Impact of a Specific Collagen Peptide Food Supplement on Periodontal Inflammation in Aftercare Patients—A Randomised Controlled Trial
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Population
2.3. Eligibility Criteria
2.4. Exclusion Criteria
2.5. Experimental Preparations
2.6. Sequence of Study Intervention
2.6.1. Visit 1 Baseline (Day 0)
2.6.2. Visit 2 Reevaluation (Day 90)
2.6.3. Assessment of Gingival Index (GI)
2.6.4. Assessment of PCR
2.6.5. Assessment of Consumption Compliance
2.6.6. Adverse Events
2.6.7. Blinding, Randomisation and Examiner Calibration
2.7. Statistical Analysis
2.7.1. Primary Study Outcome and Null Hypothesis
2.7.2. Secondary Study Outcomes
2.7.3. Sample Size Calculation
2.7.4. Statistical Data Analysis
3. Results
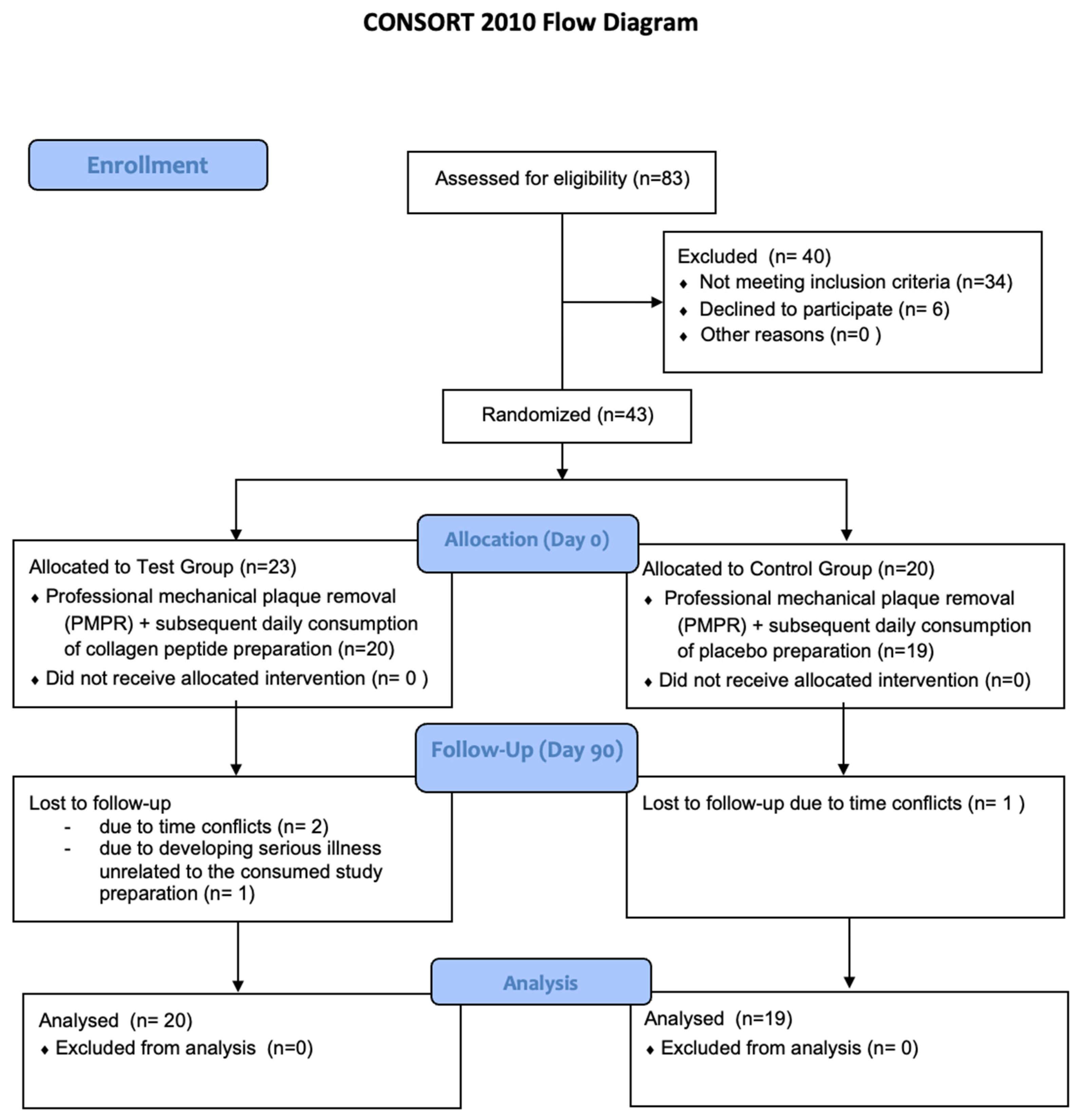
3.1. Recruitment, Drop-Outs, Protocol Violations
3.2. Reporting of Adverse Events
3.3. Periodontal and General Health Profile
3.4. Percentage of BoP-Positive Sites (Primary Outcome)
3.5. Secondary Study Outcomes
3.6. Periodontal Inflamed Surface Area (PISA)
3.7. Gingival Index (GI)
3.8. Plaque Control Record (PCR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. PEFP Workshop Participants and Methodological Consultants. Treatment of stage I–III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Franceschetti, G.; Farina, R. Effect of professional mechanical plaque removal performed on a long-term, routine basis in the secondary prevention of periodontitis: A systematic review. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S221–S236. [Google Scholar] [CrossRef] [PubMed]
- van der Weijden, F.; Slot, D.E. Oral hygiene in the prevention of periodontal diseases: The evidence. Periodontology 2000 2011, 55, 104–123. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S74–S84. [Google Scholar] [CrossRef]
- Needleman, I.; Nibali, L.; Di Iorio, A. Professional mechanical plaque removal for prevention of periodontal diseases in adults--systematic review update. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S12–S35. [Google Scholar] [CrossRef]
- Sanz, M.; Baumer, A.; Buduneli, N.; Dommisch, H.; Farina, R.; Kononen, E.; Linden, G.; Meyle, J.; Preshaw, P.M.; Quirynen, M.; et al. Effect of professional mechanical plaque removal on secondary prevention of periodontitis and the complications of gingival and periodontal preventive measures: Consensus report of group 4 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S214–S220. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef]
- Lang, N.P.; Suvan, J.E.; Tonetti, M.S. Risk factor assessment tools for the prevention of periodontitis progression a systematic review. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S59–S70. [Google Scholar] [CrossRef]
- Lang, N.P.; Joss, A.; Tonetti, M.S. Monitoring disease during supportive periodontal treatment by bleeding on probing. Periodontology 2000 1996, 12, 44–48. [Google Scholar] [CrossRef]
- Matuliene, G.; Pjetursson, B.E.; Salvi, G.E.; Schmidlin, K.; Bragger, U.; Zwahlen, M.; Lang, N.P. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J. Clin. Periodontol. 2008, 35, 685–695. [Google Scholar] [CrossRef]
- Corbella, S.; Calciolari, E.; Alberti, A.; Donos, N.; Francetti, L. Systematic review and meta-analysis on the adjunctive use of host immune modulators in non-surgical periodontal treatment in healthy and systemically compromised patients. Sci. Rep. 2021, 11, 12125. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Calciolari, E.; Brusselaers, N.; Goldoni, M.; Bostanci, N.; Belibasakis, G.N. The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 199–238. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Hasturk, H.; Lambris, J.D. C3-targeted therapy in periodontal disease: Moving closer to the clinic. Trends Immunol. 2021, 42, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Imfeld, T.; Schicht, O.; Rath, C.; Persson, R.E.; Persson, G.R. The impact of the stone age diet on gingival conditions in the absence of oral hygiene. J. Periodontol. 2009, 80, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Gartner, M.; Breuninger, L.; Anderson, A.; Konig, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dotsch, A.; Ratka-Kruger, P.; et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef]
- Bartha, V.; Exner, L.; Schweikert, D.; Woelber, J.P.; Vach, K.; Meyer, A.L.; Basrai, M.; Bischoff, S.C.; Meller, C.; Wolff, D. Effect of the Mediterranean diet on gingivitis. A randomized controlled trial. J. Clin. Periodontol. 2022, 49, 111–122. [Google Scholar] [CrossRef]
- Jockel-Schneider, Y.; Gossner, S.K.; Petersen, N.; Stolzel, P.; Hagele, F.; Schweiggert, R.M.; Haubitz, I.; Eigenthaler, M.; Carle, R.; Schlagenhauf, U. Stimulation of the nitrate-nitrite-NO-metabolism by repeated lettuce juice consumption decreases gingival inflammation in periodontal recall patients: A randomized, double-blinded, placebo-controlled clinical trial. J. Clin. Periodontol. 2016, 43, 603–608. [Google Scholar] [CrossRef]
- Jockel-Schneider, Y.; Schlagenhauf, U.; Stolzel, P.; Gossner, S.; Carle, R.; Ehmke, B.; Prior, K.; Hagenfeld, D. Nitrate-rich diet alters the composition of the oral microbiota in periodontal recall patients. J. Periodontol. 2021, 92, 1536–1545. [Google Scholar] [CrossRef]
- Widyarman, A.S.; Theodorea, C.F. Novel Indigenous Probiotic Lactobacillus reuteri Strain Produces Anti-biofilm Reuterin against Pathogenic Periodontal Bacteria. Eur. J. Dent. 2022, 16, 96–101. [Google Scholar] [CrossRef]
- Chatterjee, D.; Chatterjee, A.; Kalra, D.; Kapoor, A.; Vijay, S.; Jain, S. Role of adjunct use of omega 3 fatty acids in periodontal therapy of periodontitis. A systematic review and meta-analysis. J. Oral Biol. Craniofac. Res. 2022, 12, 55–62. [Google Scholar] [CrossRef]
- Deore, G.D.; Gurav, A.N.; Patil, R.; Shete, A.R.; Naiktari, R.S.; Inamdar, S.P. Omega 3 fatty acids as a host modulator in chronic periodontitis patients: A randomised, double-blind, palcebo-controlled, clinical trial. J. Periodontal. Implant Sci. 2014, 44, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Schlagenhauf, U.; Jakob, L.; Eigenthaler, M.; Segerer, S.; Jockel-Schneider, Y.; Rehn, M. Regular consumption of Lactobacillus reuteri-containing lozenges reduces pregnancy gingivitis: An RCT. J. Clin. Periodontol. 2016, 43, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Nik-Azis, N.M.; Mohd, N.; Mohd Fadzilah, F.; Mohamed Haflah, N.H.; Mohamed Said, M.S.; Baharin, B. Rheumatoid arthritis serotype and synthetic disease-modifying anti-rheumatic drugs in patients with periodontitis: A case-control study. PLoS ONE 2021, 16, e0252859. [Google Scholar] [CrossRef]
- Bartold, P.M.; Lopez-Oliva, I. Periodontitis and rheumatoid arthritis: An update 2012–2017. Periodontology 2000 2020, 83, 189–212. [Google Scholar] [CrossRef]
- de Molon, R.S.; Rossa, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of Periodontitis and Rheumatoid Arthritis: Current Evidence and Potential Biological Interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Howard, A.; Tahir, I.; Javed, S.; Waring, S.M.; Ford, D.; Hirst, B.H. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J. Physiol. 2010, 588, 995–1009. [Google Scholar] [CrossRef]
- Li, W.; Sun, K.; Ji, Y.; Wu, Z.; Wang, W.; Dai, Z.; Wu, G. Glycine Regulates Expression and Distribution of Claudin-7 and ZO-3 Proteins in Intestinal Porcine Epithelial Cells. J. Nutr. 2016, 146, 964–969. [Google Scholar] [CrossRef]
- Dai, Z.L.; Li, X.L.; Xi, P.B.; Zhang, J.; Wu, G.; Zhu, W.Y. L-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 2013, 45, 501–512. [Google Scholar] [CrossRef]
- Slomka, V.; Hernandez-Sanabria, E.; Herrero, E.R.; Zaidel, L.; Bernaerts, K.; Boon, N.; Quirynen, M.; Teughels, W. Nutritional stimulation of commensal oral bacteria suppresses pathogens: The prebiotic concept. J. Clin. Periodontol. 2017, 44, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Larder, C.E.; Iskandar, M.M.; Kubow, S. Gastrointestinal Digestion Model Assessment of Peptide Diversity and Microbial Fermentation Products of Collagen Hydrolysates. Nutrients 2021, 13, 2720. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.E.; Oesser, S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: A review of the literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Coronado, J.M.; Martinez-Olvera, L.; Elizondo-Omana, R.E.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Simental-Mendia, L.E.; Simental-Mendia, M. Effect of collagen supplementation on osteoarthritis symptoms: A meta-analysis of randomized placebo-controlled trials. Int. Orthop. 2019, 43, 531–538. [Google Scholar] [CrossRef]
- Puigdellivol, J.; Comellas Berenger, C.; Perez Fernandez, M.A.; Cowalinsky Millan, J.M.; Carreras Vidal, C.; Gil Gil, I.; Martinez Pagan, J.; Ruiz Nieto, B.; Jimenez Gomez, F.; Comas Figuerola, F.X.; et al. Effectiveness of a Dietary Supplement Containing Hydrolyzed Collagen, Chondroitin Sulfate, and Glucosamine in Pain Reduction and Functional Capacity in Osteoarthritis Patients. J. Diet. Suppl. 2019, 16, 379–389. [Google Scholar] [CrossRef]
- Proksch, E.; Schunck, M.; Zague, V.; Segger, D.; Degwert, J.; Oesser, S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol. Physiol. 2014, 27, 113–119. [Google Scholar] [CrossRef]
- Hexsel, D.; Zague, V.; Schunck, M.; Siega, C.; Camozzato, F.O.; Oesser, S. Oral supplementation with specific bioactive collagen peptides improves nail growth and reduces symptoms of brittle nails. J. Cosmet. Dermatol. 2017, 16, 520–526. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Brame, J.; Oesser, S.; Gollhofer, A.; Konig, D. The Influence of Specific Bioactive Collagen Peptides on Knee Joint Discomfort in Young Physically Active Adults: A Randomized Controlled Trial. Nutrients 2021, 13, 523. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Konig, D. Specific Bioactive Collagen Peptides in Osteopenia and Osteoporosis: Long-Term Observation in Postmenopausal Women. J. Bone Metab. 2021, 28, 207–213. [Google Scholar] [CrossRef]
- Nesse, W.; Abbas, F.; van der Ploeg, I.; Spijkervet, F.K.; Dijkstra, P.U.; Vissink, A. Periodontal inflamed surface area: Quantifying inflammatory burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef]
- Lobene, R.R.; Weatherford, T.; Ross, N.M.; Lamm, R.A.; Menaker, L. A modified gingival index for use in clinical trials. Clin. Prev. Dent. 1986, 8, 3–6. [Google Scholar] [PubMed]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Hefti, A.F.; Preshaw, P.M. Examiner alignment and assessment in clinical periodontal research. Periodontology 2000 2012, 59, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Grossi, S.G.; Dunford, R.G.; Ho, A.; Koch, G.; Machtei, E.E.; Genco, R.J. Sources of error for periodontal probing measurements. J. Periodontal. Res. 1996, 31, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Group, C. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010, 7, e1000251. [Google Scholar] [CrossRef]
- Figuero, E.; Herrera, D.; Tobias, A.; Serrano, J.; Roldan, S.; Escribano, M.; Martin, C. Efficacy of adjunctive anti-plaque chemical agents in managing gingivitis: A systematic review and network meta-analyses. J. Clin. Periodontol. 2019, 46, 723–739. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef]
- Raubenheimer, K.; Bondonno, C.; Blekkenhorst, L.; Wagner, K.H.; Peake, J.M.; Neubauer, O. Effects of dietary nitrate on inflammation and immune function, and implications for cardiovascular health. Nutr. Rev. 2019, 77, 584–599. [Google Scholar] [CrossRef]
- Zeiher, A.M.; Fisslthaler, B.; Schray-Utz, B.; Busse, R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ. Res. 1995, 76, 980–986. [Google Scholar] [CrossRef]
- Kubes, P.; Suzuki, M.; Granger, D.N. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA 1991, 88, 4651–4655. [Google Scholar] [CrossRef]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.J.; Sergesketter, A.R.; Offenbacher, S.; Schoenfisch, M.H. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J. Dent. Res. 2014, 93, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.J.; Worley, B.V.; Sergesketter, A.R.; Schoenfisch, M.H. Kinetic-dependent Killing of Oral Pathogens with Nitric Oxide. J. Dent. Res. 2015, 94, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Nambu, T.; Wang, D.; Mashimo, C.; Maruyama, H.; Kashiwagi, K.; Yoshikawa, K.; Yamamoto, K.; Okinaga, T. Nitric Oxide Donor Modulates a Multispecies Oral Bacterial Community-An In Vitro Study. Microorganisms 2019, 7, 353. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Blackwell, J.R.; L’Heureux, J.E.; Williams, D.W.; Smith, A.; van der Giezen, M.; Winyard, P.G.; Kelly, J.; Jones, A.M. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 2018, 124, 21–30. [Google Scholar] [CrossRef]
- Schreiber, F.; Stief, P.; Gieseke, A.; Heisterkamp, I.M.; Verstraete, W.; de Beer, D.; Stoodley, P. Denitrification in human dental plaque. BMC Biol. 2010, 8, 24. [Google Scholar] [CrossRef]
- Shim, J.S.; Park, D.S.; Baek, D.H.; Jha, N.; Park, S.I.; Yun, H.J.; Kim, W.J.; Ryu, J.J. Antimicrobial activity of NO-releasing compounds against periodontal pathogens. PLoS ONE 2018, 13, e0199998. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Chen, T.; Marsh, P.D.; Al-Hebshi, N.N. SMDI: An Index for Measuring Subgingival Microbial Dysbiosis. J. Dent. Res. 2022, 101, 331–338. [Google Scholar] [CrossRef]
- Ao, J.; Li, B. Amino acid composition and antioxidant activities of hydrolysates and peptide fractions from porcine collagen. Food Sci. Technol. Int. 2012, 18, 425–434. [Google Scholar] [CrossRef]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.W.; Wong, M.C.; Wang, H.H.; Liu, K.Q.; Lee, C.L.; Yan, B.P.; Yu, C.M.; Griffiths, S.M. Compliance with the Dietary Approaches to Stop Hypertension (DASH) diet: A systematic review. PLoS ONE 2013, 8, e78412. [Google Scholar] [CrossRef] [PubMed]
| Variable | Test Group | Control Group | p-Value |
|---|---|---|---|
| n = 20 | n = 19 | ||
| Age (yrs) median (CI) | 59.6 (56.6–62.6) | (55.2–63.1) | pU = 0.94 |
| Male gender no. (%) | 6 (33%) | 12 (67%) | Pc = 0.036 |
| No. of teeth median (CI) | 25 (22–27) | 26 (24–29) | pU = 0.23 |
| PPD baseline (mm) median (CI) | 2.9 (2.7–3.1) | 2.9 (2.6–3.1) | pU = 0.64 |
| Occasional Smoking (<10 cigarettes/day) | 2 (10%) | 2 (10.2%) | Pc = 1.0 |
| Osteoarthritis | 1 (5%) | 1 (5.3%) | Pc = 1.0 |
| Hypertension | 2 (10%) | 2 (10.5%) | Pc = 1.0 |
| Hypothyroidism | 5 (25%) | 1 (5.3 %) | Pc = 0.18 |
| Visit | Test Group n = 20 mean ± SD | Control Group n = 19 mean ± SD | pU between Groups |
|---|---|---|---|
| BoP % Baseline | 10.4 ± 7.0 | 14.2 ± 10.3 | 0.29 |
| BoP % Day 90 | 3.0 ± 3.8 | 9.4 ± 9.9 | 0.017 |
| pW within the groups | 0.00014 | 0.07 |
| Visit | Test Group n = 20 mean ± SD | Control Group n = 19 mean ± SD | pU between Groups |
|---|---|---|---|
| PISA (mm2) Baseline | 170.6 ± 129.9 | 229.4 ± 161.6 | 0.23 |
| PISA (mm2) Day 90 | 53.7 ± 70.5 | 184.3 ± 214.7 | 0.011 |
| pW within the groups | 0.00036 | 0.3 | |
| GI Baseline | 0.5 ± 0.3 | 0.4 ± 0.2 | 0.044 |
| GI Day 90 | 0.1 ± 0.2 | 0.3 ± 0.2 | 0.029 |
| pw within the groups | 0.00011 | 0.022 | |
| PCR% Baseline | 25.6 ± 19.5 | 25.4 ± 14.3 | 0.81 |
| PCR% Day 90 | 9.6 ± 10.3 | 18.1 ± 15.9 | 0.14 |
| pw within the groups | 0.0025 | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jockel-Schneider, Y.; Stoelzel, P.; Hess, J.; Haubitz, I.; Fickl, S.; Schlagenhauf, U. Impact of a Specific Collagen Peptide Food Supplement on Periodontal Inflammation in Aftercare Patients—A Randomised Controlled Trial. Nutrients 2022, 14, 4473. https://doi.org/10.3390/nu14214473
Jockel-Schneider Y, Stoelzel P, Hess J, Haubitz I, Fickl S, Schlagenhauf U. Impact of a Specific Collagen Peptide Food Supplement on Periodontal Inflammation in Aftercare Patients—A Randomised Controlled Trial. Nutrients. 2022; 14(21):4473. https://doi.org/10.3390/nu14214473
Chicago/Turabian StyleJockel-Schneider, Yvonne, Peggy Stoelzel, Jeanine Hess, Imme Haubitz, Stefan Fickl, and Ulrich Schlagenhauf. 2022. "Impact of a Specific Collagen Peptide Food Supplement on Periodontal Inflammation in Aftercare Patients—A Randomised Controlled Trial" Nutrients 14, no. 21: 4473. https://doi.org/10.3390/nu14214473
APA StyleJockel-Schneider, Y., Stoelzel, P., Hess, J., Haubitz, I., Fickl, S., & Schlagenhauf, U. (2022). Impact of a Specific Collagen Peptide Food Supplement on Periodontal Inflammation in Aftercare Patients—A Randomised Controlled Trial. Nutrients, 14(21), 4473. https://doi.org/10.3390/nu14214473






