Abstract
Associations between vitamin D deficiency and metabolic syndrome (MS) have been reported; however, the underlying biological mechanisms remain controversial. The aim of this study was to investigate the associations of CYP2R1 and VDR variants with MS and MS components in non-diabetic Brazilian adolescents. This cross-sectional study included 174 adolescents who were classified as overweight/obese. Three CYP2R1 variants and four VDR variants were identified by allelic discrimination. The CYP2R1 polymorphisms, rs12794714 (GG genotype) (odds ratio [OR] = 3.54, 95% confidence interval [CI] = 1.24–10.14, p = 0.023) and rs10741657 (recessive model—GG genotype) (OR = 3.90, 95%CI = 1.18–12.92, p = 0.026) were significantly associated with an increased risk of MS and hyperglycemia, respectively. The AG + GG genotype (dominant model) of the rs2060793 CYP2R1 polymorphism was associated with hyperglycemia protection (OR = 0.28, 95%CI = 0.08–0.92, p = 0.037). Furthermore, the CC genotype (recessive model) of the rs7975232 VDR polymorphism was significantly associated with a risk of hypertension (OR = 5.91, 95%CI = 1.91–18.32, p = 0.002). In conclusion, the CYP2R1 rs12794714 polymorphism could be considered a possible new molecular marker for predicting the risk of MS; CYP2R1 rs10741657 polymorphism and VDR rs7975232 polymorphism are associated with an increased risk of diabetes and hypertension in adolescents with overweight/obesity.
1. Introduction
Obesity is an important risk factor for the development of several metabolic abnormalities that can compromise health and quality of life in the long term; among these abnormalities, abdominal obesity, arterial hypertension, dyslipidemia, and glycemic alterations are considered components of metabolic syndrome (MS) [1,2]. MS is associated with a 1.5- to 2-fold increased risk of cardiovascular disease and other causes of mortality in adult and pediatric groups [3].
Data from large observational studies suggest that obesity is associated with an increased risk of hypovitaminosis D [4,5]. Vitamin D is a multifaceted hormone that exerts pleiotropic effects on metabolism, immunity, cell proliferation, and cell differentiation owing to its anti-inflammatory, antiatherogenic, cardioprotective, and neuroprotective properties; additionally, it plays a role in adipogenesis and insulin homeostasis [6,7,8]. However, the causality of the relationship between obesity and hypovitaminosis D remains poorly understood [9,10]. In addition to its relationship with obesity, previous studies have identified an inverse association between vitamin D concentrations and risk factors for MS, such as dyslipidemia, insulin resistance, and arterial hypertension, with serious implications for cardiometabolic risk and morbidity [11,12,13].
In this context, it is known that the biological functions of vitamin D are mediated by several genes involved in its metabolism and synthesis [14]. Furthermore, there is a possible association of the polymorphisms of genes that encode the enzymes responsible for vitamin D synthesis with different outcomes, such as the risk of obesity and MS [15]. Among the main genes involved in the bioactivation of vitamin D, CYP2R1 is responsible for the hydroxylation of vitamin D3 to 25-hydroxyvitamin D3 (25(OH)D). This process initially occurs when the 25-hydroxylase enzyme (encoded by the CYP2R1 gene) converts vitamin D (inactive precursor derived from sunlight exposure or dietary intake) to 25(OH)D (circulating form) in the liver. Human CYP2R1 belongs to the cytochrome p450 family and is located at position 11p15.2, encoding an enzyme with 501 amino acids. A mutation in this gene is naturally associated with a deficiency in the active form of vitamin D [16,17]. Despite the limited number of studies on CYP2R1, there is evidence suggesting that CYP2R1 variants affect body mass index (BMI) regardless of vitamin D concentrations. Furthermore, no studies in the literature have investigated the association of MS with variants of this gene [18,19].
Unlike in the case of CYP2R1, variants of the vitamin D receptor (VDR) gene are the main markers that have been investigated in previous studies on the association of vitamin D with metabolic outcomes. The genomic actions of 1.25 dihydroxyvitamin D3 (1,25(OH)2D3) are mediated by the VDR, which belongs to the family of steroid receptors and is expressed in various body tissues (adipocytes, kidneys, pancreas, and immune system) [20]. Along with the VDR, 1,25(OH)2D3 regulates the transcription of target genes by heterodimerization with the retinoid X receptor (RXR). The VDR-RXR complex can translocate to the cell nucleus and interact with deoxyribonucleic acid (DNA) sequence elements or vitamin D response elements (VDREs), which are found in the promoter regions of target genes. Thus, genetic alterations in the VDR can lead to important defects in gene activation, cell proliferation and differentiation, calcium homeostasis, and other related biological mechanisms [21].
VDR polymorphisms, such as rs7975232 (ApaI), rs1544410 (BsmI), rs2228570 (FokI), and rs731236 (TaqI), which are the most commonly studied, have been associated with MS and its components, including anthropometric and biochemical parameters, in different populations [14,22]. However, corresponding information is not only scarce but also inconclusive. Recently, Jin et al. determined that VDR polymorphisms, including ApaI, BsmI, FokI, and TaqI, were not associated with the risk of MS; however, the ApaI variant was associated with hypertriglyceridemia, and the BsmI and TaqI variants were associated with high-density lipoprotein cholesterol (HDL-c) in adults [23]. By contrast, in Chinese children, the FokI polymorphism of the VDR was associated with a higher risk of MS [24].
In this study, we aimed to investigate the association of CYP2R1 polymorphisms (rs10741657, rs2060793, rs12794714) and VDR polymorphisms (rs2228570, rs1544410, rs7975232, rs731236) with MS, MS components (waist circumference, fasting blood glucose and triglycerides, blood pressure, HDL-c), and vitamin D serum levels in Brazilian non-diabetic adolescents.
2. Materials and Methods
2.1. Study Design and Participants
This was a cross-sectional study involving adolescents aged 10–19 years of both sexes who were overweight/obese. All participants were recruited from the Pediatric Endocrinology Outpatient Clinic of Onofre Lopes University Hospital (HUOL/Natal-RN/Brazil) between September 2017 and March 2020. The determination of the sample size of 174 adolescents was based on a power of 62% required to reveal a difference between the serum levels of vitamin D corresponding to the three groups of alleles of each analyzed polymorphism, on assuming a standard deviation of 0.5 (3.5 ng/mL) and adopting a significance level of 0.017. This sample size made it possible to obtain population estimates of the proportion of individuals with each polymorphism, with a maximum error of 6.2% for a confidence level (CI) of 95%.
The study participants were classified according to the anthropometric nutritional status using the World Health Organization (WHO) BMI/age curves according to sex; z-scores between +1 and +2 indicated overweight, z-scores between +2 and +3 indicated obesity, and z-scores ≥ +3 indicated severe obesity [25]. Then, the adolescents were subdivided into groups with MS (n = 48) and without MS (n = 126), according to the criteria proposed by the International Diabetes Federation (IDF) [26].
The exclusion criteria were the presence of genetic syndromes associated with obesity or other chronic diseases; pregnancy and lactation; use of vitamin D supplementation; use of drugs to treat insulin resistance or type 2 Diabetes mellitus; acute or chronic liver, kidney, thyroid dysfunction, heart failure; cancer; or other conditions that alter vitamin D metabolism.
This study was approved by the Research Ethics Committee of the University Hospital Onofre Lopes (CEP/HUOL/UFRN; CAAE 56763716.7.0000.5292) and was performed in accordance with the principles of the Declaration of Helsinki. Written consent was obtained from all participants and their legal guardians.
2.2. Collection of Data and Blood Samples
All participants underwent weight, height, waist circumference (WC), and blood pressure (BP) measurements. All measurements were performed by trained researchers. Weight and height were measured using a digital scale and stadiometer (Omron Health Care, Kyoto, Japan), respectively. WC was measured with an inelastic tape placed at the midpoint between the last rib and iliac crest, with the patient standing up. The WC cutoff point was defined as ≥the 90th percentile. BMI z-scores based on age were calculated according to WHO guidelines [25]. BP was measured after a 5-min rest period using an automatic sphygmomanometer (Omron Health Care, Kyoto, Japan) according to the collection and classification guidelines established by the Brazilian Guidelines on Arterial Hypertension [27].
Blood samples were collected after fasting for 12–14 h. The collected blood serum was used to obtain the concentrations of 25(OH)D, fasting glucose, total cholesterol and its fractions, and triglycerides. Biochemical determinations were performed using Wiener kits, according to the methodology described by the manufacturer, and a CMD-800 biochemical analyzer (Wiener Laboratories, Rosario, Argentina). The 25(OH)D analyses were performed using the automated electrochemiluminescence immunoassay kit manufactured by Roche Diagnostics GmbH, COBAS Series 6000 Modular Analyzer (Mannheim, Germany). A diagnosis of vitamin D deficiency was reached when the 25(OH)D concentration was <20 ng/dL [28].
A diagnosis of MS was reached in the adolescents according to the criteria of the IDF: the presence of abdominal obesity (≥p90) associated with two or more clinical criteria, such as high BP (systolic/diastolic BP ≥ 130 or ≥85), hyperglycemia (≥100 mg/dL), hypertriglyceridemia (≥150 mg/dL), and low HDL-c levels (<40 mg/dL) [26].
2.3. Genetics Analyses
Genomic DNA was obtained from whole blood using the commercial QIAamp DNA Blood Mini Kit (Qiagen, CA, USA) by following the manufacturer’s recommendations. The obtained material was stored in a −20 °C freezer until analysis. The integrity of the DNA samples was evaluated by electrophoretic separation on 0.8% agarose gel in tris borate EDTA (TBE) buffer (pH 8.0), followed by staining with red gel and photodocumentation using a SmartView Pro 1200 Imager System (Major Science, CA, USA). DNA was quantified using the Qubit® 2.0 fluorometer (Foster City, CA, USA).
The search for polymorphisms in the CYP2R1 and VDR was performed with real-time polymerase chain reaction (PCR) in the ABI 7500 Fast device (Applied Biosystems, Foster City, CA, USA) using the allelic discrimination technique (TaqMan® system) and assays provided by Applied Biosystems (Foster City, CA, USA). The following polymorphisms were analyzed: rs10741657 (A > G) (C___2958430_10), rs2060793 (A > G) (C___2958431_10), and rs12794714 (A > G) (C___1131665_10) in the CYP2R1 and rs2228570 (A > G) (C__12060045_20), rs1544410) (T > C) (C___8716062_20), rs7975232 (A > C) (C__28977635_10), and rs731236 (A > G) (C___2404008_10) in the VDR.
2.4. Statistical Analysis
Statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Data were tested for the normal distribution of continuous variables using the Kolmogorov–Smirnov test. Continuous variables were expressed as mean and standard deviation of the mean or median and interquartile range. Variables with parametric distributions were analyzed using Student’s t test or a one-way analysis of variance (ANOVA), followed by Tukey’s post-test. Variables that were considered non-parametric were analyzed using the Mann–Whitney test or Kruskal–Wallis test, followed by Dunn’s post-test. Differences between categorical variables, sex, genotype/allele frequencies, and Hardy–Weinberg equilibrium were tested using the chi-square (χ2) test.
The associations between polymorphisms, vitamin D deficiency, MS, and MS components were evaluated using binary logistic regression, according to the following genetic models (dominant and recessive), which were adjusted for sex and BMI variables [29]. Odds ratios (OR) and 95% CIs were used to assess the strength of the associations. The reference genotype was homozygous for the wild-type allele of each polymorphism. The software used in these assessments was SPSS version 22.0. Linkage disequilibrium (LD) was estimated from the combined data of all groups by calculating r2 [30]. The structure of the haplotype block was determined using the CI algorithm [31], and the haplotype frequencies were estimated using the expectation maximization algorithm [32] in Haploview version 4.1 (Broad Institute, Cambridge, MA, USA). In all the analyses, a p-value < 0.05 was considered statistically significant.
3. Results
This study included a total of 174 adolescents who were assigned to the non-MS and MS groups. Table 1 shows the characteristics of these subjects. The mean age was similar between the groups (11 ± 10.1 years) (p = 0.530), and the frequency of MS was higher in male adolescents (14.9%) than in female adolescents (12.6%). The results showed significantly higher BMI (p = 0.003), WC (p = 0.017), triglyceride (p < 0.001), and fasting glucose (p < 0.001) values and significantly lower HDL-c values (p < 0.001) in adolescents with MS than in those without MS (Table 1). However, the systolic BP (p = 0.072), diastolic BP (p = 0.302), low-density lipoprotein cholesterol (LDL-c) (p = 0.381), total cholesterol (p = 0.980), and 25(OH)D (p = 0.114) levels did not differ between the groups.

Table 1.
Characteristics of the adolescents in the non-MS and MS groups.
Hardy–Weinberg equilibrium was verified in the analysis of the CYP2R1 and VDR polymorphisms (Table 2). To ensure scoring quality, 10% of the samples were re-genotyped at random, and all the results were consistent. Genotypes and allele frequencies of the CYP2R1 and VDR polymorphisms in the adolescents in the non-MS and MS groups are shown in Table 2.

Table 2.
Genotype and allele frequencies of the CYP2R1 and VDR polymorphisms in the adolescents in the non-MS and MS groups.
During this evaluation, it was observed that in the case of the single nucleotide polymorphism (SNP), rs12794714, in the CYP2R1, the GG genotype (OR = 3.54, 95% CI = 1.24–10.14, p = 0.023) and G allele (OR = 1.74, 95% CI = 1.09–2.84, p = 0.023) were significantly associated with the risk of MS. In the case of the other CYP2R1 and VDR polymorphisms, no significant associations with the risk of MS development were observed.
Table 3 presents the results of the logistic regression analysis of the association of CYP2R1 and VDR polymorphisms, as well as 25(OH)D levels with MS development. In this evaluation, it was observed that the GG genotype of the CYP2R1 (SNP rs12794714) polymorphism was associated with the risk of MS (OR = 2.74, 95% CI = 1.14–6.58, p = 0.024). No significant associations were observed with the other CYP2R1 and VDR polymorphisms, as well as with the 25(OH)D levels.

Table 3.
Logistic regression analysis of the association of the CYP2R1 and VDR polymorphisms and 25(OH)D levels with MS development.
To investigate the relationship of each CYP2R1 (Table 4) and VDR (Table 5) polymorphism with MS and vitamin D status, the components of MS and vitamin D deficiency in adolescents with MS were evaluated according to the genetic inheritance model.

Table 4.
Association of CYP2R1 polymorphisms with components of MS and vitamin D deficiency.

Table 5.
Association of VDR polymorphisms with components of MS and vitamin D deficiency.
Regarding CYP2R1 (Table 4), it was observed that the GG genotype (recessive model) of the SNP, rs10741657 (OR = 3.90, 95% CI = 1.18–12.92, p = 0.026), and AG + GG genotype (dominant model) of the SNP rs2060793 (OR = 0.28, 95% CI = 0.08–0.92, p = 0.037) were significantly associated with hyperglycemia protection. No significant associations of the components of MS and vitamin D deficiency with the other CYP2R1 polymorphisms were observed.
In the evaluation of the VDR polymorphisms (Table 5), it was observed that the CC genotype (recessive model) of the SNP rs7975232 was significantly associated with hypertension (OR = 5.91, 95% CI = 1.91–18.32, p = 0.002). In the case of the other VDR polymorphisms, no significant associations were observed with the MS components and vitamin D deficiency.
The results of the LD analysis of the CYP2R1 and VDR polymorphisms are shown in Figure 1A and 1B, respectively. A single haplotypic block (Figure 1C) was constructed for the SNPs, rs10741657 and rs2060793, in the CYP2R1. However, there was no significant association with MS (p > 0.05).
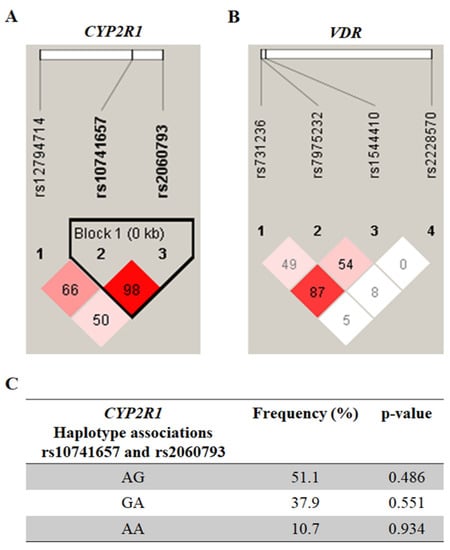
Figure 1.
Pairwise linkage disequilibrium plot of CYP2R1/rs12794714, rs10741657, and rs2060793 (A), as well as of VDR/rs731236, rs7975232, rs1544410, and rs2228570 (B) in the combined sample (cases & controls) showing r2 (×100) values. The block for CYP2R1 (C) is designed according to the internally developed solid spine of linkage disequilibrium (LD). The value within each diamond represents the pairwise correlation between pairs of single-nucleotide polymorphisms (SNPs) (measured as 100 × r2) defined by the upper left and upper right sides of the diamond. The frequency of each haplotype is shown below the blocks.
4. Discussion
The findings of the present study have revealed an important association between the CYP2R1 polymorphism, rs12794714, and an increased risk of MS. There are few reports in the literature indicating that CYP2R1 is associated with metabolic outcomes, such as obesity, hypertension, and diabetes, which are important conditions related to MS. To the best of our knowledge, this is the first time that a variant of the CYP2R1 has been associated with the risk of MS. The variant rs12794714, which was found to be associated with MS in our study, is located in the promoter region of the CYP2R1, which may influence gene transcription, and thus could be considered a possible new molecular marker for predicting the risk of MS [18].
Xu et al. (2022) found that in 766 individuals with incident hypertension, the rs12794714 polymorphism was significantly associated with an increased risk of hypertension (OR = 1.26, 95% CI = 1.01–1.56, p = 0.041). Furthermore, interactions between rs12794714 and general (OR = 3.93, CI = 2.72–5.68, p < 0.001) and central obesity (OR = 3.22, CI = 2.29–4.52, p < 0.001) exerted significant effects on the susceptibility to hypertension in the study population [18].
On individually evaluating the MS components, we identified an association between SNP rs10741657 (recessive model) of the CYP2R1 and hyperglycemia. Furthermore, in the group of individuals with hyperglycemia in our study population, the frequency of the presence of the GG genotype of the SNP rs107441657 (32.3%) was higher than that of the presence of the AA genotype (22.6%). Our findings have revealed, for the first time, that new variants of the CYP2R1, which have not yet been reported in the literature, are associated with hyperglycemia. A possible explanation for the association between CYP2R1 variant and hyperglycemia, which was identified in our study, is that the changes in vitamin D levels caused by these polymorphisms may influence the extracellular calcium concentrations in pancreatic β cells, which in turn may affect calcium-dependent insulin secretion [33]. On the other hand, SNP rs2060793 (dominant model) of the CYP2R1 was associated with protection from hyperglycemia.
Other SNPs of the CYP2R1 have been reported to increase the risk of diabetes. Wang et al. (2018) found that the rs1993116 and rs10766197 polymorphisms were significantly associated with the risk of type 2 Diabetes mellitus. Carriers of the AG + GG genotype of the rs1993116 and rs10766197 polymorphisms had a higher risk of developing type 2 Diabetes mellitus than AA carriers. However, they found no association between the variants, rs10741657 and rs12794714, and type 2 Diabetes mellitus [34].
Consistent with the results of previous studies, our results demonstrated an association between a VDR variant and an almost 6-fold increase in the risk of hypertension [35,36]. The role of vitamin D in the risk of hypertension has been demonstrated in experimental studies, which have indicated that vitamin D may be an endocrine negative regulator of the renin-angiotensin-aldosterone system (RAAS), a key stabilizer of BP balance. This downregulation of 1,25-dihydroxyvitamin D3-mediated renin expression and RAAS activity occurs through its interaction with the vitamin D receptor. Global VDR-knockout mice have been reported to have higher BP and to develop cardiac hypertrophy due to increased renin expression and subsequent RAAS activation [37,38,39].
However, few studies were able to reproduce a direct relationship between hypertension and rs7975232 (ApaI). This is an important variant that is located in the three prime untranslated (3’-UTR) region of the VDR and may influence messenger ribonucleic acid (mRNA) stability and VDR protein expression. Hajj et al. (2016) observed an association between ApaI and BP in a population of 369 adults. Women with the TT genotype had higher BP values [40]. However, the role of VDR variants in BP remains controversial. A large genetic study failed to reproduce any association between VDR-related SNPs and BP, suggesting that further research is needed to elucidate this association [41].
Our study population consisted solely of adolescents with overweight. It is known that obesity exerts an influence on vitamin D concentrations, thereby hindering its biological role within cells, and that vitamin D deficiency can influence weight gain and other metabolic changes, which are well established in the literature [5,10]. Thus, the influence of genetics on these outcomes has been increasingly investigated in order to identify new molecular markers that can predict the risk of disease in vulnerable populations. There is evidence of a relationship of both the genes evaluated in our study with the risk of obesity in other populations. Although we did not identify an association between polymorphisms and obesity or vitamin D deficiency itself in our study, it was possible to identify an association between these variants and important risk factors for MS. These findings reinforce the importance of evaluating populations globally, as vitamin D deficiency, hyperglycemia, hypertension, and MS are increasingly being investigated because these conditions are directly linked to obesity; further, when these conditions are present in pediatric populations, they increase the risk of morbidity and mortality in adulthood.
Our study has some limitations. First, the sample size was relatively small for studies on genetic polymorphisms, which may have influenced the fact that we did not find an association between the studied variants and obesity. Further comprehensive research is required to validate our results. Furthermore, the stage of sexual maturation was not included while analyzing the results; this stage may influence some metabolic parameters at the start of puberty. Finally, not all the VDR and CYP2R1 polymorphisms were included in the assessment of the study population. Therefore, it was not possible to fully assess the influence of SNPs on MS and its components.
Finally, our study has some strengths. First, our sample consisted entirely of adolescents with overweight/obesity; however, the lack of data on comorbidities and use of medications could have affected the analysis of our results. Second, the seven selected variants were previously published in genome-wide association studies (GWAS) that evaluated their biological effects. Third, all the patients were selected from a referral endocrinology center in the state of Rio de Grande do Norte, reinforcing the representative nature of the sample. Finally, our results reinforce the importance of evaluating molecular markers and anthropometric and biochemical variables together in order to predict the risk of MS.
5. Conclusions
The present study demonstrated that the rs12794714 polymorphism in the CYP2R1 was significantly associated with an increased risk of MS, regardless of 25(OH)D concentrations; this finding revealed its potential as a novel molecular biomarker for predicting the risk of MS in our population. Furthermore, we observed that both the rs10741657 and rs2060793 polymorphisms of the CYP2R1 can significantly influence glucose metabolism, while the rs7975232 polymorphism of the VDR was significantly associated with the risk of hypertension.
These results reinforce the importance of looking for genetic markers associated with vitamin D metabolism in overweight/obese children and adolescents in order to identify, at an early stage, variants associated with MS components, since morbidity and mortality progress more rapidly when starts in young individuals, with unfavorable prognostic implications and a potential increase in individual and collective costs.
Author Contributions
Conceptualization, S.C.V.d.C.L., R.F.A. and A.A.d.R.; methodology, S.C.V.d.C.L., R.F.A. and A.A.d.R.; validation, S.C.V.d.C.L., R.F.A. and A.A.d.R.; formal analysis, K.S.C.d.S.; investigation, E.P.d.S.A.; data curation, E.P.d.S.A., O.A.G. and K.S.C.d.S.; writing—original draft preparation, E.P.d.S.A. and K.S.C.d.S.; writing—review and editing, S.C.V.d.C.L., A.A.d.R., E.P.d.S.A. and K.S.C.d.S.; visualization, S.C.V.d.C.L., A.A.d.R., E.P.d.S.A., O.A.G. and K.S.C.d.S.; supervision, S.C.V.d.C.L. and A.A.d.R.; project administration, S.C.V.d.C.L., R.F.A. and A.A.d.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Coordination of Improvement of Higher Education Personnel—Brazil, grant number 001.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Onofre Lopes University Hospital (CAAE 56763716.7.0000.5292).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Nehus, E.; Mitsnefes, M. Childhood Obesity and the Metabolic Syndrome. Pediatr. Clin. N. Am. 2019, 66, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Flemming, G.M.C.; Bussler, S.; Körner, A.; Kiess, W. Definition and early diagnosis of metabolic syndrome in children. J. Pediatr. Endocrinol. Metab. 2020, 33, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalidi, B.; Kimball, S.M.; Kuk, J.L.; Ardern, C.I. Metabolically healthy obesity, vitamin D, and all-cause and cardiometabolic mortality risk in NHANES III. Clin. Nutr. 2019, 38, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Massaro, J.M.; Fox, C.S.; Larson, M.G.; Keyes, M.J.; McCabe, E.L.; Robins, S.J.; O’Donnell, C.J.; Hoffmann, U.; Jacques, P.F.; et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes 2010, 59, 242–248. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat Weight Disord. 2017, 22, 27–41. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Lubberts, E.; Bener, A. Editorial: Immune-Modulatory Effects of Vitamin D. Front. Immunol. 2020, 11, 596611. [Google Scholar] [CrossRef]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.-F. Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies. Nutrients 2022, 14, 2049. [Google Scholar] [CrossRef]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.S.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Kosmopoulos, M.; Nikas, I.P.; Spartalis, M.; Kassi, E.; Goulis, D.G.; Lambrinoudaki, I.; Siasos, G. The Impact of Obesity on the Association between Vitamin D Deficiency and Cardiovascular Disease. Nutrients 2019, 14, 2458. [Google Scholar]
- Al-Daghri, N.M.; Amer, O.E.; Khattak, M.N.K.; Sabico, S.; Ghouse, A.A.M.; Al-Saleh, Y.; Aljohani, N.; Alfawaz, H.; Alokail, M.S. Effects of different vitamin D supplementation strategies in reversing metabolic syndrome and its component risk factors in adolescents. J. Steroid Biochem. Mol. Biol. 2019, 191, 105378. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Walton, R.T.; Griffiths, C.J.; Martineau, A.R. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: Review of genetic association studies. J. Steroid Biochem. Mol. Biol. 2016, 164, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Totonchi, H.; Rezaei, R.; Noori, S.; Azarpira, N.; Mokarram, P.; Imani, D. Vitamin D Receptor Gene Polymorphisms and the Risk of Metabolic Syndrome (MetS): A Meta-Analysis. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 943–955. [Google Scholar] [CrossRef]
- Thacher, T.D.; Levine, M.A. CYP2R1 mutations causing vitamin D-deficiency rickets. J. Steroid Biochem. Mol. Biol. 2017, 173, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xue, Z.; Ji, H.; Zhang, D.; Wang, Y. Effects of CYP2R1 gene variants on vitamin D levels and status: A systematic review and meta-analysis. Gene 2018, 15, 361–369. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, D.; Lin, J.; Li, X.; Liu, Y.; Gao, J.; Xue, Y.; Zhang, Y.; Ding, R.; Huang, G.; et al. The influence of CYP2R1 polymorphisms and gene-obesity interaction with hypertension risk in a Chinese rural population. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 241–248. [Google Scholar] [CrossRef]
- Bakos, B.; Szili, B.; Szabó, B.; Horváth, P.; Kirschner, G.; Kósa, J.P.; Toldy, E.; Lakatos, P.; Tabák, A.G.; Takács, I. Genetic variants of VDR and CYP2R1 affect BMI independently of serum vitamin D concentrations. BMC Med. Genet. 2020, 21, 129. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Mangino, M.; Andrews, P.; Moore, J.H.; Spector, T.D.; Power, C.; Järvelin, M.R.; Hyppönen, E. Interaction between allelic variations in vitamin D receptor and retinoid X receptor genes on metabolic traits. BMC Genet. 2014, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.; Grineva, E.; Belyaeva, O.; Bystrova, A.; Jude, E.B.; Andreeva, A.; Kostareva, A.; Pludowski, P. Relationship Between Vitamin D Status and Vitamin D Receptor Gene Polymorphisms With Markers of Metabolic Syndrome Among Adults. Front. Endocrinol. 2018, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Lu, W.; Gong, X.; Zhou, J.; Wu, F. Association of vitamin D receptor polymorphisms with metabolic syndrome-related components: A cross-sectional study. J. Clin. Lab. Anal. 2021, 35, e23829. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Su, K.; Ding, Z.; Zhang, Z.; Wang, C. Association of Vitamin D Receptor Gene Polymorphisms with Metabolic Syndrome in Chinese Children. Int. J. Gen. Med. 2021, 14, 57–66. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneve, Switzerland, 2006. [Google Scholar]
- Zimmet, P.; Alberti, G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The metabolic syndrome in children and adolescents. Lancet 2007, 369, 2059–2061. [Google Scholar] [CrossRef]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.M.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Júnior, D.M.; et al. Brazilian Guidelines of Hypertension—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava, B.A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, R.; Guinó, E.; Moreno, V. Análisis estadístico de polimorfismos genéticos en estudios epidemiológicos. Gac. Sanit 2005, 19, 333–341. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef]
- Qin, Z.S.; Niu, T.; Liu, J.S. Partition-ligation-expectation-maximization algorithm for haplotype inference with single-nucleotide polymorphisms. Am. J. Hum. Genet. 2002, 71, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, G.; Li, Y.; Liu, X.; Liu, L.; Yang, K.; Wang, C.; Wei, S. Evaluation of the Associations of GC and CYP2R1 Genes and Gene-Obesity Interactions with Type 2 Diabetes Risk in a Chinese Rural Population. Ann. Nutr. Metab. 2020, 76, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, F.; Yu, S.; Zhang, D.; Wang, J.; Han, H.; Sun, H.; Xue, Y.; Ba, Y.; Wang, C.; et al. Triangular relationship between CYP2R1 gene polymorphism, serum 25(OH)D3 levels and T2DM in a Chinese rural population. Gene 2018, 678, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, L.; Li, C.; Gai, Z.; Li, Y. Vitamin D and Vitamin D Receptor: New Insights in the Treatment of Hypertension. Curr. Protein Pept. Sci. 2019, 20, 984–995. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.; Manson, J.E.; Buring, J.E.; Gaziano, J.M.; Sesso, H.D. A prospective study of plasma vitamin D metabolites, vitamin D receptor gene polymorphisms, and risk of hypertension in men. Eur. J. Nutr. 2013, 52, 1771–1779. [Google Scholar] [CrossRef]
- Legarth, C.; Grimm, D.; Wehland, M.; Bauer, J.; Krüger, M. The Impact of Vitamin D in the Treatment of Essential Hypertension. Int. J. Mol. Sci. 2018, 19, 455. [Google Scholar] [CrossRef]
- Kheiri, B.; Abdalla, A.; Osman, M.; Ahmed, S.; Hassan, M.; Bachuwa, G. Vitamin D deficiency and risk of cardiovascular diseases: A narrative review. Clin. Hypertens. 2018, 24, 9. [Google Scholar] [CrossRef]
- Simpson, R.U.; Hershey, S.H.; Nibbelink, K.A. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J. Steroid. Biochem. Mol. Biol. 2007, 103, 521–524. [Google Scholar] [CrossRef]
- Hajj, A.; Chedid, R.; Chouery, E.; Megarbané, A.; Gannagé-Yared, M.H. Relationship between vitamin D receptor gene polymorphisms, cardiovascular risk factors and adiponectin in a healthy young population. Pharmacogenomics 2016, 17, 1675–1686. [Google Scholar] [CrossRef]
- Wang, L.; Chu, A.; Buring, J.E.; Ridker, P.M.; Chasman, D.I.; Sesso, H.D. Common genetic variations in the vitamin D pathway in relation to blood pressure. Am. J. Hypertens. 2014, 27, 1387–1395. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).