Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors
Abstract
1. Introduction to Advanced Glycation End Products (AGEs)
1.1. Introduction
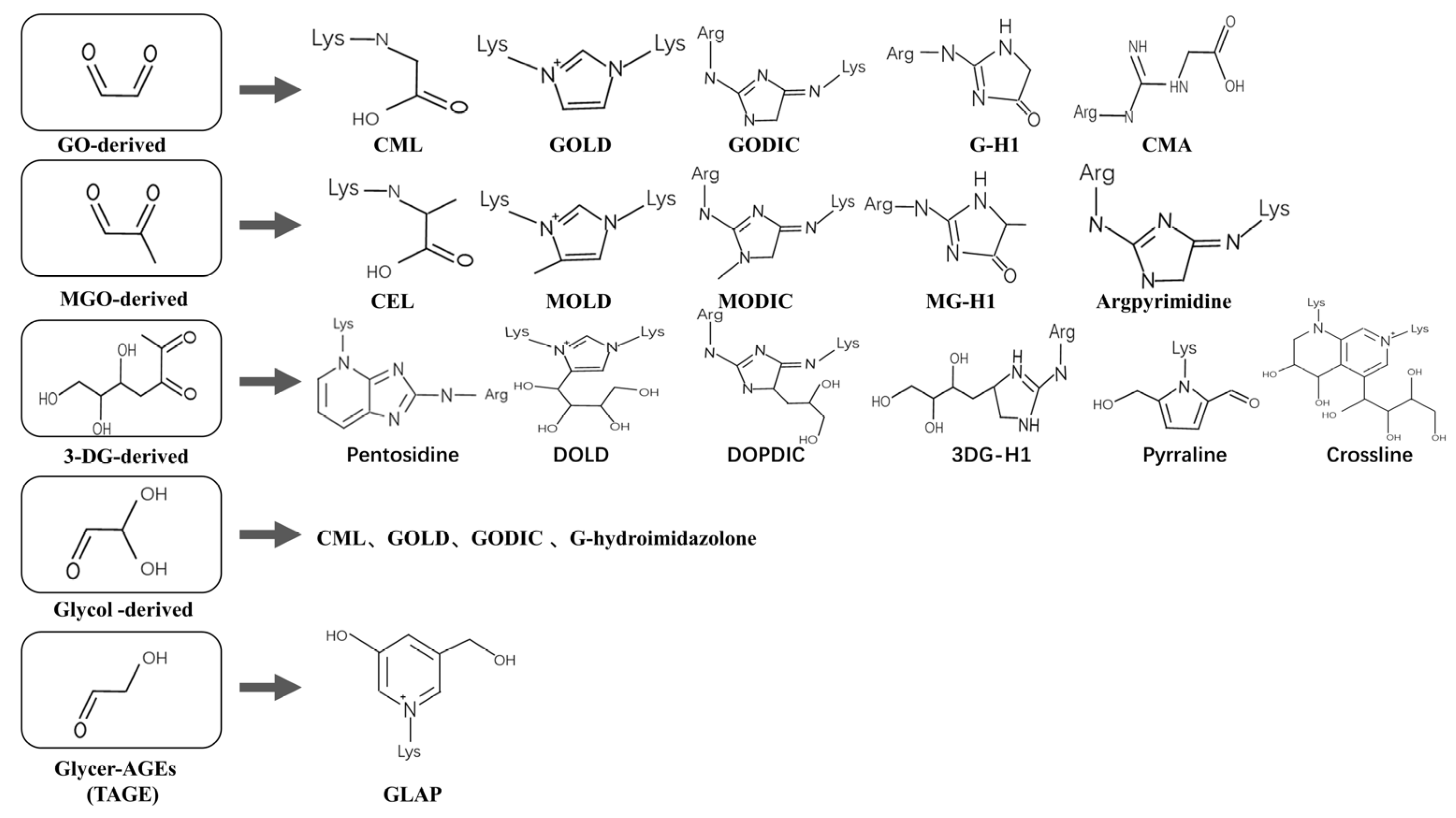
1.2. Composition
1.3. Sources
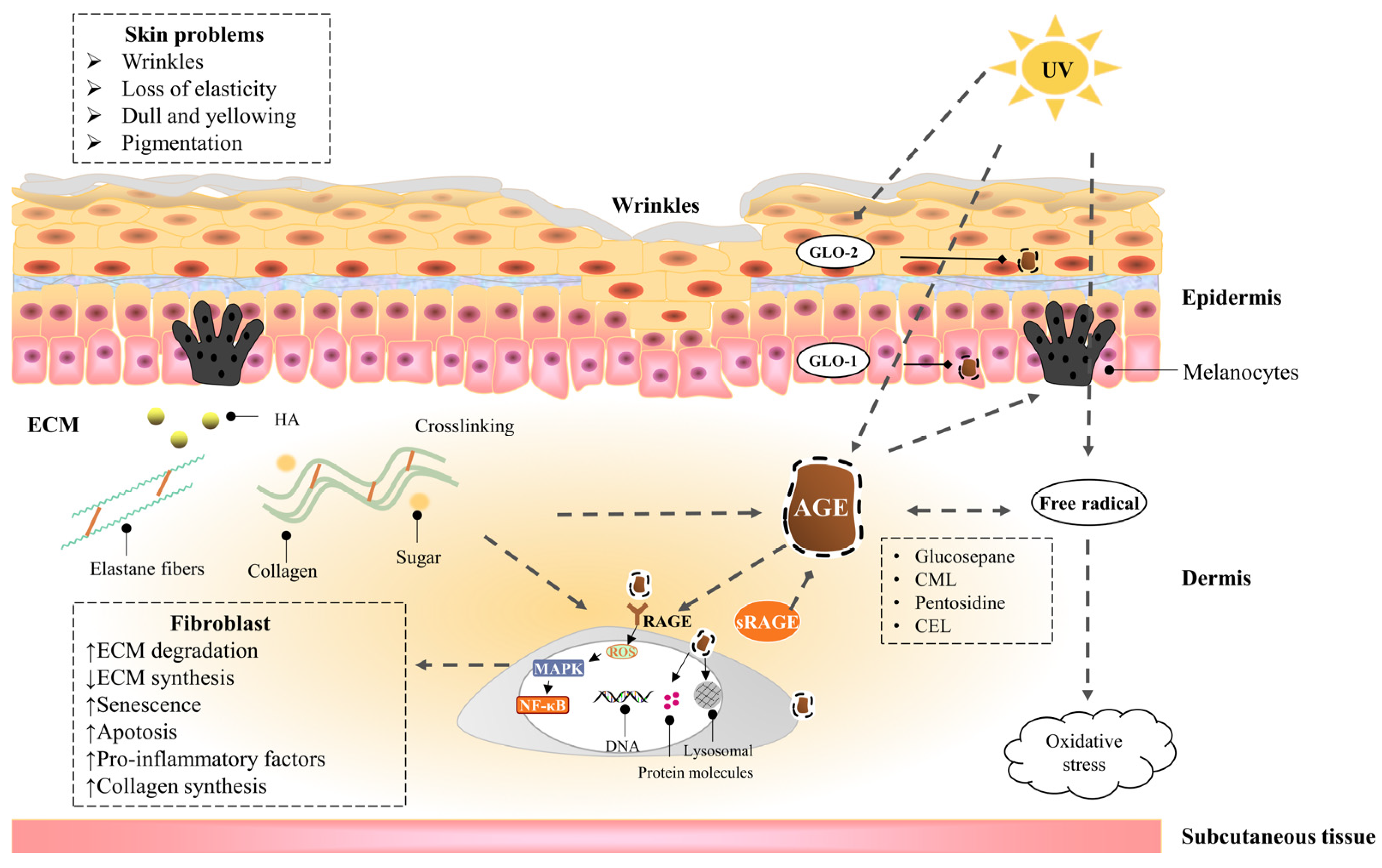
1.4. Mechanism of Injury
1.5. Reducing AGEs—Strategies to Improve Healthy Lifespan
2. The Hazards of Skin Glycation
2.1. The Harm of High Glucose to the Skin
2.2. Advanced Glycaion End Products Induce Skin Aging
2.2.1. Epidermis
2.2.2. Dermis—Fibroblast
2.2.3. Dermis—Extracellular matrix (ECM)
2.3. UVA Induces Advanced Glycation End Products of the Skin
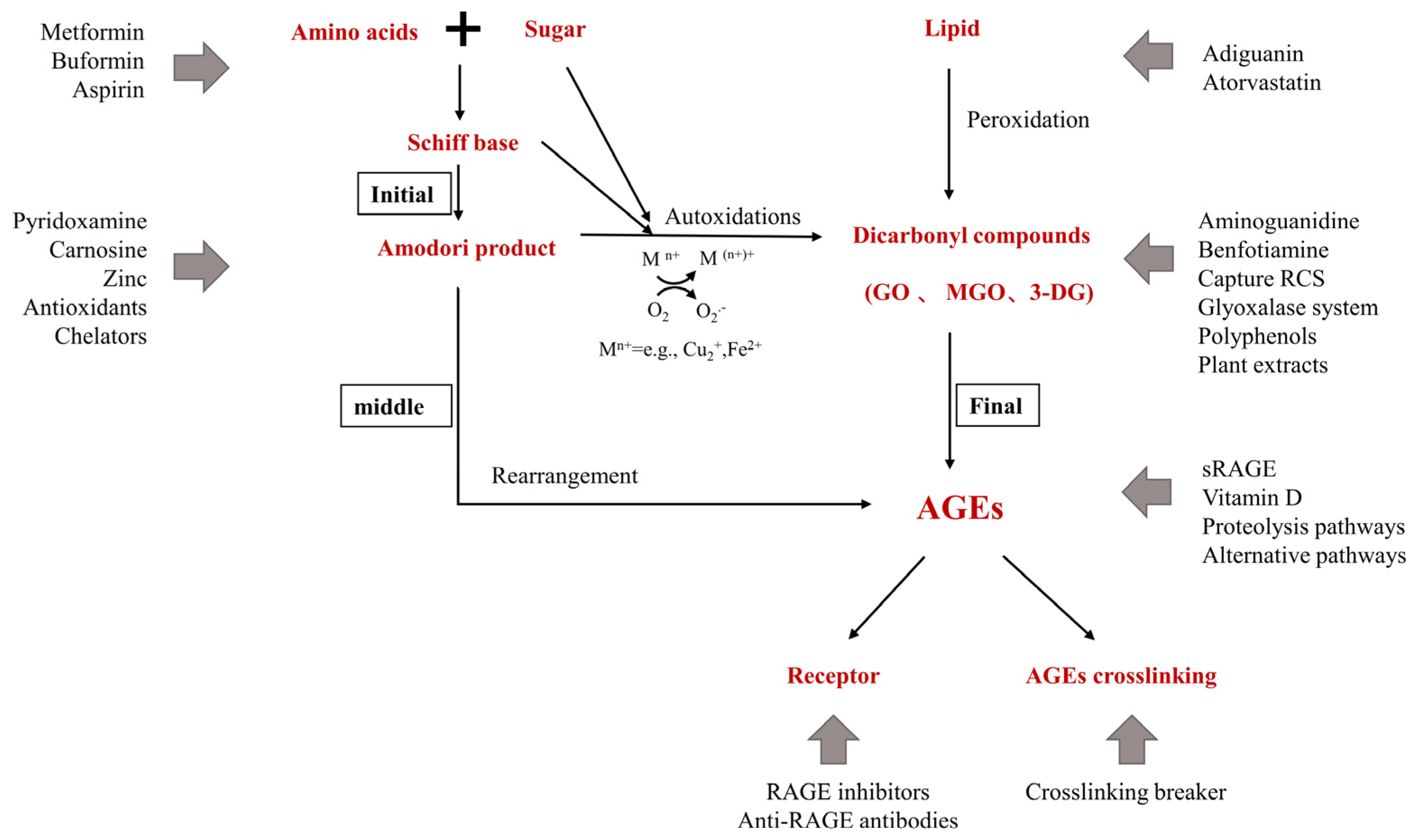
3. Inhibitors of AGEs
3.1. Pre-Amadori Inhibitors
3.2. Post-Amadori Inhibitors
3.3. Crosslinking Breaker
3.4. Indirect AGEs Inhibitors
3.5. Natural AGEs Inhibitors
3.6. Polyphenolic Compounds
3.7. Other AGEs Inhibitors
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Waqas, K.; Tan, R.C.; Voortman, T.; Ikram, M.A.; Nijsten, T.; de Groot, L.; Uitterlinden, A.G.; Zillikens, M.C. The Association between Dietary and Skin Advanced Glycation End Products: The Rotterdam Study. Am. J. Clin. Nutr. 2020, 112, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does Accumulation of Advanced Glycation End Products Contribute to the Aging Phenotype? J. Gerontol. Ser. A 2010, 65, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; van Ypersele, D.S.C.; Kurokawa, K.; Baynes, J.W. Alterations in Nonenzymatic Biochemistry in Uremia: Origin and Significance of "Carbonyl Stress" in Long-Term Uremic Complications. Kidney Int. 1999, 55, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ang, A.; Talegawkar, S.; Crasto, C.; Dalal, M.; Jardack, P.; Traber, M.G.; Ferrucci, L.; Arab, L. Dietary Intake Associated with Serum Versus Urinary Carboxymethyl-Lysine, a Major Advanced Glycation End Product, in Adults: The Energetics Study. Eur. J. Clin. Nutr. 2012, 66, 3–9. [Google Scholar] [CrossRef]
- Semba, R.D.; Najjar, S.S.; Sun, K.; Lakatta, E.G.; Ferrucci, L. Serum Carboxymethyl-Lysine, an Advanced Glycation End Product, is Associated with Increased Aortic Pulse Wave Velocity in Adults. Am. J. Hypertens. 2009, 22, 74–79. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, S.; Zhang, L.; Xiao, J.; Luo, Q.; Chen, Y.; Zhou, M.; Feng, N.; Wang, C. The Inhibitory Effect of the Catechin Structure On Advanced Glycation End Product Formation in Alcoholic Media. Food Funct. 2020, 11, 5396–5408. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Pugliese, G. Do Advanced Glycation End Products Contribute to the Development of Long-Term Diabetic Complications? Nutr. Metab. Cardiovasc. Dis. 2008, 18, 457–460. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced Glycation Endproducts--Role in Pathology of Diabetic Complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete, S.A.; Grune, T.; Simm, A. Role of Advanced Glycation End Products in Cellular Signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Ban, I.; Sugawa, H.; Nagai, R. Protein Modification with Ribose Generates N(Delta)-(5-Hydro-5-Methyl-4-Imidazolone-2-Yl)-Ornithine. Int. J. Mol. Sci. 2022, 23, 1224. [Google Scholar] [CrossRef] [PubMed]
- Kuzan, A. Toxicity of Advanced Glycation End Products (Review). Biomed. Rep. 2021, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sakasai-Sakai, A.; Takata, T.; Takino, J.I.; Koriyama, Y.; Kikuchi, C.; Furukawa, A.; Nagamine, K.; Hori, T.; Matsunaga, T. Intracellular Toxic Ages (Tage) Triggers Numerous Types of Cell Damage. Biomolecules 2021, 11, 387. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, T.L. Dietary Advanced Glycation End-Products Elicit Toxicological Effects by Disrupting Gut Microbiome and Immune Homeostasis. J. Immunotoxicol. 2021, 18, 93–104. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Bohm, M. Advanced Glycation End Products: Key Players in Skin Aging? Dermato-Endocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Selvaraj, N.; Bobby, Z.; Sridhar, M.G. Oxidative Stress: Does It Play a Role in the Genesis of Early Glycated Proteins? Med. Hypotheses 2008, 70, 265–268. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Bialkowska, A.M.; Koziolkiewicz, M. Advanced Glycation End-Products (Ages): Formation, Chemistry, Classification, Receptors, and Diseases Related to Ages. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Asgharpour, D.F.; Ranjkesh, Z.; Goodarzi, M.T. A Systematic Review of Antiglycation Medicinal Plants. Diabetes Metab. Syndr.-Clin. Res. Rev. 2019, 13, 1225–1229. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bugel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced Glycation Endproducts in Food and their Effects On Health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Piarulli, F.; Sartore, G.; Lapolla, A. Glyco-Oxidation and Cardiovascular Complications in Type 2 Diabetes: A Clinical Update. Acta Diabetol. 2013, 50, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Del, C.M.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macias-Cervantes, M.H.; Markowicz, B.D.; Medrano, A.; Menini, T.; et al. Dietary Advanced Glycation End Products and their Role in Health and Disease. Adv. Nutr. Int. Rev. J. 2015, 6, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Coughlan, M. Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T. Effect of Diet-Derived Advanced Glycation End Products On Inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.D.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced Glycation End Products and Rage: A Common Thread in Aging, Diabetes, Neurodegeneration, and Inflammation. Glycobiology 2005, 15, 16R–28R. [Google Scholar] [CrossRef]
- Nam, H.K.; Jeong, S.R.; Pyo, M.C.; Ha, S.K.; Nam, M.H.; Lee, K.W. Methylglyoxal-Derived Advanced Glycation End Products (Age4) Promote Cell Proliferation and Survival in Renal Cell Carcinoma Cells through the Rage/Akt/Erk Signaling Pathways. Biol. Pharm. Bull. 2021, 44, 1697–1706. [Google Scholar] [CrossRef]
- Basta, G.; Lazzerini, G.; Del, T.S.; Ratto, G.M.; Schmidt, A.M.; De Caterina, R. At Least 2 Distinct Pathways Generating Reactive Oxygen Species Mediate Vascular Cell Adhesion Molecule-1 Induction by Advanced Glycation End Products. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1401–1407. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Links, T.P.; Graaff, R.; Hoogenberg, K.; Lefrandt, J.D.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Increased Accumulation of Skin Advanced Glycation End-Products Precedes and Correlates with Clinical Manifestation of Diabetic Neuropathy. Diabetologia 2005, 48, 1637–1644. [Google Scholar] [CrossRef]
- Zeng, C.; Li, Y.; Ma, J.; Niu, L.; Tay, F.R. Clinical/Translational Aspects of Advanced Glycation End-Products. Trends Endocrinol. Metab. 2019, 30, 959–973. [Google Scholar] [CrossRef]
- Ruiz, H.H.; Ramasamy, R.; Schmidt, A.M. Advanced Glycation End Products: Building On the Concept of the "Common Soil" in Metabolic Disease. Endocrinology 2020, 161. [Google Scholar] [CrossRef]
- Braley, A.; Kwak, T.; Jules, J.; Harja, E.; Landgraf, R.; Hudson, B.I. Regulation of Receptor for Advanced Glycation End Products (Rage) Ectodomain Shedding and its Role in Cell Function. J. Biol. Chem. 2016, 291, 12057–12073. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Przywara-Chowaniec, B.; Damasiewicz-Bodzek, A.; Blachut, D.; Nowalany-Kozielska, E.; Tyrpien-Golder, K. Advanced Glycation End-Products (Ages) and their Soluble Receptor (Srage) in Women Suffering From Systemic Lupus Erythematosus (Sle). Cells 2021, 10, 3523. [Google Scholar] [CrossRef] [PubMed]
- Damasiewicz-Bodzek, A.; Labuz-Roszak, B.; Kumaszka, B.; Tadeusiak, B.; Tyrpien-Golder, K. The Assessment of Serum Concentrations of Ages and their Soluble Receptor (Srage) in Multiple Sclerosis Patients. Brain Sci. 2021, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Aghabozorg, A.S.; Omrani, M.D.; Arsang-Jang, S.; Ganji, M.; Noroozi, R.; Taheri, M.; Ghafouri-Fard, S. Soluble Receptor for Advanced Glycation End Products (Srage) is Up-Regulated in Multiple Sclerosis Patients Treated with Interferon Beta-1a. Cell Physiol Biochem. 2018, 46, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Shubbar, E.; Vegfors, J.; Carlstrom, M.; Petersson, S.; Enerback, C. Psoriasin (S100a7) Increases the Expression of Ros and Vegf and Acts through Rage to Promote Endothelial Cell Proliferation. Breast Cancer Res. Treat. 2012, 134, 71–80. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (Ages): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. Age-Receptor-1 Counteracts Cellular Oxidant Stress Induced by Ages Via Negative Regulation of P66Shc-Dependent Fkhrl1 Phosphorylation. Am. J. Physiol. Physiol. 2008, 294, C145–C152. [Google Scholar] [CrossRef]
- Saeed, M.; Kausar, M.A.; Singh, R.; Siddiqui, A.J.; Akhter, A. The Role of Glyoxalase in Glycation and Carbonyl Stress Induced Metabolic Disorders. Curr. Protein Pept. Sci. 2020, 21, 846–859. [Google Scholar] [CrossRef]
- Aragones, G.; Rowan, S.; Francisco, S.G.; Whitcomb, E.A.; Yang, W.; Perini-Villanueva, G.; Schalkwijk, C.G.; Taylor, A.; Bejarano, E. The Glyoxalase System in Age-Related Diseases: Nutritional Intervention as Anti-Ageing Strategy. Cells 2021, 10, 1852. [Google Scholar] [CrossRef]
- Radjei, S.; Gareil, M.; Moreau, M.; Leblanc, E.; Schnebert, S.; Friguet, B.; Nizard, C.; Petropoulos, I. The Glyoxalase Enzymes are Differentially Localized in Epidermis and Regulated During Ageing and Photoageing. Exp. Dermatol. 2016, 25, 492–494. [Google Scholar] [CrossRef]
- Rowan, S.; Bejarano, E.; Taylor, A. Mechanistic Targeting of Advanced Glycation End-Products in Age-Related Diseases. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 3631–3643. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Shekhtman, A.; Schmidt, A.M. The Multiple Faces of Rage--Opportunities for Therapeutic Intervention in Aging and Chronic Disease. Expert Opin. Ther. Targets 2016, 20, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Jahan, H.; Choudhary, M.I. Glycation, Carbonyl Stress and Ages Inhibitors: A Patent Review. Expert Opin. Ther. Pat. 2015, 25, 1267–1284. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging-From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.; Deng, Y.; Liu, P.; Yang, H.; Du, X.; Du, Y. Polygoni Multiflori Radix Preparat Delays Skin Aging by Inducing Mitophagy. BioMed Res. Int. 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Arnold, F.; Peno-Mazzarino, L.; Bouzoud, D.; Luu, M.T.; Lati, E.; Mercier, M. Glycation Induction and Antiglycation Activity of Skin Care Ingredients On Living Human Skin Explants. Int. J. Cosmet. Sci. 2011, 33, 366–370. [Google Scholar] [CrossRef]
- Danby, F.W. Nutrition and Aging Skin: Sugar and Glycation. Clin. Dermatol. 2010, 28, 409–411. [Google Scholar] [CrossRef]
- Cibrian, D.; de la Fuente, H.; Sánchez-Madrid, F. Metabolic Pathways that Control Skin Homeostasis and Inflammation. Trends Mol. Med. 2020, 26, 975–986. [Google Scholar] [CrossRef]
- Devarakonda, K.; Mobbs, C.V. Mechanisms and Significance of Brain Glucose Signaling in Energy Balance, Glucose Homeostasis, and Food-Induced Reward. Mol. Cell. Endocrinol. 2016, 438, 61–69. [Google Scholar] [CrossRef]
- Quondamatteo, F. Skin and Diabetes Mellitus: What Do we Know? Cell Tissue Res. 2014, 355, 1–21. [Google Scholar] [CrossRef]
- Argyropoulos, A.J.; Robichaud, P.; Balimunkwe, R.M.; Fisher, G.J.; Hammerberg, C.; Yan, Y.; Quan, T. Alterations of Dermal Connective Tissue Collagen in Diabetes: Molecular Basis of Aged-Appearing Skin. PLoS ONE 2016, 11, e153806. [Google Scholar] [CrossRef] [PubMed]
- Mentink, C.J.; Hendriks, M.; Levels, A.A.; Wolffenbuttel, B.H. Glucose-Mediated Cross-Linking of Collagen in Rat Tendon and Skin. Clin. Chim. Acta 2002, 321, 69–76. [Google Scholar] [CrossRef]
- Van Putte, L.; De Schrijver, S.; Moortgat, P. The Effects of Advanced Glycation End Products (Ages) On Dermal Wound Healing and Scar Formation: A Systematic Review. Scars Burn. Heal. 2016, 2, 1011166500. [Google Scholar] [CrossRef]
- Shu, F.; Gao, H.; Wu, W.; Yu, S.; Zhang, L.; Liu, H.; Xiao, S.; Xia, Z.; Zheng, Y. Amniotic Epithelial Cells Accelerate Diabetic Wound Healing by Protecting Keratinocytes and Fibroblasts From High-Glucose-Induced Senescence. Cell Biol. Int. 2022, 46, 755–770. [Google Scholar] [CrossRef]
- Sruthi, C.R.; Raghu, K.G. Advanced Glycation End Products and their Adverse Effects: The Role of Autophagy. J. Biochem. Mol. Toxicol. 2021, 35, e22710. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G.; Baynes, J.W.; Hudson, B.G. Amadorins: Novel Post-Amadori Inhibitors of Advanced Glycation Reactions. Biochem. Biophys. Res. Commun. 1999, 257, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T.; Kato, Y. Protective Role of Antioxidative Food Factors in Oxidative Stress Caused by Hyperglycemia. Ann. N. Y. Acad. Sci. 2005, 1043, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Yevdokimova, N.Y. High Glucose-Induced Alterations of Extracellular Matrix of Human Skin Fibroblasts are Not Dependent On Tsp-1-Tgfbeta1 Pathway. J. Diabetes Complicat. 2003, 17, 355–364. [Google Scholar] [CrossRef]
- Bian, X.; Li, B.; Yang, J.; Ma, K.; Sun, M.; Zhang, C.; Fu, X. Regenerative and Protective Effects of Dmsc-Sevs On High-Glucose-Induced Senescent Fibroblasts by Suppressing Rage Pathway and Activating Smad Pathway. Stem Cell Res. Ther. 2020, 11, 166. [Google Scholar] [CrossRef]
- Li, B.; Bian, X.; Hu, W.; Wang, X.; Li, Q.; Wang, F.; Sun, M.; Ma, K.; Zhang, C.; Chang, J.; et al. Regenerative and Protective Effects of Calcium Silicate On Senescent Fibroblasts Induced by High Glucose. Wound Repair Regen. 2020, 28, 315–325. [Google Scholar] [CrossRef]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Pawaputanon, N.M.C.; Kido, D.; Takeda, K.; Izumi, Y. High Glucose-Induced Oxidative Stress Impairs Proliferation and Migration of Human Gingival Fibroblasts. PLoS ONE 2018, 13, e201855. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Y.; Wang, B.; Yuan, X.; Fang, B. High Levels of Glucose Induced the Caspase-3/Parp Signaling Pathway, Leading to Apoptosis in Human Periodontal Ligament Fibroblasts. Cell Biophys. 2013, 66, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Soydas, T.; Sayitoglu, M.; Sarac, E.Y.; Cinar, S.; Solakoglu, S.; Tiryaki, T.; Sultuybek, G.K. Metformin Reverses the Effects of High Glucose On Human Dermal Fibroblasts of Aged Skin via Downregulating Rela/P65 Expression. J. Physiol. Biochem. 2021, 77, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, H.; Jiang, S.; Xu, Y. Microrna-495 Inhibits the High Glucose-Induced Inflammation, Differentiation and Extracellular Matrix Accumulation of Cardiac Fibroblasts through Downregulation of Nod1. Cell. Mol. Biol. Lett. 2018, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Yue, E.; Yu, Y.; Wang, X.; Liu, B.; Bai, Y.; Yang, B. Anthocyanin Protects Cardiac Function and Cardiac Fibroblasts From High-Glucose Induced Inflammation and Myocardial Fibrosis by Inhibiting Il-17. Front. Pharmacol. 2020, 11, 593633. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.C.; Fu, M.M.; Yang, T.S.; Fu, E.; Chiang, C.Y.; Tu, H.P.; Chin, Y.T.; Lin, F.G.; Shih, K.C. Effect of High Glucose, Porphyromonas Gingivalis Lipopolysaccharide and Advanced Glycation End-Products On Production of Interleukin-6/-8 by Gingival Fibroblasts. J. Periodont. Res. 2017, 52, 268–276. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, E.T.; Lim, J.M.; Park, S.G. Development of the Facial Glycation Imaging System for in Situ Human Face Skin Glycation Index Measurement. J. Cosmet. Dermatol. 2021, 20, 2963–2968. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Katta, R. Sugar Sag: Glycation and the Role of Diet in Aging Skin. Ski. Ther. Lett. 2015, 20, 1–5. [Google Scholar]
- Da, M.S.C.; Webb, M.; Waller, H.; Khunti, K.; Davies, M. Skin Autofluorescence, a Non-Invasive Marker of Advanced Glycation End Products: Clinical Relevance and Limitations. Postgrad. Med. J. 2017, 93, 289–294. [Google Scholar] [CrossRef]
- Smit, A.J.; van de Zande, S.C.; Mulder, D.J. Skin Autofluorescence as Tool for Cardiovascular and Diabetes Risk Prediction. Curr. Opin. Nephrol. Hypertens. 2022, 31, 522–526. [Google Scholar] [CrossRef]
- André, A.; Touré, A.K.; Stien, D.; Eparvier, V. 2,5-Diketopiperazines Mitigate the Amount of Advanced Glycation End Products Accumulated with Age in Human Dermal Fibroblasts. Int. J. Cosmet. Sci. 2020, 42, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Low, E.; Alimohammadiha, G.; Smith, L.A.; Costello, L.F.; Przyborski, S.A.; von Zglinicki, T.; Miwa, S. How Good is the Evidence that Cellular Senescence Causes Skin Ageing? Ageing Res. Rev. 2021, 71, 101456. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, M.; Lemma, T.; Calcagnile, A.; Proietti, D.S.L.; Dogliotti, E. Cell Type and Dna Damage Specific Response of Human Skin Cells to Environmental Agents. Mutat. Res. Mol. Mech. Mutagen. 2007, 614, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, M.; Yamamoto, Y.; Kitayama, Y.; Higuchi, K.; Fujimura, T.; Hase, T.; Yamamoto, H. Epidermal Expression of Receptor for Advanced Glycation End Products (Rage) is Related to Inflammation and Apoptosis in Human Skin. Exp. Dermatol. 2016, 25, 235–237. [Google Scholar] [CrossRef]
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.X.; Ran, J.M.; Yan, L. Involvement of Rage, Mapk and Nf-Kappab Pathways in Ages-Induced Mmp-9 Activation in Hacat Keratinocytes. Exp. Dermatol. 2012, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Tian, M. Effects of Advanced Glycation End-Products (Ages) On Skin Keratinocytes by Nuclear Factor-Kappa B (Nf-Κb) Activation. Afr. J. Biotechnol. 2012, 11, 11132–11142. [Google Scholar] [CrossRef][Green Version]
- Farrar, M.D. Advanced Glycation End Products in Skin Ageing and Photoageing: What are the Implications for Epidermal Function? Exp. Dermatol. 2016, 25, 947–948. [Google Scholar] [CrossRef]
- Yumnam, S.; Subedi, L.; Kim, S.Y. Glyoxalase System in the Progression of Skin Aging and Skin Malignancies. Int. J. Mol. Sci. 2020, 22, 310. [Google Scholar] [CrossRef]
- Reichert, O.; Fleming, T.; Neufang, G.; Schmelz, M.; Genth, H.; Kaever, V.; Wenck, H.; Stab, F.; Terstegen, L.; Kolbe, L.; et al. Impaired Glyoxalase Activity is Associated with Reduced Expression of Neurotrophic Factors and Pro-Inflammatory Processes in Diabetic Skin Cells. Exp. Dermatol. 2017, 26, 44–50. [Google Scholar] [CrossRef]
- Pageon, H.; Bakala, H.; Monnier, V.M.; Asselineau, D. Collagen Glycation Triggers the Formation of Aged Skin in Vitro. Eur. J. Dermatol. 2007, 17, 12–20. [Google Scholar] [CrossRef]
- Okano, Y.; Masaki, H.; Sakurai, H. Dysfunction of Dermal Fibroblasts Induced by Advanced Glycation End-Products (Ages) and the Contribution of a Nonspecific Interaction with Cell Membrane and Ages. J. Dermatol. Sci. 2002, 29, 171–180. [Google Scholar] [CrossRef]
- Guarneri, F.; Custurone, P.; Papaianni, V.; Gangemi, S. Involvement of Rage and Oxidative Stress in Inflammatory and Infectious Skin Diseases. Antioxidants 2021, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, Y.; Huang, Y.; Chen, J.; Gong, Z.; Li, Y.; Lu, C.; Lai, W.; Xu, Q. Cathepsin D Contributes to the Accumulation of Advanced Glycation End Products During Photoaging. J. Dermatol. Sci. 2018, 90, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations On Human Health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis. 2018, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H. Reaction of Glycation and Human Skin: The Effects On the Skin and its Components, Reconstructed Skin as a Model. Pathol. Biol. 2010, 58, 226–231. [Google Scholar] [CrossRef]
- Jaisson, S.; Gillery, P. Methods to Assess Advanced Glycation End-Products. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 411–415. [Google Scholar] [CrossRef]
- Sjoberg, J.S.; Bulterijs, S. Characteristics, Formation, and Pathophysiology of Glucosepane: A Major Protein Cross-Link. Rejuvenation Res. 2009, 12, 137–148. [Google Scholar] [CrossRef]
- Pennacchi, P.C.; de Almeida, M.E.; Gomes, O.L.; Faiao-Flores, F.; de Araujo, C.M.; Dos, S.M.; de Moraes, B.S.; Maria-Engler, S.S. Glycated Reconstructed Human Skin as a Platform to Study the Pathogenesis of Skin Aging. Tissue Eng. Part A 2015, 21, 2417–2425. [Google Scholar] [CrossRef]
- Han, A.R.; Nam, M.H.; Lee, K.W. Plantamajoside Inhibits Uvb and Advanced Glycation End Products-Induced Mmp-1 Expression by Suppressing the Mapk and Nf-Kappab Pathways in Hacat Cells. Photochem. Photobiol. 2016, 92, 708–719. [Google Scholar] [CrossRef]
- Crisan, M.; Taulescu, M.; Crisan, D.; Cosgarea, R.; Parvu, A.; Catoi, C.; Drugan, T. Expression of Advanced Glycation End-Products On Sun-Exposed and Non-Exposed Cutaneous Sites During the Ageing Process in Humans. PLoS ONE 2013, 8, e75003. [Google Scholar] [CrossRef] [PubMed]
- Okano, Y.; Masaki, H.; Sakurai, H. Pentosidine in Advanced Glycation End-Products (Ages) During Uva Irradiation Generates Active Oxygen Species and Impairs Human Dermal Fibroblasts. J. Dermatol. Sci. 2001, 27 (Suppl. 1), S11–S18. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting Against Skin Aging: The Way From Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Zucchi, H.; Ricois, S.; Bastien, P.; Asselineau, D. Uva Exposure Combined with Glycation of the Dermis are Two Catalysts for Skin Aging and Promotes a Favorable Environment to the Appearance of Elastosis. J. Aging Res. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced Glycation End Products (Ages) Promote Melanogenesis through Receptor for Ages. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef]
- Sanajou, D.; Ghorbani, H.A.; Argani, H.; Aslani, S. Age-Rage Axis Blockade in Diabetic Nephropathy: Current Status and Future Directions. Eur. J. Pharmacol. 2018, 833, 158–164. [Google Scholar] [CrossRef]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A Multimodal Rage-Specific Inhibitor Reduces Amyloid Beta-Mediated Brain Disorder in a Mouse Model of Alzheimer Disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef]
- Burstein, A.H.; Sabbagh, M.; Andrews, R.; Valcarce, C.; Dunn, I.; Altstiel, L. Development of Azeliragon, an Oral Small Molecule Antagonist of the Receptor for Advanced Glycation Endproducts, for the Potential Slowing of Loss of Cognition in Mild Alzheimer’s Disease. J. Prev. Alzheimer′s Dis. 2018, 5, 149–154. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Rabbani, P.; Egana-Gorrono, L.; Quadri, N.; Frye, L.; Zhou, B.; Reverdatto, S.; Ramirez, L.S.; Dansereau, S.; Pan, J.; et al. Small-Molecule Antagonism of the Interaction of the Rage Cytoplasmic Domain with Diaph1 Reduces Diabetic Complications in Mice. Sci. Transl. Med. 2021, 13, f7084. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.S.; Alouffi, S.; Khan, S.; Khan, M.; Akashah, R.; Faisal, M.; Shahab, U. Gold Nanoparticle-Bioconjugated Aminoguanidine Inhibits Glycation Reaction: An in Vivo Study in a Diabetic Animal Model. BioMed Res. Int. 2021, 2021, 5591851. [Google Scholar] [CrossRef]
- Degenhardt, T.P.; Fu, M.X.; Voss, E.; Reiff, K.; Neidlein, R.; Strein, K.; Thorpe, S.R.; Baynes, J.W.; Reiter, R. Aminoguanidine Inhibits Albuminuria, but Not the Formation of Advanced Glycation End-Products in Skin Collagen of Diabetic Rats. Diabetes Res. Clin. Pr. 1999, 43, 81–89. [Google Scholar] [CrossRef]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase Iia Clinical Trial. J. Alzheimer′s Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G.; Chen, Y.; Wassenberg, J.J. Post-Amadori Age Inhibition as a Therapeutic Target for Diabetic Complications: A Rational Approach to Second-Generation Amadorin Design. Ann. N. Y. Acad. Sci. 2005, 1043, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Adrover, M.; Vilanova, B.; Frau, J.; Munoz, F.; Donoso, J. The Pyridoxamine Action On Amadori Compounds: A Reexamination of its Scavenging Capacity and Chelating Effect. Bioorganic Med. Chem. 2008, 16, 5557–5569. [Google Scholar] [CrossRef] [PubMed]
- Ramis, R.; Ortega-Castro, J.; Caballero, C.; Casasnovas, R.; Cerrillo, A.; Vilanova, B.; Adrover, M.; Frau, J. How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of its Primary Antioxidant Activity. Antioxidants 2019, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Voziyan, P.A.; Hudson, B.G. Pyridoxamine: The Many Virtues of a Maillard Reaction Inhibitor. Ann. New York Acad. Sci. 2005, 1043, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of Advanced Glycation End Product (Age) Formation and Accumulation. Handb. Exp. Pharmacol. 2021, 264, 395–423. [Google Scholar] [CrossRef]
- Lunceford, N.; Gugliucci, A. Ilex Paraguariensis Extracts Inhibit Age Formation More Efficiently than Green Tea. Fitoterapia 2005, 76, 419–427. [Google Scholar] [CrossRef]
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard Reaction and Age Breakers as Therapeutics for Multiple Diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef]
- Yagi, M.; Inoue, K.; Sato, Y.; Ishizaki, K.; Sakiyama, C.; Yonei, Y. Antiglycative Effect of Black Galangal, Kaempferia Parviflora Wall. Ex. Baker (Zingiberaceae). Glycative Stress Res. 2021, 8, 1–7. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally Occurring Inhibitors Against the Formation of Advanced Glycation End-Products. Food Funct. 2011, 2, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, K.; Matsui, T.; Inoue, H. A Novel Pleiotropic Effect of Atorvastatin On Advanced Glycation End Product (Age)-Related Disorders. Med. Hypotheses 2007, 69, 338–340. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Metformin Inhibits Advanced Glycation End Products (Ages)-Induced Renal Tubular Cell Injury by Suppressing Reactive Oxygen Species Generation Via Reducing Receptor for Ages (Rage) Expression. Horm. Metab. Res. 2012, 44, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Kiho, T.; Kato, M.; Usui, S.; Hirano, K. Effect of Buformin and Metformin On Formation of Advanced Glycation End Products by Methylglyoxal. Clin. Chim. Acta 2005, 358, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Mesias, M.; Navarro, M.; Gokmen, V.; Morales, F.J. Antiglycative Effect of Fruit and Vegetable Seed Extracts: Inhibition of Age Formation and Carbonyl-Trapping Abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Yousof, A.M.; Jannat, S.; Mizanur, R.M. Ginsenoside Derivatives Inhibit Advanced Glycation End-Product Formation and Glucose-Fructose Mediated Protein Glycation in Vitro Via a Specific Structure-Activity Relationship. Bioorganic Chem. 2021, 111, 104844. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.A.; Kim, M.; Kum, H.; Jung, E.; Park, D. Anti-Glycation Activities of Phenolic Constituents From Silybum Marianum (Milk Thistle) Flower in Vitro and On Human Explants. Molecules 2015, 20, 3549–3564. [Google Scholar] [CrossRef]
- Chayaratanasin, P.; Adisakwattana, S.; Thilavech, T. Protective Role of Clitoria Ternatea L. Flower Extract On Methylglyoxal-Induced Protein Glycation and Oxidative Damage to Dna. Bmc Complement. Med. Ther. 2021, 21, 80. [Google Scholar] [CrossRef]
- Fernandes, A.; Vieira, N.C.; Santana, A.L.; Gandra, R.; Rubia, C.; Castro-Gamboa, I.; Macedo, J.A.; Macedo, G.A. Peanut Skin Polyphenols Inhibit Toxicity Induced by Advanced Glycation End-Products in Raw264.7 Macrophages. Food Chem. Toxicol. 2020, 145, 111619. [Google Scholar] [CrossRef]
- Ramful, D.; Tarnus, E.; Rondeau, P.; Da, S.C.; Bahorun, T.; Bourdon, E. Citrus Fruit Extracts Reduce Advanced Glycation End Products (Ages)- And H2O2-Induced Oxidative Stress in Human Adipocytes. J. Agric. Food Chem. 2010, 58, 11119–11129. [Google Scholar] [CrossRef]
- Shin, S.; Son, D.; Kim, M.; Lee, S.; Roh, K.B.; Ryu, D.; Lee, J.; Jung, E.; Park, D. Ameliorating Effect of Akebia Quinata Fruit Extracts On Skin Aging Induced by Advanced Glycation End Products. Nutrients 2015, 7, 9337–9352. [Google Scholar] [CrossRef] [PubMed]
- Suantawee, T.; Wesarachanon, K.; Anantsuphasak, K.; Daenphetploy, T.; Thien-Ngern, S.; Thilavech, T.; Pasukamonset, P.; Ngamukote, S.; Adisakwattana, S. Protein Glycation Inhibitory Activity and Antioxidant Capacity of Clove Extract. J. Food Sci. Technol. 2015, 52, 3843–3850. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, L.; Della, P.S.; Fanali, C.; Dugo, L.; De Gara, L. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted From Hazelnut Skin On Advanced Glycation End-Products (Ages) Formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Ahmad, S.; Husain, A.; Rehman, S.; Farooqui, A.; Yusuf, M.A. Glycation and Antioxidants: Hand in the Glove of Antiglycation and Natural Antioxidants. Curr. Protein Pept. Sci. 2020, 21, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, K.W.; Ma, J.; Chen, B.; Ho, C.T.; Lo, C.; Chen, F.; Wang, M. Cinnamon Bark Proanthocyanidins as Reactive Carbonyl Scavengers to Prevent the Formation of Advanced Glycation Endproducts. J. Agric. Food Chem. 2008, 56, 1907–1911. [Google Scholar] [CrossRef]
- Khedher, M.R.B.; Hafsa, J.; Haddad, M.; Hammami, M. Inhibition of Protein Glycation by Combined Antioxidant and Antiglycation Constituents from a Phenolic Fraction of Sage (Salvia officinalis L.). Plant Foods Hum. Nutr. 2020, 75, 505–511. [Google Scholar] [CrossRef]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and Neuroprotective Activity of Colon-Derived Polyphenol Catabolites. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S35–S43. [Google Scholar] [CrossRef]
- Liu, W.; Ma, H.; Frost, L.; Yuan, T.; Dain, J.A.; Seeram, N.P. Pomegranate Phenolics Inhibit Formation of Advanced Glycation Endproducts by Scavenging Reactive Carbonyl Species. Food Funct. 2014, 5, 2996–3004. [Google Scholar] [CrossRef]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory Effect of Phenolic Compounds and Plant Extracts On the Formation of Advance Glycation End Products: A Comprehensive Review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef]
- Edeas, M.; Attaf, D.; Mailfert, A.S.; Nasu, M.; Joubet, R. Maillard Reaction, Mitochondria and Oxidative Stress: Potential Role of Antioxidants. Pathol. Biol. 2010, 58, 220–225. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Sahebkar, A.; Zabetian-Targhi, F.; Maleki, V. The Impact of Resveratrol On Toxicity and Related Complications of Advanced Glycation End Products: A Systematic Review. BioFactors 2019, 45, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Khangholi, S.; Majid, F.A.; Berwary, N.J.; Ahmad, F.; Aziz, R.B. The Mechanisms of Inhibition of Advanced Glycation End Products Formation through Polyphenols in Hyperglycemic Condition. Planta Med. 2016, 82, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H. Anti-Glycative Effects of Asiatic Acid in Human Keratinocyte Cells. BioMedicine 2014, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Alouffi, S.; Khan, S.; Khan, M.; Akasha, R.; Ashraf, J.M.; Farhan, M.; Shahab, U.; Khan, M.Y. Physicochemical Characterization of in Vitro Ldl Glycation and its Inhibition by Ellagic Acid (Ea): An in Vivo Approach to Inhibit Diabetes in Experimental Animals. BioMed Res. Int. 2022, 2022, 5583298. [Google Scholar] [CrossRef]
- Abbas, G.; Al-Harrasi, A.S.; Hussain, H.; Hussain, J.; Rashid, R.; Choudhary, M.I. Antiglycation Therapy: Discovery of Promising Antiglycation Agents for the Management of Diabetic Complications. Pharm. Biol. 2016, 54, 198–206. [Google Scholar] [CrossRef]
- Khan, M.; Otaibi, A.A.; Alsukaibi, A.; Alshammari, E.M.; Al-Zahrani, S.A.; Sherwani, S.; Khan, W.A.; Saha, R.; Verma, S.R.; Ahmed, N. Biophysical, Biochemical, and Molecular Docking Investigations of Anti-Glycating, Antioxidant, and Protein Structural Stability Potential of Garlic. Molecules 2022, 27, 1868. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Shahab, U.; Tabrez, S.; Lee, E.J.; Choi, I.; Ahmad, S. Quercetin as a Finer Substitute to Aminoguanidine in the Inhibition of Glycation Products. Int. J. Biol. Macromol. 2015, 77, 188–192. [Google Scholar] [CrossRef]
- Alam, M.M.; Ahmad, I.; Naseem, I. Inhibitory Effect of Quercetin in the Formation of Advance Glycation End Products of Human Serum Albumin: An in Vitro and Molecular Interaction Study. Int. J. Biol. Macromol. 2015, 79, 336–343. [Google Scholar] [CrossRef]
- Chen, X.Y.; Huang, I.M.; Hwang, L.S.; Ho, C.T.; Lo, C.Y. Anthocyanins in Blackcurrant Effectively Prevent the Formation of Advanced Glycation End Products by Trapping Methylglyoxal. J. Funct. Foods 2014, 8, 259–268. [Google Scholar] [CrossRef]
- Meeprom, A.; Sompong, W.; Chan, C.B.; Adisakwattana, S. Isoferulic Acid, a New Anti-Glycation Agent, Inhibits Fructose- and Glucose-Mediated Protein Glycation in Vitro. Molecules 2013, 18, 6439–6454. [Google Scholar] [CrossRef]
- Arfin, S.; Siddiqui, G.A.; Naeem, A.; Moin, S. Inhibition of Advanced Glycation End Products by Isoferulic Acid and its Free Radical Scavenging Capacity: An in Vitro and Molecular Docking Study. Int. J. Biol. Macromol. 2018, 118, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-Anun, S. Cinnamic Acid and its Derivatives Inhibit Fructose-Mediated Protein Glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, R.; Kheirouri, S. Carnosine and Advanced Glycation End Products: A Systematic Review. Amino Acids 2018, 50, 1177–1186. [Google Scholar] [CrossRef]
- Narda, M.; Peno-Mazzarino, L.; Krutmann, J.; Trullas, C.; Granger, C. Novel Facial Cream Containing Carnosine Inhibits Formation of Advanced Glycation End-Products in Human Skin. Ski. Pharmacol. Physiol. 2018, 31, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Kheirouri, S.; Alizadeh, M. Vitamin D and Advanced Glycation End Products and their Receptors. Pharmacol. Res. 2020, 158, 104879. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M.; Maleki, V. Zinc Against Advanced Glycation End Products. Clin. Exp. Pharmacol. Physiol. 2018, 45, 491–498. [Google Scholar] [CrossRef]
- Dos, S.C.; Talpo, T.C.; Motta, B.P.; Kaga, A.K.; Baviera, A.M.; Castro, R.N.; Da, S.V.; de Sousa-Junior, P.T.; Wessjohann, L.; de Carvalho, M.G. New Compounds of Siolmatra Brasiliensis and Inhibition of in Vitro Protein Glycation Damage. Fitoterapia 2019, 133, 109–119. [Google Scholar] [CrossRef]
- Lin, H.; Lin, T.Y.; Lin, J.A.; Cheng, K.C.; Santoso, S.P.; Chou, C.H.; Hsieh, C.W. Effect of Pholiota Nameko Polysaccharides Inhibiting Methylglyoxal-Induced Glycation Damage in Vitro. Antioxidants 2021, 10, 1589. [Google Scholar] [CrossRef]
- Sri, H.P.; Lavelli, V.; Scarafoni, A. Protective Ability of Phenolics From White Grape Vinification by-Products Against Structural Damage of Bovine Serum Albumin Induced by Glycation. Food Chem. 2014, 156, 220–226. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Thilavech, T.; Chusak, C. Mesona Chinensis Benth Extract Prevents Age Formation and Protein Oxidation Against Fructose-Induced Protein Glycation in Vitro. BMC Complement. Altern. Med. 2014, 14, 130. [Google Scholar] [CrossRef]
- Senevirathne, I.; Abeysekera, W.; Abeysekera, W.; Jayanath, N.Y.; Galbada, A.S.; Wijewardana, D. Antiamylase, Antiglucosidase, and Antiglycation Properties of Millets and Sorghum From Sri Lanka. Evid. -Based Complement. Altern. Med. 2021, 2021, 5834915. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, K.; Kus, P.; Fecka, I. Investigation of the Phytochemical Composition, Antioxidant Activity, and Methylglyoxal Trapping Effect of Galega Officinalis L. Herb in Vitro. Molecules 2020, 25, 5810. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.; Sabitha, K.E.; Srinivasan, P.; Shyamaladevi, C.S. Green Tea Attenuates Diabetes Induced Maillard-Type Fluorescence and Collagen Cross-Linking in the Heart of Streptozotocin Diabetic Rats. Pharmacol. Res. 2007, 55, 433–440. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients 2022, 14, 4588. https://doi.org/10.3390/nu14214588
Zheng W, Li H, Go Y, Chan XH, Huang Q, Wu J. Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients. 2022; 14(21):4588. https://doi.org/10.3390/nu14214588
Chicago/Turabian StyleZheng, Wenge, Huijuan Li, Yuyo Go, Xi Hui (Felicia) Chan, Qing Huang, and Jianxin Wu. 2022. "Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors" Nutrients 14, no. 21: 4588. https://doi.org/10.3390/nu14214588
APA StyleZheng, W., Li, H., Go, Y., Chan, X. H., Huang, Q., & Wu, J. (2022). Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients, 14(21), 4588. https://doi.org/10.3390/nu14214588





