.7) | 29.8 (13.8) | 38.5 (1.4) | 0.970 |
| Total leukocyte count (×103/µL) | 8.30 (0.40) a | 8.64 (0.48) a.b | 6.04 (0.39) a.c | 0.007 |
| Platelets (×105/µL) | 3.93 (0.53) a | 4.32 (0.51) a.b | 3.02 (0.83) a.c | 0.021 |
| Fasting blood glucose (mg/dL) | 151 (34.3) a | 167.5 (17.5) a.b | 107.4 (7.1) a.c | 0.015 |
| Total cholesterol (mg/dL) | 84.1 (7.6) a | 79.4 (12.2) a | 58.6 (8.5) b | 0.003 |
| HDL-c (mg/dL) | 27.7 (2.2) a | 34.6 (3.9) b | 22.0 (2.2) c | 0.000 |
| LDL-c (mg/dL) | 40.9 (5.2) a | 30.6 (10.7) a.b | 22.8 (6.4) b | 0.011 |
| VLDL-c (mg/dL) | 15.5 (1.6) | 14.2 (1.3) | 15.8 (1.7) | 0.267 |
| GOT (U/L) | 45.8 (4.5) | 36.2 (7.3) | 59.0 (8.6) | 0.093 |
| GPT (U/L) | 43.6 (3.5) | 40.7 (9.2) | 46.6 (3.4) | 0.339 |
| GGT (U/L) | 28.1 (1.9) | 27.9 (2.3) | 28.7 (1.1) | 0.808 |
| Alkaline phosphatase (U/L) | 65.1 (5.4) | 67.3 (4.8) | 62.4 (4.6) | 0.321 |
| Urea (mg/dL) | 26.1 (2.0) | 25.9 (2.3) | 27.1 (2.8) | 0.684 |
| Creatinine (mg/dL) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.865 |
| Total proteins (mg/dL) | 6.5 (0.4) | 6.3 (0.3) | 6.7 (0.2) | 0.241 |
| Albumin (mg/dL) | 3.0 (0.2) a | 3.5 (0.2) b | 2.8 (0.3) a | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, M.S.R.; Gonçalves, C.; Neto, M.D.; Macedo, M.H.; Queiroz, J.L.C.d.; da Silva, V.C.; Costa, I.d.S.; Camillo, C.d.S.; Santos, P.P.d.A.; Lima, A.A.M.; et al. Anti-Inflammatory Protein Isolated from Tamarind Promotes Better Histological Aspects in the Intestine Regardless of the Improvement of Intestinal Permeability in a Preclinical Study of Diet-Induced Obesity. Nutrients 2022, 14, 4669. https://doi.org/10.3390/nu14214669
Lima MSR, Gonçalves C, Neto MD, Macedo MH, Queiroz JLCd, da Silva VC, Costa IdS, Camillo CdS, Santos PPdA, Lima AAM, et al. Anti-Inflammatory Protein Isolated from Tamarind Promotes Better Histological Aspects in the Intestine Regardless of the Improvement of Intestinal Permeability in a Preclinical Study of Diet-Induced Obesity. Nutrients. 2022; 14(21):4669. https://doi.org/10.3390/nu14214669
Chicago/Turabian StyleLima, Mayara S. R., Catarina Gonçalves, Mafalda D. Neto, Maria Helena Macedo, Jaluza L. C. de Queiroz, Valéria C. da Silva, Izael de S. Costa, Christina da S. Camillo, Pedro Paulo de A. Santos, Aldo A. M. Lima, and et al. 2022. "Anti-Inflammatory Protein Isolated from Tamarind Promotes Better Histological Aspects in the Intestine Regardless of the Improvement of Intestinal Permeability in a Preclinical Study of Diet-Induced Obesity" Nutrients 14, no. 21: 4669. https://doi.org/10.3390/nu14214669
APA StyleLima, M. S. R., Gonçalves, C., Neto, M. D., Macedo, M. H., Queiroz, J. L. C. d., da Silva, V. C., Costa, I. d. S., Camillo, C. d. S., Santos, P. P. d. A., Lima, A. A. M., Pastrana, L., Maciel, B. L. L., & Morais, A. H. A. (2022). Anti-Inflammatory Protein Isolated from Tamarind Promotes Better Histological Aspects in the Intestine Regardless of the Improvement of Intestinal Permeability in a Preclinical Study of Diet-Induced Obesity. Nutrients, 14(21), 4669. https://doi.org/10.3390/nu14214669







