A Review of Current Evidence on the Relationship between Phosphate Metabolism and Metabolic Syndrome
Abstract
1. Introduction
2. Search Strategy
3. The Association between Phosphate Metabolism with MetS and Its Associated Conditions
3.1. Phosphate Metabolism and MetS
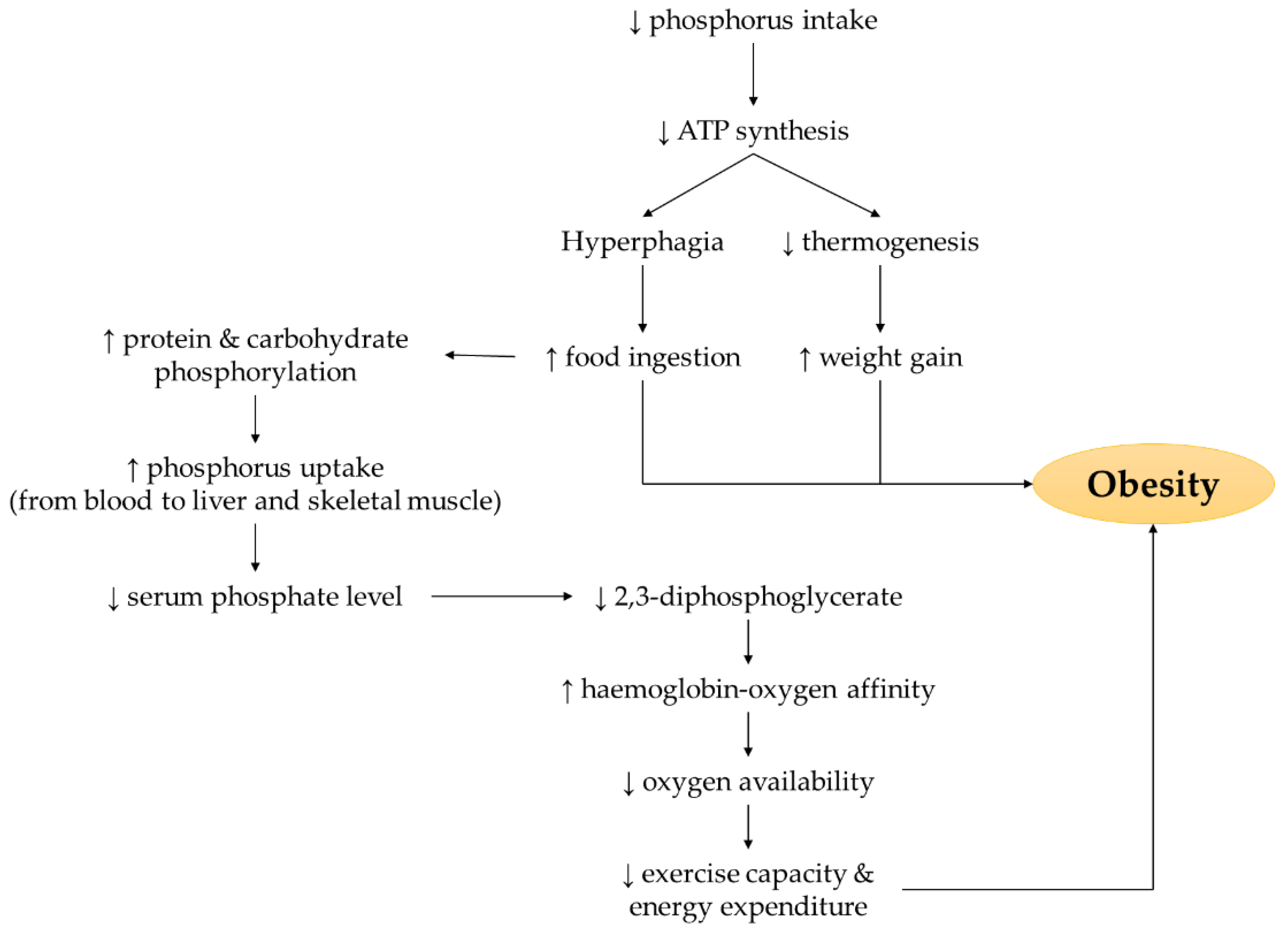
3.2. Phosphate Metabolism and Obesity
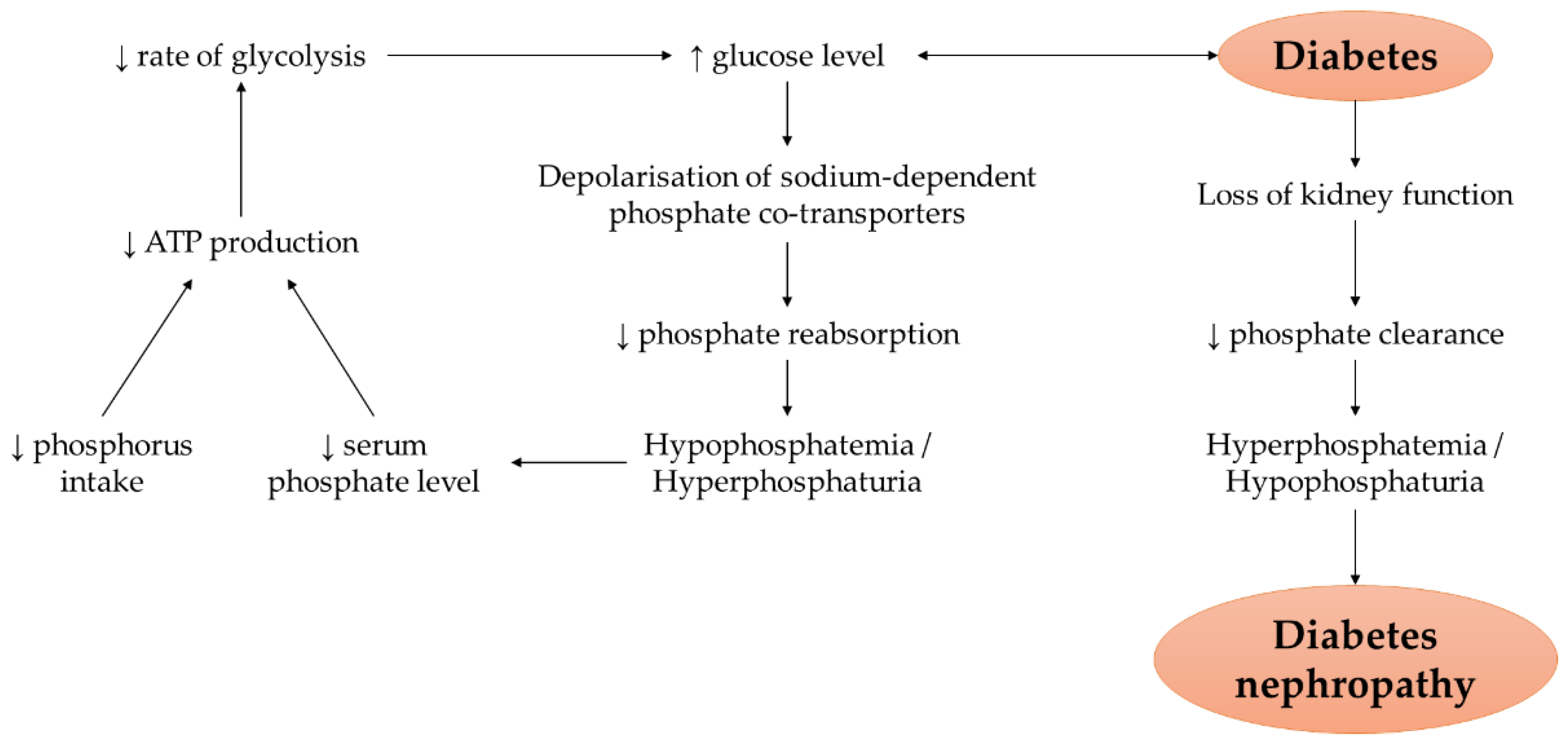
3.3. Phosphate Metabolism and Hyperglycaemia
| Researcher (Year) | Study Type | Model/Subjects | Phosphorus/Phosphate-Related Parameters | Phosphorus/Phosphate-Related Outcomes | Reference |
|---|---|---|---|---|---|
| Abuduli et al. (2016) | Animal experimentation | Male Sprague-Dawley rats | Diet containing low (0.2%), normal (0.6%), or high (1.2%) phosphate |
| [7] |
| Akter et al. (2020) | Cross-sectional study | Apparently healthy adults (n = 1657, age: 18–78 years) | Phosphorus intake (514 ± 98 mg/day (quartile 1); 519 ± 110 mg/day (quartile 2); 514 ± 100 mg/day (quartile 3); 505 ± 106 mg/day (quartile 4)) |
| [38] |
| Haap et al. (2006) | Cross-sectional and longitudinal study | Healthy, non-diabetic adults (n = 881, age: 38 ± 1 years) | Serum phosphate level (3.4 ± 0.02 mg/dL) |
| [37] |
| Håglin et al. (2001) | Cross-sectional study | Men (n = 993, age: 50.8 ± 9.4 years) and women (n = 1272, age: 50.1 ± 10.7 years) | Serum phosphate level (0.98 ± 0.21 mmol/L (men); 1.06 ± 0.22 mmol/L (women)) |
| [32] |
| Håglin et al. (2014) | Cross-sectional study | Men and women (n = 2504, age: 50.4 ± 10.1 years) | Serum phosphate level (0.98 ± 0.20 mmol/L (men); 1.05 ± 0.21 mmol/L (women)) |
| [31] |
| Hazim et al. (2014) | Pilot cross-over study | Healthy male subjects (n = 8, age: 19.25 ± 0.41 years) | Phosphorus supplementation (500 mg) |
| [39] |
| Celik & Andiran (2011) | Case control study | Normal and obese children (n = 177, age: 6–12 years) and adolescents (n = 121, age: 12–16 years) | Serum phosphate level (4.8 ± 0.4 mg/dL (obese children); 5.1 ± 0.5 mg/dL (control–children); 4.4 ± 0.5 mg/dL (obese adolescents); 4.5 ± 0.6 mg/dL (control–adolescents)) |
| [30] |
| Khattab et al. (2015) | Cross-over study | Healthy male subjects (n = 7, age: 23.22 ± 1.83 years) | Phosphorus supplementation (500 mg)–given together with glucose solution (75 g) |
| [40] |
| Healthy male subjects (n = 8, age: 27.3 ± 1.68 years) | Phosphorus supplementation (500 mg)–given 60 min prior to glucose ingestion (75 g) |
| |||
| Lorenzo et al. (2014) | Longitudinal study | Non-diabetic adults (n = 863, age: 40–69 years) | Serum phosphate level (1.01 ± 0.001 mmol/L (non-diabetes); 1.04 ± 0.01 mmol/L (diabetes)) and calcium-phosphate product (2.32 ± 0.02 mmol2/L2 (non-diabetes); 2.41 ± 0.03 mmol2/L2 (diabetes)) |
| [41] |
| Mancini et al. (2018) | Prospective cohort study | French women (n = 71270) | Dietary phosphorus intake (1477 ± 391 mg/day) |
| [42] |
| Xiang et al. (2018) | Cohort study | Patients with diabetic nephropathy (n = 591) | Serum phosphate level (1.0 ± 0.1 mmol/L (quartile 1); 1.2 ± 0.1 mmol/L (quartile 2); 1.3 ± 0.1 mmol/L (quartile 3); 1.5 ± 0.2 mmol/L (quartile 4)) |
| [46] |
| Yoon et al. (2017) | Prospective community-based cohort study | Subjects with diabetes mellitus (n = 873; aged 55.6 ± 8.7 years) and without diabetes (n = 5846; aged 51.4 ± 8.6 years) | Dietary phosphorus intake (< 0.46 mg/kcal (quartile 1); 0.46 to <0.50 mg/kcal (quartile 2); 0.50 to <0.55 mg/kcal (quartile 3); ≥0.55 mg/kcal (quartile 4)) |
| [47] |
3.4. Phosphate Metabolism and Hypertension
| Researcher (Year) | Study Type | Model/Subjects | Phosphorus/Phosphate-Related Parameters | Phosphorus/Phosphate-Related Outcomes | Reference |
|---|---|---|---|---|---|
| Suzuki et al. (2014) | Animal experimentation | Male spontaneous hypertensive rats and Wistar Kyoto rats | Control diet (0.3% phosphorus) or high phosphorus (1.2%) and zinc-free diet |
| [49] |
| Bozic et al. (2014) | Animal experimentation | Male Sprague-Dawley rats | Moderate-phosphate (0.6%) or high-phosphate (1.2%) diet |
| [51] |
| Mizuno et al. (2016) | Animal experimentation | Male Sprague-Dawley rats | Normal phosphate (0.6%) or high phosphorus (1.2%) diet |
| [50] |
| Elliott et al. (2008) | Cross-sectional study | Adult men and women (n = 4680, age: 40–59 years) | Phosphorus intake (439–662 mg/day) |
| [53] |
| Alonso et al. (2010) | Cohort study | Adult men and women free of cardiovascular diseases (n = 13,444, age: 45–84 years) | Phosphorus intake (787 mg/day (quintile 1); 968 mg/day (quintile 2); 1063 mg/day (quintile 3); 1171 mg/day (quintile 4); 1526 mg/day (quintile 5)) |
| [52] |
| Longitudinal study | Adult men and women free of cardiovascular diseases (n = 11,109, age: 45–84 years) |
| |||
| McClure et al. (2020) | Longitudinal study | Adult men and women with non-optimal blood pressure (n = 806, age: 25–79 years) | Phosphorus intake (1154 ± 408 mg/day) and excretion (937 ± 384 mg/day) |
| [54] |
| Olivo et al. (2019) | Cross-sectional study | African American adults (n = 973, age: 59.3 ± 10.8 years) | Phosphorus intake (231–801 mg/day (quartile 1); 802–1055 mg/day (quartile 2); 1056–1420 mg/day (quartile 3); 1421–3769 mg/day (quartile 4)) |
| [55] |
| Mohammad et al. (2018) | Prospective study | Young adults (low phosphate group (n = 10, age: 23.4 ± 3.4 years); high phosphate group (n = 10, age: 23.1 ± 3.2 years)) | Low phosphate (0.7 mmol/kg sodium chloride) or high phosphate (1 mmol/kg/day sodium phosphate) diet |
| [56] |
| Zhang et al. (2021) | Open-label prospective cross-over study | Young healthy male volunteers with normal nutritional status and without any medication use (n = 6, age: 29 ± 2 years) | Low (500 mg/day), normal (1500 mg/day), or high (2300 mg/day) phosphorus diet |
| [57] |
| Håglin et al. (2001) | Cross-sectional study | Men (n = 993, age: 50.8 ± 9.4 years) and women (n = 1272, age: 50.1 ± 10.7 years) | Serum phosphate level (0.98 ± 0.21 mmol/L (men); 1.06 ± 0.22 mmol/L (women)) |
| [32] |
| Kesteloot & Joossens (1988) | Epidemiological survey | Men and women (n = 8058, mean age: 49 years) | Serum phosphate level (1.05 ± 0.17 mmol/L (men); 1.08 ± 0.16 mmol/L (women)) |
| [58] |
| Huang et al. (2008) | Cross-sectional and longitudinal study | Haemodialysis patients (n = 707, age: ≥17 years) | Serum phosphate level (<4.42 mg/dL (quintile 1); 4.42–5.21 mg/dL (quintile 2); 5.21–6.07 mg/dL (quintile 3); >6.07 mg/dL (quintile 4)) |
| [59] |
| Patel et al. (2015) | Longitudinal study | Hypertensive adults (n = 9260, age: 51.7 ± 14.6 years) | Serum phosphate level (1.0 ± 0.2 mmol/L (men); 1.1 ± 0.2 mmol/L (women)) |
| [60] |
| Kanbay et al. (2007) | Cross-sectional study | Dipper (n = 76, age: 51.4 ± 13.4 years) and non-dippers (n = 114, age: 53.4 ± 12.8 years) hypertensive patients | Serum phosphate level (3.3 ± 0.4 mg/dL (dippers); 3.6 ± 0.5 mg/dL (non-dippers)) |
| [61] |
3.5. Phosphate Metabolism and Dyslipidaemia
4. Perspectives
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvo, M.S.; Lamberg-Allardt, C.J. Phosphorus. Adv. Nutr. 2015, 6, 860–862. [Google Scholar] [CrossRef]
- Peacock, M. Chapter 31—Primary Hyperparathyroidism and the Kidney. In The Parathyroids, 3rd ed.; Bilezikian, J.P., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 455–467. [Google Scholar]
- Lockitch, G.; Halstead, A.C.; Albersheim, S.; MacCallum, C.; Quigley, G. Age- and sex-specific pediatric reference intervals for biochemistry analytes as measured with the Ektachem-700 analyzer. Clin. Chem. 1988, 34, 1622–1625. [Google Scholar] [CrossRef]
- Michigami, T.; Kawai, M.; Yamazaki, M.; Ozono, K. Phosphate as a Signaling Molecule and Its Sensing Mechanism. Physiol. Rev. 2018, 98, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Meyers, L.D.; Hellwig, J.P.; Otten, J.J. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Abuduli, M.; Ohminami, H.; Otani, T.; Kubo, H.; Ueda, H.; Kawai, Y.; Masuda, M.; Yamanaka-Okumura, H.; Sakaue, H.; Yamamoto, H.; et al. Effects of dietary phosphate on glucose and lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E526–E538. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A. (Ed.) Chapter 1—Glycolysis. In Sweet Biochemistry; Academic Press: Cambridge, MA, USA, 2018; pp. 1–5. [Google Scholar]
- Adeva-Andany, M.M.; González-Lucán, M.; Donapetry-García, C.; Fernández-Fernández, C.; Ameneiros-Rodríguez, E. Glycogen metabolism in humans. Biochim. Biophys. Acta Clin. 2016, 5, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Bamba, T.; Suyama, T.; Ishijima, T.; Fukusaki, E.; Abe, K.; Nakai, Y. A High Phosphorus Diet Affects Lipid Metabolism in Rat Liver: A DNA Microarray Analysis. PLoS ONE 2016, 11, e0155386. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Han, J. Mineral Balance and Metabolic Syndrome in Adolescents: Focus on Calcium and Phosphorus Intake. Healthcare 2021, 9, 1525. [Google Scholar] [CrossRef]
- Osadnik, K.; Osadnik, T.; Delijewski, M.; Lejawa, M.; Fronczek, M.; Reguła, R.; Gąsior, M.; Pawlas, N. Calcium and Phosphate Levels are Among Other Factors Associated with Metabolic Syndrome in Patients with Normal Weight. Diabetes Metab. Syndr. Obes. 2020, 13, 1281–1288. [Google Scholar] [CrossRef]
- Shimodaira, M.; Okaniwa, S.; Nakayama, T. Reduced Serum Phosphorus Levels Were Associated with Metabolic Syndrome in Men But Not in Women: A Cross-Sectional Study among the Japanese Population. Ann. Nutr. Metab. 2017, 71, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Stoian, M.; Stoica, V. The role of distubances of phosphate metabolism in metabolic syndrome. Maedica 2014, 9, 255–260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Terzi, R.; Dindar, S.; Terzi, H.; Demirtaş, Ö. Relationships among the metabolic syndrome, bone mineral density, bone turnover markers, and hyperglycemia. Metab. Syndr. Relat. Disord. 2015, 13, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Effects of Vitamin E from Elaeis guineensis (Oil Palm) in a Rat Model of Bone Loss Due to Metabolic Syndrome. Int. J. Environ. Res. Public Health 2018, 15, 1828. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct. Foods 2018, 44, 246–254. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from Bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells. Int. J. Environ. Res. Public Health 2019, 16, 3313. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Fikri, N.I.A.; Munesveran, K.; Hisham, N.S.N.; Lau, S.H.J.; Chin, K.-Y.; Fahami, N.A.M.; Saad, Q.H.M.; Kamisah, Y.; Abdullah, A.; et al. Effects of tocotrienol on osteocyte-mediated phosphate metabolism in high-carbohydrate high-fat diet-induced osteoporotic rats. J. Funct. Foods 2022, 96, 105213. [Google Scholar] [CrossRef]
- Gattineni, J.; Friedman, P.A. Chapter Nine—Regulation of Hormone-Sensitive Renal Phosphate Transport. In Vitamins & Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 249–306. [Google Scholar]
- Park, W.; Kim, B.S.; Lee, J.E.; Huh, J.K.; Kim, B.J.; Sung, K.C.; Kang, J.H.; Lee, M.H.; Park, J.R.; Rhee, E.J.; et al. Serum phosphate levels and the risk of cardiovascular disease and metabolic syndrome: A double-edged sword. Diabetes Res. Clin. Pract. 2009, 83, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Grima, P.; Guido, M.; Chiavaroli, R.; Stano, F.; Tundo, P.; Tana, M.; de Donno, A.; Zizza, A. Altered phosphate metabolism in HIV-1-infected patients with metabolic syndrome. Scand. J. Infect. Dis. 2012, 44, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Ghanei, L.; Ziaee, A.; Rostami, P.; Oveisi, S.; Esmailzadehha, N.; Kazemifar, A.M.; Zargar, A. Association of serum 25-hydroxyvitamin d levels and vitamin D dietary intake with metabolic syndrome: A case control study. J. Res. Health Sci. 2015, 15, 32–36. [Google Scholar]
- Kalaitzidis, R.; Tsimihodimos, V.; Bairaktari, E.; Siamopoulos, K.C.; Elisaf, M. Disturbances of phosphate metabolism: Another feature of metabolic syndrome. Am. J. Kidney Dis. 2005, 45, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Vyssoulis, G.; Karpanou, E.; Tzamou, V.; Kyvelou, S.M.; Michaelidis, A.; Stefanadis, C. Serum phosphate in white-coat hypertensive patients: Focus on dipping status and metabolic syndrome. Hypertens. Res. 2010, 33, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, H.; Strand, A.H.; Kjeldsen, S.E.; Høieggen, A.; Os, I. Serum phosphate, blood pressure, and the metabolic syndrome—20-year follow-up of middle-aged men. J. Clin. Hypertens. 2008, 10, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Jhuang, Y.H.; Kao, T.W.; Peng, T.C.; Chen, W.L.; Chang, P.K.; Wu, L.W. Serum Phosphorus as a Risk Factor of Metabolic Syndrome in the Elderly in Taiwan: A Large-Population Cohort Study. Nutrients 2019, 11, 2340. [Google Scholar] [CrossRef] [PubMed]
- Zhukouskaya, V.V.; Rothenbuhler, A.; Colao, A.; Di Somma, C.; Kamenický, P.; Trabado, S.; Prié, D.; Audrain, C.; Barosi, A.; Kyheng, C.; et al. Increased prevalence of overweight and obesity in children with X-linked hypophosphatemia. Endocr. Connect. 2020, 9, 144–153. [Google Scholar] [CrossRef]
- Celik, N.; Andiran, N. The relationship between serum phosphate levels with childhood obesity and insulin resistance. J. Pediatr. Endocrinol. Metab. 2011, 24, 81–83. [Google Scholar]
- Håglin, L.M.; Törnkvist, B.; Bäckman, L.O. High serum phosphate and triglyceride levels in smoking women and men with CVD risk and type 2 diabetes. Diabetol. Metab. Syndr. 2014, 6, 39. [Google Scholar] [CrossRef]
- Håglin, L.; Lindblad, A.; Bygren, L.O. Hypophosphataemia in the metabolic syndrome. Gender differences in body weight and blood glucose. Eur. J. Clin. Nutr. 2001, 55, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, J.J.; Samra, M.J.; Hlais, S.A.; Bassil, M.S.; Obeid, O.A. Effect of phosphorus supplementation on weight gain and waist circumference of overweight/obese adults: A randomized clinical trial. Nutr. Diabetes 2015, 5, e189. [Google Scholar] [CrossRef]
- Assaad, M.; El Mallah, C.; Obeid, O. Phosphorus ingestion with a high-carbohydrate meal increased the postprandial energy expenditure of obese and lean individuals. Nutrition 2019, 57, 59–62. [Google Scholar] [CrossRef]
- Brown, R.B. Obesity and Cancer: Potential Mediation by Dysregulated Dietary Phosphate. Obesities 2022, 2, 64–75. [Google Scholar] [CrossRef]
- Obeid, O.A. Low phosphorus status might contribute to the onset of obesity. Obes. Rev. 2013, 14, 659–664. [Google Scholar] [CrossRef]
- Haap, M.; Heller, E.; Thamer, C.; Tschritter, O.; Stefan, N.; Fritsche, A. Association of serum phosphate levels with glucose tolerance, insulin sensitivity and insulin secretion in non-diabetic subjects. Eur. J. Clin. Nutr. 2006, 60, 734–739. [Google Scholar] [CrossRef]
- Akter, S.; Eguchi, M.; Kochi, T.; Kabe, I.; Nanri, A.; Mizoue, T. Association of Serum Calcium and Phosphate Concentrations with Glucose Metabolism Markers: The Furukawa Nutrition and Health Study. Nutrients 2020, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Hazim, J.; Hlais, S.; Ghattas, H.; Shatila, D.; Bassil, M.; Obeid, O. Phosphorus supplement alters postprandial lipemia of healthy male subjects: A pilot cross-over trial. Lipids Health Dis. 2014, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.; Abi-Rashed, C.; Ghattas, H.; Hlais, S.; Obeid, O. Phosphorus ingestion improves oral glucose tolerance of healthy male subjects: A crossover experiment. Nutr. J. 2015, 14, 112. [Google Scholar] [CrossRef]
- Lorenzo, C.; Hanley, A.J.; Rewers, M.J.; Haffner, S.M. Calcium and phosphate concentrations and future development of type 2 diabetes: The Insulin Resistance Atherosclerosis Study. Diabetologia 2014, 57, 1366–1374. [Google Scholar] [CrossRef]
- Mancini, F.R.; Affret, A.; Dow, C.; Balkau, B.; Clavel-Chapelon, F.; Bonnet, F.; Boutron-Ruault, M.C.; Fagherazzi, G. High dietary phosphorus intake is associated with an increased risk of type 2 diabetes in the large prospective E3N cohort study. Clin. Nutr. 2018, 37, 1625–1630. [Google Scholar] [CrossRef]
- Vorum, H.; Ditzel, J. Disturbance of Inorganic Phosphate Metabolism in Diabetes Mellitus: Its Relevance to the Pathogenesis of Diabetic Retinopathy. J. Ophthalmol. 2014, 2014, 135287. [Google Scholar] [CrossRef]
- Ditzel, J.; Lervang, H.H. Disturbance of inorganic phosphate metabolism in diabetes mellitus: Temporary therapeutic intervention trials. Diabetes Metab. Syndr. Obes. 2009, 2, 173–177. [Google Scholar]
- van der Vaart, A.; Yeung, S.M.H.; van Dijk, P.R.; Bakker, S.J.L.; de Borst, M.H. Phosphate and fibroblast growth factor 23 in diabetes. Clin. Sci. 2021, 135, 1669–1687. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhang, H.; Zhou, M.; Jiang, S.; Zhang, L.; Chen, D.; Liu, Z. Phosphorus is an independent risk factor for the progression of diabetic nephropathy. Adv. Clin. Exp. Med. 2018, 27, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.Y.; Park, J.T.; Jhee, J.H.; Noh, J.; Kee, Y.K.; Seo, C.; Lee, M.; Cha, M.U.; Kim, H.; Park, S.; et al. High dietary phosphorus density is a risk factor for incident chronic kidney disease development in diabetic subjects: A community-based prospective cohort study. Am. J. Clin. Nutr. 2017, 106, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Mizuno, M.; Vongpatanasin, W. Phosphate, the forgotten mineral in hypertension. Curr. Opin. Nephrol. Hypertens. 2019, 28, 345–351. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mitsushima, S.; Kato, A.; Yamaguchi, T.; Ichihara, S. High-phosphorus/zinc-free diet aggravates hypertension and cardiac dysfunction in a rat model of the metabolic syndrome. Cardiovasc. Pathol. 2014, 23, 43–49. [Google Scholar] [CrossRef]
- Mizuno, M.; Mitchell, J.H.; Crawford, S.; Huang, C.L.; Maalouf, N.; Hu, M.C.; Moe, O.W.; Smith, S.A.; Vongpatanasin, W. High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R39–R48. [Google Scholar] [CrossRef]
- Bozic, M.; Panizo, S.; Sevilla, M.A.; Riera, M.; Soler, M.J.; Pascual, J.; Lopez, I.; Freixenet, M.; Fernandez, E.; Valdivielso, J.M. High phosphate diet increases arterial blood pressure via a parathyroid hormone mediated increase of renin. J. Hypertens. 2014, 32, 1822–1832. [Google Scholar] [CrossRef]
- Alonso, A.; Nettleton, J.A.; Ix, J.H.; de Boer, I.H.; Folsom, A.R.; Bidulescu, A.; Kestenbaum, B.R.; Chambless, L.E.; Jacobs, D.R., Jr. Dietary phosphorus, blood pressure, and incidence of hypertension in the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Hypertension 2010, 55, 776–784. [Google Scholar] [CrossRef]
- Elliott, P.; Kesteloot, H.; Appel, L.J.; Dyer, A.R.; Ueshima, H.; Chan, Q.; Brown, I.J.; Zhao, L.; Stamler, J. Dietary phosphorus and blood pressure: International study of macro-and micro-nutrients and blood pressure. Hypertension 2008, 51, 669–675. [Google Scholar] [CrossRef]
- McClure, S.T.; Rebholz, C.M.; Mitchell, D.C.; Selvin, E.; Appel, L.J. The association of dietary phosphorus with blood pressure: Results from a secondary analysis of the PREMIER trial. J. Hum. Hypertens. 2020, 34, 132–142. [Google Scholar] [CrossRef]
- Olivo, R.E.; Hale, S.L.; Diamantidis, C.J.; Bhavsar, N.A.A.; Tyson, C.C.; Tucker, K.L.; Carithers, T.C.; Kestenbaum, B.; Muntner, P.; Tanner, R.M.; et al. Dietary Phosphorus and Ambulatory Blood Pressure in African Americans: The Jackson Heart Study. Am. J. Hypertens. 2019, 32, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, J.; Scanni, R.; Bestmann, L.; Hulter, H.N.; Krapf, R. A Controlled Increase in Dietary Phosphate Elevates BP in Healthy Human Subjects. J. Am. Soc. Nephrol. 2018, 29, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; You, H.Z.; Wang, M.J.; Zhang, Q.; Dong, X.Y.; Liu, J.F.; Chen, J. High-phosphorus diet controlled for sodium elevates blood pressure in healthy adults via volume expansion. J. Clin. Hypertens. 2021, 23, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Kesteloot, H.; Joossens, J.V. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension 1988, 12, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Plantinga, L.C.; Fink, N.E.; Melamed, M.L.; Coresh, J.; Powe, N.R. Phosphate levels and blood pressure in incident hemodialysis patients: A longitudinal study. Adv. Chronic. Kidney Dis. 2008, 15, 321–331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, R.K.; Jeemon, P.; Stevens, K.K.; McCallum, L.; Hastie, C.E.; Schneider, A.; Jardine, A.G.; Mark, P.B.; Padmanabhan, S. Association between serum phosphate and calcium, long-term blood pressure, and mortality in treated hypertensive adults. J. Hypertens. 2015, 33, 2046–2053. [Google Scholar] [CrossRef]
- Kanbay, M.; Isik, B.; Akcay, A.; Ozkara, A.; Karakurt, F.; Turgut, F.; Alkan, R.; Uz, E.; Bavbek, N.; Yigitoglu, R.; et al. Relation between serum calcium, phosphate, parathyroid hormone and ‘nondipper’ circadian blood pressure variability profile in patients with normal renal function. Am. J. Nephrol. 2007, 27, 516–521. [Google Scholar] [CrossRef]
- Simeoni, M.; Perna, A.F.; Fuiano, G. Secondary Hyperparathyroidism and Hypertension: An Intriguing Couple. J. Clin. Med. 2020, 9, 629. [Google Scholar] [CrossRef]
- Freundlich, M.; Gamba, G.; Rodriguez-Iturbe, B. Fibroblast growth factor 23—Klotho and hypertension: Experimental and clinical mechanisms. Pediatr. Nephrol. 2021, 36, 3007–3022. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Yamamoto, H.; Nakahashi, O.; Kagawa, T.; Ishiguro, M.; Masuda, M.; Kozai, M.; Ikeda, S.; Taketani, Y.; Takeda, E. Dietary phosphate restriction induces hepatic lipid accumulation through dysregulation of cholesterol metabolism in mice. Nutr. Res. 2013, 33, 586–593. [Google Scholar] [CrossRef]
- Tanaka, S.; Yamamoto, H.; Nakahashi, O.; Ishiguro, M.; Takei, Y.; Masuda, M.; Kozai, M.; Ikeda, S.; Taketani, Y.; Miyamoto, K.; et al. Hypercholesterolemia and effects of high cholesterol diet in type IIa sodium-dependent phosphate co-transporter (Npt2a) deficient mice. J. Med. Investig. 2013, 60, 191–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grundmann, S.M.; Schutkowski, A.; Berger, C.; Baur, A.C.; König, B.; Stangl, G.I. High-phosphorus diets reduce aortic lesions and cardiomyocyte size and modify lipid metabolism in Ldl receptor knockout mice. Sci. Rep. 2020, 10, 20748. [Google Scholar] [CrossRef] [PubMed]
- Ditscheid, B.; Keller, S.; Jahreis, G. Cholesterol metabolism is affected by calcium phosphate supplementation in humans. J. Nutr. 2005, 135, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004, 5, 248. [Google Scholar] [CrossRef]
- Ma, S.; Sun, W.; Gao, L.; Liu, S. Therapeutic targets of hypercholesterolemia: HMGCR and LDLR. Diabetes Metab. Syndr. Obes. 2019, 12, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, G.; Torres-Gonzalez, M.; McGrane, M.M.; Volek, J.S.; Fernandez, M.L. Carbohydrate restriction and dietary cholesterol modulate the expression of HMG-CoA reductase and the LDL receptor in mononuclear cells from adult men. Lipids Health Dis. 2007, 6, 34. [Google Scholar] [CrossRef]
- Ferré, P.; Foufelle, F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm Res. 2007, 68, 72–82. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef]
- Bilotta, M.T.; Petillo, S.; Santoni, A.; Cippitelli, M. Liver X Receptors: Regulators of Cholesterol Metabolism, Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2020, 11, 584303. [Google Scholar] [CrossRef]
- Ferré, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12 (Suppl. 2), 83–92. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Lv, Y.; Wang, S.; Hu, T.; Hong, H.; Fu, Z. PPARγ overexpression regulates cholesterol metabolism in human L02 hepatocytes. J. Pharmacol. Sci. 2019, 139, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Videla, L.A. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011, 2, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, S.O.; Borèn, J. Apolipoprotein B: A clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 2005, 258, 395–410. [Google Scholar] [CrossRef]
- Masuda, D.; Sakai, N.; Sugimoto, T.; Kitazume-Taneike, R.; Yamashita, T.; Kawase, R.; Nakaoka, H.; Inagaki, M.; Nakatani, K.; Yuasa-Kawase, M.; et al. Fasting serum apolipoprotein B-48 can be a marker of postprandial hyperlipidemia. J. Atheroscler. Thromb. 2011, 18, 1062–1070. [Google Scholar] [CrossRef]
- Ooi, T.C.; Nordestgaard, B.G. Methods to study postprandial lipemia. Curr. Vasc. Pharmacol. 2011, 9, 302–308. [Google Scholar]
- Jeon, H.; Blacklow, S.C. Structure and physiologic function of the low-density lipoprotein receptor. Annu. Rev. Biochem. 2005, 74, 535–562. [Google Scholar] [CrossRef]


| Researcher (Year) | Study Type | Model/Subjects | Definition of MetS | Phosphorus/Phosphate-Related Parameters | Phosphorus/Phosphate-Related Outcomes | Reference |
|---|---|---|---|---|---|---|
| Shimodaira et al. (2017) | Cross-sectional study | Japanese men (n = 9076) and women (n = 6965; age: ≤80 years) | Fulfilment of ≥3 components:
| Serum phosphate level (3.04 ± 0.44 mg/dL (men with MetS); 3.13 ± 0.42 mg/dL men with non-MetS); 3.53 ± 0.41 mg/dL (women with MetS); 3.54 ± 0.41 mg/dL (women with non-MetS)) |
| [13] |
| Park et al. (2009) | Cross-sectional study | Subjects without a previous medical history (n = 46798, age: ≥20 years | Fulfilment of ≥3 components:
| Serum phosphate level (3.41 ± 0.43 mg/dL (men); 3.64 ± 0.42 mg/dL (women)) |
| [22] |
| Grima et al. (2012) | Prospective, cross-sectional, single-centre study | Human immunodeficiency virus-1 (HIV-1)-infected patients (n = 121, age: >18 years) | Fulfilment of ≥3 components:
| Serum phosphate level (3.5 ± 0.6 mg/dL (control); 3.1 ± 0.6 mg/dL (MetS)) |
| [23] |
| Ghanei et al. (2015) | Case control study | Patients with MetS (n = 122, age: 46.3 ± 11.8 years) and without MetS (n = 128, age: 45.3 ± 12.3 years) | Fulfilment of ≥3 components:
| Phosphorus intake (1336.0 ± 485.5 mg/day (control); 1439 ± 372.1 mg/day (MetS)) and serum phosphate level (3.9 ± 0.3 mg/dL (control); 3.3 ± 0.2 mg/dL (MetS)) |
| [24] |
| Stoian & Stoica (2014) | Case-control study | Subjects with (n = 64, age: 48.8 years) and without MetS (n = 91, age: 48.7 years) | Fulfilment of ≥3 components:
| Serum phosphate level (3.3 ± 0.5 mg/dL (control); 3.0 ± 0.5 mg/dL (MetS)) |
| [14] |
| Kalaitzidis et al. (2005) | Case control study | Individuals with MetS (n = 64, age: 48.8 ± 11.1 years) and controls (n = 191, age: 48.7 ± 9.9 years) | Fulfilment of ≥3 components:
| Serum phosphate level (3.3 ± 0.5 mg/dL (control); 3.0 ± 0.5 mg/dL (MetS)) |
| [25] |
| Gudmundsdottir et al. (2008) | Longitudinal study | Caucasian middle-aged men (n = 56, age: 42.1 ± 0.5 years) | Characteristics of MetS subjects:
| Serum phosphate level (1.02 ± 0.13 mmol/L (normotensive); 0.86 ± 0.13 mmol/L (hypertensive)) |
| [27] |
| Vyssoulis et al. (2010) | Cohort study | White-coat hypertensive patients (n = 2600, age: ≥18 years) | Fulfilment of hypertension (blood pressure: ≥140/90 mm Hg) and at least 2 other components:
| Serum phosphate level (3.53 ± 0.36 mg/dL (1 MetS component); 3.50 ± 0.38 mg/dL (2 MetS components); 3.49 ± 0.38 mg/dL (3 MetS components); 3.44 ± 0.36 mg/dL (4 MetS components); 3.35 ± 0.31 mg/dL (5 MetS components)) |
| [26] |
| Park & Han (2021) | Cross-sectional study | Korean adolescents (n = 895, age: 12–18 years) | Fulfilment of ≥3 components:
| Phosphorus intake (1271.34 ± 594.53 mg/day (male); 938.41 ± 418.75 mg/day (female)) |
| [11] |
| Osadnik et al. (2020) | Cross-sectional study | Normal weight adults (n = 460, age: 18–35 years) | Fulfilment of ≥2 components:
| Serum phosphorus level (1.14 ± 0.15 mmol/L (non-MetS); 1.06 ± 0.18 mmol/L (MetS)) |
| [12] |
| Jhuang et al. (2019) | Cohort study | Taiwan population (n = 1491, age: 29.26 ± 5.57 years (group 1); 49.21 ± 5.64 years (group 2); 69.21 ± 8.05 years (group 3)) | Fulfilment of central obesity (waist circumference, men: ≥90 cm; women: ≥80 cm) and at least 2 other components:
| Serum phosphorus level (3.80 ± 0.63 mg/dL (age < 40 years); 4.01 ± 2.34 mg/dL (40–60 years); 4.17 ± 1.389 mg/dL (≥60 years)) |
| [28] |
| Terzi et al. (2015) | Prospective case–control study | Postmenopausal women with or without MetS (n = 230, age: 45–65 years) | Fulfilment of central obesity (waist circumference, men: ≥90 cm; women: ≥80 cm) and at least 2 other components:
| Serum phosphorus level (3.7 ± 0.6 mg/dL (non-MetS); 3.6 ± 0.4 mg/dL (MetS)) |
| [15] |
| Researcher (Year) | Study Type | Subjects | Phosphorus/Phosphate-Related Parameters | Phosphorus/Phosphate-Related Outcomes | Reference |
|---|---|---|---|---|---|
| Zhukouskaya et al. (2020) | Retrospective longitudinal observational study | Children with and without X-linked hypophosphatemia (n = 172, age: 5–20 years) |
| [29] | |
| Celik & Andiran (2011) | Case control study | Normal and obese children (n = 177, age: 6–12 years) and adolescents (n = 121, age: 12–16 years) | Serum phosphate level (4.8 ± 0.4 mg/dL (obese children); 5.1 ± 0.5 mg/dL (control–children); 4.4 ± 0.5 mg/dL (obese adolescents); 4.5 ± 0.6 mg/dL (control–adolescents)) |
| [30] |
| Håglin et al. (2001) | Cross-sectional study | Men (n = 993, age: 50.8 ± 9.4 years) and women (n = 1272, age: 50.1 ± 10.7 years) | Serum phosphate level (0.98 ± 0.21 mmol/L (men); 1.06 ± 0.22 mmol/L (women)) |
| [32] |
| Håglin et al. (2014) | Cross-sectional study | Diabetic and non-diabetic men and women (n = 2504, age: 50.4 ± 10.1 years) | Serum phosphate level (0.98 ± 0.20 mmol/L (men); 1.05 ± 0.21 mmol/L (women)) |
| [31] |
| Ayoub et al. (2015) | Double-blind, randomised, placebo-controlled trial | Adults with BMI of ≥25 kg m−2 and normal kidney function (n = 63, age: 18–45 years) | Phosphorus supplementation (375 mg/day) for 12 weeks |
| [33] |
| Assaad et al. (2019) | Randomised blinded cross-over study | Lean (n = 8) and obese (n = 7) male subjects (age: 20–29 years) | Phosphorus supplementation (500 mg/day) |
| [34] |
| Researcher (Year) | Study Type | Model/Subjects | Phosphorus/Phosphate-Related Parameters | Phosphorus/Phosphate-Related Outcomes | Reference |
|---|---|---|---|---|---|
| Tanaka et al. (2013b) | Animal experimentation | C57BL/6J mice | Phosphate-restricted (0.1%) or phosphate-sufficient diet (1.2%) with or without 2% cholesterol |
| [64] |
| Tanaka et al. (2013a) | Animal experimentation | Npt2a−/− or wild type mice | Diet with or without 2% cholesterol |
| [65] |
| Grundmann et al. (2020) | Animal experimentation | Male Ldlr−/− mice | Diet containing adequate (0.3%) or high (1.5%) phosphorus with adequate (1000 IU/kg) or low (50 IU/kg) vitamin D |
| [66] |
| Abuduli et al. (2016) | Animal experimentation | Male Sprague-Dawley rats | Diet containing low (0.2%), normal (0.6%), or high (1.2%) phosphate |
| [7] |
| Ditscheid et al. (2005) | Placebo-controlled, double-blind, cross-over study | Young healthy volunteers (n = 31, age: 21–29 years) | Bread incorporated with pentacalcium hydroxy-triphosphate (1060 mg calcium; 490 mg phosphorus), 4 weeks |
| [67] |
| Hazim et al. (2014) | Pilot cross-over study | Healthy male subjects (n = 8, age: 19.25 ± 0.41 years) | Phosphorus supplementation (500 mg) |
| [39] |
| Håglin et al. (2014) | Cross-sectional study | Diabetic and non-diabetic men and women (n = 2504, age: 50.4 ± 10.1 years) | Serum phosphate level (0.98 ± 0.20 (men); 1.05 ± 0.21 (women)) |
| [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.K. A Review of Current Evidence on the Relationship between Phosphate Metabolism and Metabolic Syndrome. Nutrients 2022, 14, 4525. https://doi.org/10.3390/nu14214525
Wong SK. A Review of Current Evidence on the Relationship between Phosphate Metabolism and Metabolic Syndrome. Nutrients. 2022; 14(21):4525. https://doi.org/10.3390/nu14214525
Chicago/Turabian StyleWong, Sok Kuan. 2022. "A Review of Current Evidence on the Relationship between Phosphate Metabolism and Metabolic Syndrome" Nutrients 14, no. 21: 4525. https://doi.org/10.3390/nu14214525
APA StyleWong, S. K. (2022). A Review of Current Evidence on the Relationship between Phosphate Metabolism and Metabolic Syndrome. Nutrients, 14(21), 4525. https://doi.org/10.3390/nu14214525






