Protective Effects of Fish (Alaska Pollock) Protein Intake against Short-Term Memory Decline in Senescence-Accelerated Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Nutritional Composition
2.3. Animals and Diets
2.4. Behavioral Tests
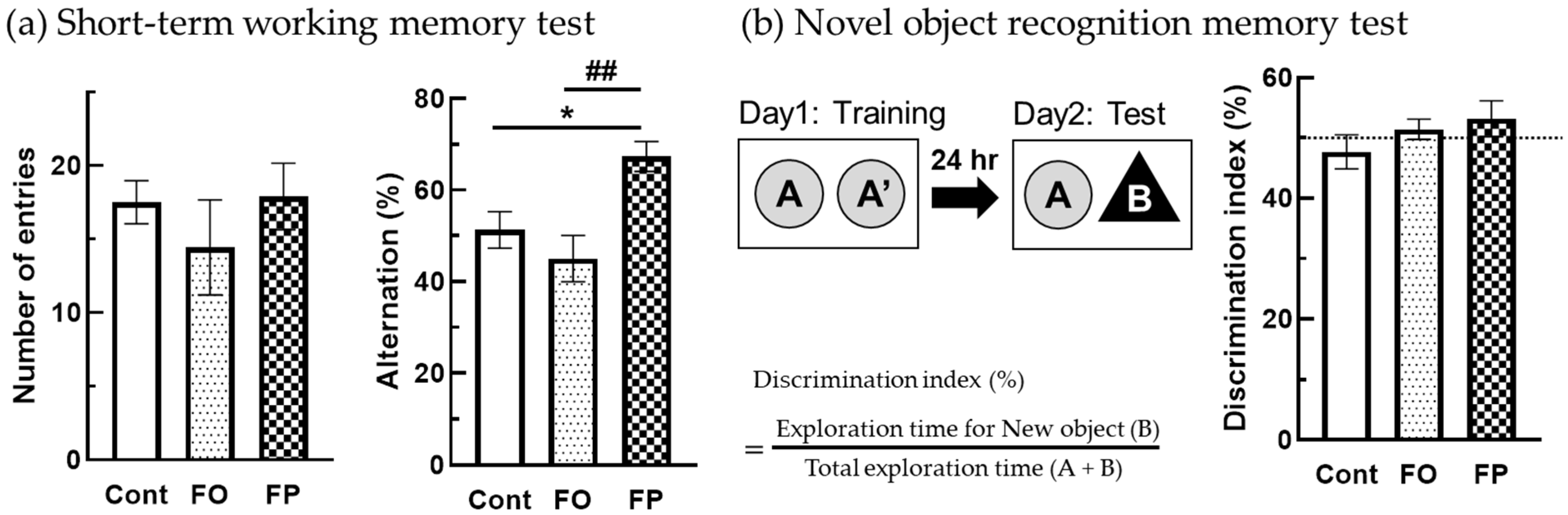
2.4.1. Y-maze Test
2.4.2. Novel-Object Recognition Test (NORT)
2.5. Sample Collection
2.6. Immunohistochemistry
2.7. Measurement of Serum Biochemical Parameters and the Levels of Plasmalogens (Pls) in Tissues
2.8. Statistical Analysis
3. Results
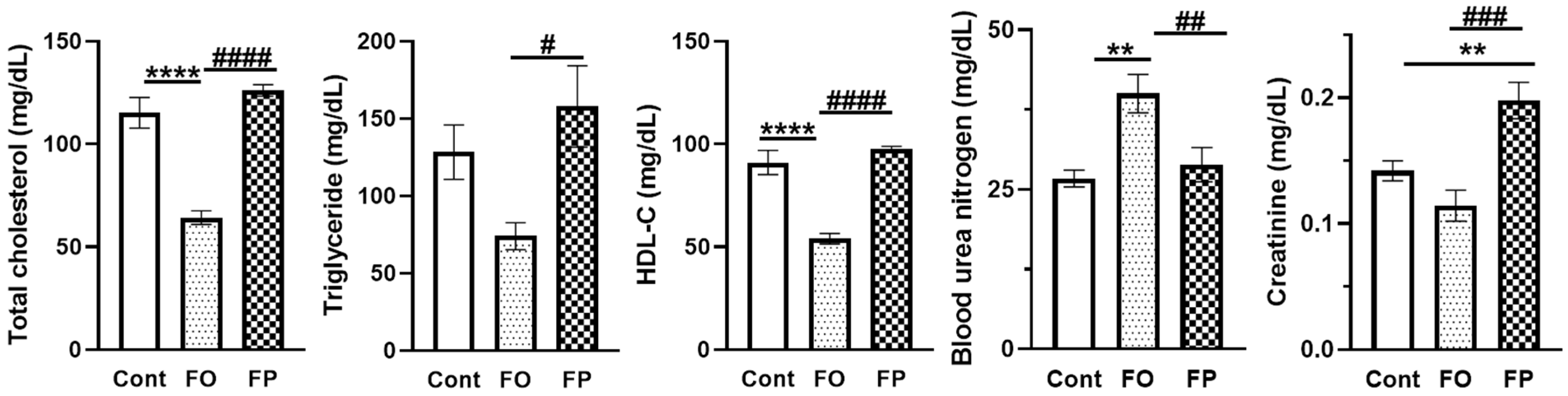
3.1. Effect of Diets Containing FO or FP on Growth Parameters, Relative Organ Weights, and Serum Biochemical Parameters
3.2. Effect of Diets Containing FO or FP on Short-Term Memory
3.3. Effect of Diets Containing FO or FP on the Morphological Changes in the CA1 Hipp Region
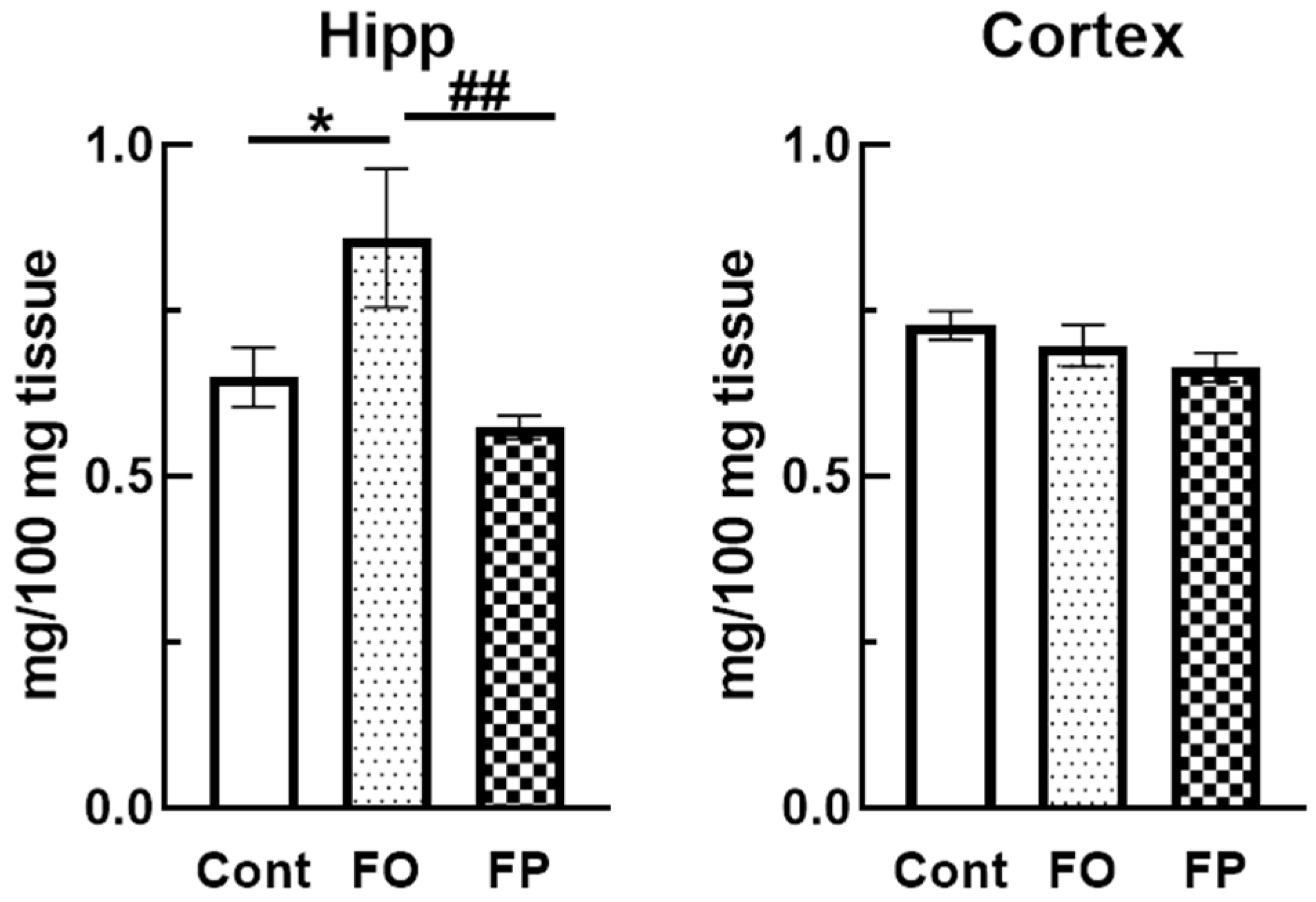
3.4. Effect of Diets Containing FO or FP on the Levels of Pls in an SAM Brain at 6 Months of Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nozaki, S.; Sawada, N.; Matsuoka, Y.J.; Shikimoto, R.; Mimura, M.; Tsugane, S. Association Between Dietary Fish and PUFA Intake in Midlife and Dementia in Later Life: The JPHC Saku Mental Health Study. J. Alzheimer’s Dis. 2021, 79, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-F.; Cao, Y.; Liang, W.-X.; Bao, W.-H.; Pan, J.-K.; Wang, Q.; Liu, J.; Liang, H.-D.; Xie, H.; Chai, Y.-T.; et al. An exploration of the role of a fish-oriented diet in cognitive decline: A systematic review of the literature. Oncotarget 2017, 8, 39877–39895. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Crivello, F.; Mazoyer, B.; Debette, S.; Tzourio, C.; Samieri, C. Fish Intake and MRI Burden of Cerebrovascular Disease in Older Adults. Neurology 2021, 97, e2213–e2222. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Troesch, B.; Weber, P. Inadequate supply of vitamins and DHA in the elderly: Implications for brain aging and Alzheimer-type dementia. Nutrition 2015, 31, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Gao, X.; Shi, B.; Chen, S.; Zhou, X.; Li, Z.; Gan, Y.; Cui, L.; Kang, J.X.; Li, W.; et al. Enriched endogenous n-3 polyunsaturated fatty acids alleviate cognitive and behavioral deficits in a mice model of Alzheimer’s disease. Neuroscience 2016, 333, 345–355. [Google Scholar] [CrossRef]
- Cederholm, T. Fish consumption and omega-3 fatty acid supplementation for prevention or treatment of cognitive decline, dementia or Alzheimer’s disease in older adults—any news? Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 104–109. [Google Scholar] [CrossRef]
- Burckhardt, M.; Herke, M.; Wustmann, T.; Watzke, S.; Langer, G.; Fink, A. Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst. Rev. 2016, 4, CD009002. [Google Scholar] [CrossRef]
- Werner, T.; Kumar, R.; Horvath, I.; Scheers, N.; Wittung-Stafshede, P. Abundant fish protein inhibits α-synuclein amyloid formation. Sci. Rep. 2018, 8, 5465. [Google Scholar] [CrossRef]
- Hosomi, R.; Nishimoto, A.; Kobayashi, T.; Ikeda, Y.; Mitsui, M.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Alaska pollock protein alters insulin sensitivity and gut microbiota composition in rats. J. Food Sci. 2020, 85, 3628–3637. [Google Scholar] [CrossRef]
- Onodera, T.; Watanabe, R.; Tha, K.K.; Hayashi, Y.; Murayama, T.; Okuma, Y.; Ono, C.; Oketani, Y.; Hosokawa, M.; Nomura, Y. Depressive Behavior and Alterations in Receptors for Dopamine and 5-Hydroxyiryptamine in the Brain of the Senescence Accelerated Mouse (SAM)-P10. Jpn. J. Pharmacol. 2000, 83, 312–318. [Google Scholar] [CrossRef]
- Shimada, A.; Hasegawa-Ishii, S. Senescence-accelerated Mice (SAMs) as a Model for Brain Aging and Immunosenescence. Aging Dis. 2011, 2, 414–435. [Google Scholar] [PubMed]
- Shimada, A.; Keino, H.; Satoh, M.; Kishikawa, M.; Hosokawa, M. Age-related loss of synapses in the frontal cortex of SAMP10 mouse: A model of cerebral degeneration. Synapse 2003, 48, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-W.; Agarwal, A.; Smith, M.D.; Khuder, S.S.; Baxi, E.G.; Thomas, A.G.; Rojas, C.; Moniruzzaman, M.; Slusher, B.S.; Bergles, D.E.; et al. Inhibition of neutral sphingomyelinase 2 promotes remyelination. Sci. Adv. 2020, 6, eaba5210. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kang, M.S.; Choi, Y.M.; Suh, Y.H.; Kim, D.K. Sphingomyelinase Activity Is Enhanced in Cerebral Cortex of Senescence-Accelerated Mouse-P/10 with Advancing Age. Biochem. Biophys. Res. Commun. 1997, 237, 583–587. [Google Scholar] [CrossRef]
- Nave, K.-A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 2010, 11, 275–283. [Google Scholar] [CrossRef]
- Bartzokis, G. Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer′s disease. Neurobiol. Aging 2004, 25, 5–18. [Google Scholar] [CrossRef]
- Bartzokis, G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging 2011, 32, 1341–1371. [Google Scholar] [CrossRef]
- Bouhrara, M.; Reiter, D.A.; Bergeron, C.M.; Zukley, L.M.; Ferrucci, L.; Resnick, S.M.; Spencer, R.G. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimer’s Dement. 2018, 14, 998–1004. [Google Scholar] [CrossRef]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Et Biophys. Acta Mol. Basis Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A. Book Review: Plasmalogens: Workhorse Lipids of Membranes in Normal and Injured Neurons and Glia. Neuroscientist 2001, 7, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Rouser, G.; Yamamoto, A. Curvilinear regression course of human brain lipid composition changes with age. Lipids 1968, 3, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, S.; Ohara, S.; Taniwaki, Y.; Tsuboi, Y.; Maruyama, T.; Fujino, T. Improvement of Blood Plasmalogens and Clinical Symptoms in Parkinson’s Disease by Oral Administration of Ether Phospholipids: A Preliminary Report. Park. Dis. 2020, 2020, 2671070. [Google Scholar] [CrossRef] [PubMed]
- Schedin, S.; Sindelar, P.J.; Pentchev, P.; Brunk, U.; Dallner, G. Peroxisomal Impairment in Niemann-Pick Type C Disease. J. Biol. Chem. 1997, 272, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- George, W.; Latimer, J.R. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Fukunaga, K.; Hosomi, R.; Fukao, M.; Miyauchi, K.; Kanda, S.; Nishiyama, T.; Yoshida, M. Hypolipidemic Effects of Phospholipids (PL) Containing n-3 Polyunsaturated Fatty Acids (PUFA) Are Not Dependent on Esterification of n-3 PUFA to PL. Lipids 2016, 51, 279–289. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Murakami, Y.; Imamura, Y.; Kasahara, Y.; Yoshida, C.; Momono, Y.; Fang, K.; Nishiyama, T.; Sakai, D.; Konishi, Y. The Effects of Maternal Interleukin-17A on Social Behavior, Cognitive Function, and Depression-Like Behavior in Mice with Altered Kynurenine Metabolites. Int. J. Tryptophan Res. 2021, 14, 11786469211026639. [Google Scholar] [CrossRef]
- Murakami, Y.; Imamura, Y.; Saito, K.; Sakai, D.; Motoyama, J. Altered kynurenine pathway metabolites in a mouse model of human attention-deficit hyperactivity/autism spectrum disorders: A potential new biological diagnostic marker. Sci. Rep. 2019, 9, 13182. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Gottfried, E.L.; Rapport, M.M. The biochemistry of plasmalogens. I. Isolation and characterization of phosphatidal choline, a pure native plasmalogen. J. Biol. Chem. 1962, 237, 329–333. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hosomi, R.; Fukunaga, K.; Arai, H.; Kanda, S.; Nishiyama, T.; Yoshida, M. Effect of combination of dietary fish protein and fish oil on lipid metabolism in rats. J. Food Sci. Technol. 2013, 50, 266–274. [Google Scholar] [CrossRef]
- Roach, P.D.; Kambouris, A.M.; Trimble, R.P.; Topping, D.L.; Nestel, P.J. The effects of dietary fish oil on hepatic high density and low density lipoprotein receptor activities in the rat. FEBS Lett. 1987, 222, 159–162. [Google Scholar] [CrossRef]
- Arai, T.; Kim, H.-J.; Chiba, H.; Matsumoto, A. Interaction of Fenofibrate and Fish Oil in Relation to Lipid Metabolism in Mice. J. Atheroscler. Thromb. 2009, 16, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. In Pre-Clinical Models: Techniques and Protocols, Methods in Molecular BIOLOGY; Guest, P.C., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1916, pp. 105–111. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Murray, E.A.; Richmond, B.J. Role of perirhinal cortex in object perception, memory, and associations. Curr. Opin. Neurobiol. 2001, 11, 188–193. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nakai, T.; Masutani, T.; Unno, K.; Akao, Y. Improvement of Learning and Memory in Senescence-Accelerated Mice by S-Allylcysteine in Mature Garlic Extract. Nutrients 2020, 12, 1834. [Google Scholar] [CrossRef]
- Takahashi, E.; Ono, E. Effects of semi-purified diet on depressive behaviors in aged mice. Biochem. Biophys. Rep. 2021, 28, 101152. [Google Scholar] [CrossRef]
- Wang, J.; Lei, H.; Hou, J.; Liu, J. Involvement of oxidative stress in SAMP10 mice with age-related neurodegeneration. Neurol. Sci. 2015, 36, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Ohta, A.; Akiguchi, I.; Takeda, T. Inbred SAM-P/10 as a Mouse Model of Spontaneous, Inherited Brain Atrophy. J. Neuropathol. Exp. Neurol. 1992, 51, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-J.; Kim, K.; Suh, Y.-H. Age-related changes in the expression of Alzheimer’s βAPP in the brain of senescence accelerated mouse (SAM)-P/10. NeuroReport 1997, 8, 1733–1737. [Google Scholar] [CrossRef]
- Jeffery, K.; Casali, G. Hippocampal Neurons: Simulating the Spatial Structure of a Complex Maze. Curr. Biol. 2014, 24, R643–R645. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, K.Z.; Pevzner, A.; Hamidi, A.B.; Nakazawa, Y.; Graham, J.; Wiltgen, B.J. Cortical Representations Are Reinstated by the Hippocampus during Memory Retrieval. Neuron 2014, 84, 347–354. [Google Scholar] [CrossRef]
- Sacket, S.J.; Chung, H.-Y.; Okajima, F.; Im, D.-S. Increase in sphingolipid catabolic enzyme activity during aging. Acta Pharmacol. Sin. 2009, 30, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Goodenowe, D.B.; Cook, L.L.; Liu, J.; Lu, Y.; Jayasinghe, D.A.; Ahiahonu, P.W.K.; Heath, D.; Yamazaki, Y.; Flax, J.; Krenitsky, K.F.; et al. Peripheral ethanolamine plasmalogen deficiency: A logical causative factor in Alzheimer’s disease and dementia. J. Lipid Res. 2007, 48, 2485–2498. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The Role of Lipids in Parkinson’s Disease. Cells 2019, 8, 27. [Google Scholar] [CrossRef]
- Ferreira, H.B.; Melo, T.; Monteiro, A.; Paiva, A.; Domingues, P.; Domingues, M.R. Serum phospholipidomics reveals altered lipid profile and promising biomarkers in multiple sclerosis. Arch. Biochem. Biophys. 2021, 697, 108672. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Sun, G.Y. Free Fatty Acids, Neutral Glycerides, and Phosphoglycerides in Transient Focal Cerebral Ischemia. J. Neurochem. 1995, 64, 1688–1695. [Google Scholar] [CrossRef]
- Katayama, S.; Imai, R.; Sugiyama, H.; Nakamura, S. Oral Administration of Soy Peptides Suppresses Cognitive Decline by Induction of Neurotrophic Factors in SAMP8 Mice. J. Agric. Food Chem. 2014, 62, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Herculano, B.; Tamura, M.; Ohba, A.; Shimatani, M.; Kutsuna, N.; Hisatsune, T. β-Alanyl-L-Histidine Rescues Cognitive Deficits Caused by Feeding a High Fat Diet in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Iga, Y.; Ikegaya, S.; Kakiuchi, T.; Ohba, H.; Nishiyama, S.; Fukomoto, D.; Kanazawa, M.; Harada, N.; Tsukada, H.; et al. In vivo alterations of mitochondrial activity and amyloidosis in early-stage senescence-accelerated mice: A positron emission tomography study. J. Neuroinflammation 2021, 18, 288. [Google Scholar] [CrossRef] [PubMed]

| Experimental Proteins | ||
|---|---|---|
| Casein | FP | |
| Water (g/100 g) | 2.5 | 2.5 |
| Ash (g/100 g) | 1.6 | 4.6 |
| Crude protein (g/100 g) | 93.9 | 92.1 |
| Amino acid composition (wt%) | ||
| Alanine | 3.07 | 6.40 |
| Arginine | 3.56 | 6.65 |
| Aspartic acid 1 | 6.81 | 10.64 |
| Cysteine | 0.38 | 1.11 |
| Glutamic acid 2 | 21.07 | 16.19 |
| Glycine | 1.81 | 4.63 |
| Histidine | 2.67 | 2.04 |
| Isoleucine | 5.23 | 4.99 |
| Leucine | 9.03 | 8.65 |
| Lysine | 8.04 | 10.14 |
| Methionine | 2.55 | 3.31 |
| Phenylalanine | 4.88 | 4.16 |
| Proline | 10.6 | 3.36 |
| Serine | 4.92 | 4.27 |
| Threonine | 3.85 | 4.42 |
| Tyrosine | 5.30 | 3.72 |
| Valine | 6.23 | 5.32 |
| Crude fat (g/100 g) | 1.3 | 0.5 |
| EPA + DHA (g/100 g) | N.D. | 0.1 |
| Experimental Oils | ||
|---|---|---|
| Soybean Oil | FO | |
| Fatty acid composition (wt%) | ||
| C14: 0 | N.D. | 8.8 |
| C16: 0 | 11.3 | 18.2 |
| C16: 1 | N.D. | 13.1 |
| C18: 0 | 3.5 | 3.1 |
| C18: 1n-9 | 21.8 | 6.9 |
| C18: 1n-7 | N.D. | 3.5 |
| C18: 2n-6 | 53.6 | 1.6 |
| C18: 3n-3 | 5.5 | 1.4 |
| C20: 4n-6 (AA) | N.D. | 1.1 |
| C20: 5n-3 (EPA) | N.D. | 16.4 |
| C22: 6n-3 (DHA) | N.D. | 11.7 |
| Others | 4.3 | 14.2 |
| Components | Experimental Groups | ||
|---|---|---|---|
| Control | FO | FP | |
| Casein | 200 | 200 | — |
| Fish protein | — | — | 203.9 |
| Soybean oil | 67.4 | 20 | 68.98 |
| Fish oil | — | 50 | — |
| tert-Butylhydroquinone | 0.014 | 0.014 | 0.014 |
| Corn starch | 400.1 | 397.5 | 394.6 |
| Dextrinized corn starch | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 100 |
| Cellulose | 50 | 50 | 50 |
| L-Cystine | 3 | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| AIN-93 vitamin mixture | 10 | 10 | 10 |
| AIN-93G mineral mixture | 35 | 35 | 35 |
| Experimental Groups | |||
|---|---|---|---|
| Control | FO | FP | |
| Growth parameters | |||
| Initial BW (g) | 19.1 ± 0.84 | 19.1 ± 0.68 | 18.9 ± 0.95 |
| Final BW (g) | 34.4 ± 1.36 | 37.0 ± 1.51 | 35.5 ± 1.35 |
| BW gain (g/day) | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 |
| Food intake (g/day) | 4.87 ± 0.17 | 4.97 ± 0.23 | 5.24 ± 0.16 |
| Organ weights (g/100 g BW) | |||
| Liver | 4.12 ± 0.17 | 4.63 ± 0.09 | 4.38 ± 0.16 |
| Spleen | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.19 ± 0.02 |
| Kidney | 1.84 ± 0.08 | 1.93 ± 0.14 | 1.98 ± 0.11 |
| Cecum | 0.71 ± 0.07 | 0.80 ± 0.06 | 0.68 ± 0.06 |
| Serum biochemical parameters | |||
| Total proteins (g/dL) | 4.86 ± 0.08 | 4.83 ± 0.06 | 4.69 ± 0.06 |
| Albumin (g/dL) | 2.86 ± 0.06 | 2.84 ± 0.04 | 2.74 ± 0.03 |
| LDH (U/L) | 191.7 ± 8.94 | 186.7 ± 19.0 | 206.8 ± 15.1 |
| AST (U/L) | 46.3 ± 1.72 | 50.7 ± 2.08 | 48.6 ± 1.69 |
| ALT (U/L) | 21.2 ± 2.23 | 30.9 ± 3.24 | 27.7 ± 2.79 |
| CPK (U/L) | 24.3 ± 3.04 | 27.4 ± 2.61 | 26.2 ± 3.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, Y.; Hosomi, R.; Nishimoto, A.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Protective Effects of Fish (Alaska Pollock) Protein Intake against Short-Term Memory Decline in Senescence-Accelerated Mice. Nutrients 2022, 14, 4618. https://doi.org/10.3390/nu14214618
Murakami Y, Hosomi R, Nishimoto A, Nishiyama T, Yoshida M, Fukunaga K. Protective Effects of Fish (Alaska Pollock) Protein Intake against Short-Term Memory Decline in Senescence-Accelerated Mice. Nutrients. 2022; 14(21):4618. https://doi.org/10.3390/nu14214618
Chicago/Turabian StyleMurakami, Yuki, Ryota Hosomi, Ayano Nishimoto, Toshimasa Nishiyama, Munehiro Yoshida, and Kenji Fukunaga. 2022. "Protective Effects of Fish (Alaska Pollock) Protein Intake against Short-Term Memory Decline in Senescence-Accelerated Mice" Nutrients 14, no. 21: 4618. https://doi.org/10.3390/nu14214618
APA StyleMurakami, Y., Hosomi, R., Nishimoto, A., Nishiyama, T., Yoshida, M., & Fukunaga, K. (2022). Protective Effects of Fish (Alaska Pollock) Protein Intake against Short-Term Memory Decline in Senescence-Accelerated Mice. Nutrients, 14(21), 4618. https://doi.org/10.3390/nu14214618





