Toxicity Investigations of (R)-3-Hydroxybutyrate Glycerides In Vitro and in Male and Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Substance
2.2. Bacterial Reverse Mutation Test (Ames Test)
2.3. In Vitro Micronucleus Assay
2.4. 14-Day Range Finding/Palatability Study in Rats
2.5. 90-Day Repeat Dose Study in Rats
2.6. Statistical Methods
3. Results
3.1. Mutagenicity
3.2. Micronucleus Study
3.3. 14-Day Range Finding/Palatability Study in Rats
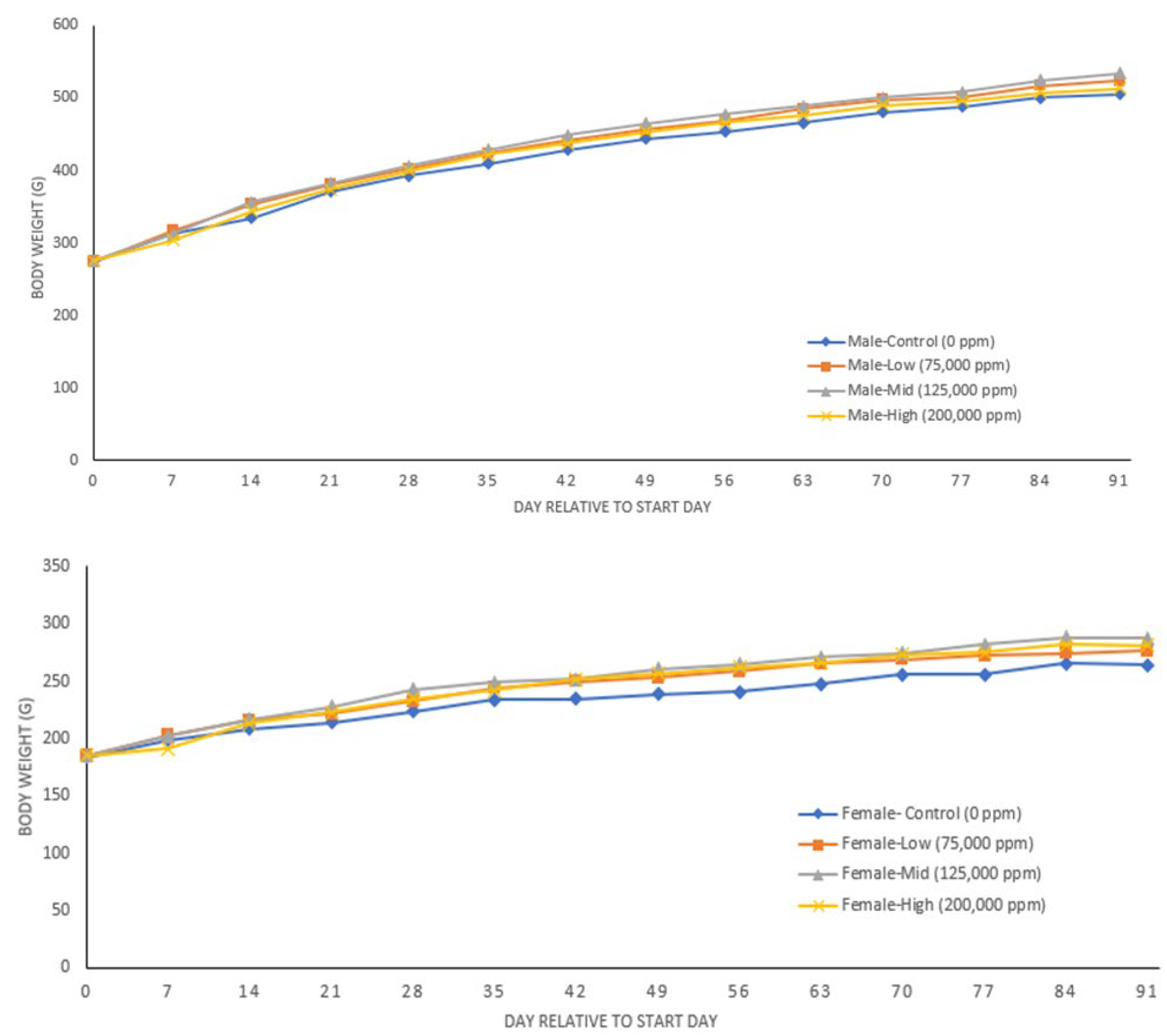
3.4. 90-Day Study
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broom, G.M.; Shaw, I.C.; Rucklidge, J.J. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition 2019, 60, 118–121. [Google Scholar] [CrossRef]
- LaFountain, R.A.; Miller, V.J.; Barnhart, E.C.; Hyde, P.N.; Crabtree, C.D.; McSwiney, F.T.; Beeler, M.K.; Buga, A.; Sapper, T.N.; Short, J.A.; et al. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019, 184, e538–e547. [Google Scholar] [CrossRef]
- Ma, S.; Suzuki, K. Keto-Adaptation and Endurance Exercise Capacity, Fatigue Recovery, and Exercise-Induced Muscle and Organ Damage Prevention: A Narrative Review. Sports 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Martin-McGill, K.J.; Lambert, B.; Whiteley, V.J.; Wood, S.; Neal, E.G.; Simpson, Z.R.; Schoeler, N.E.; on behalf of the Ketogenic Dietitians Research Network (KDRN). Understanding the core principles of a ‘modified ketogenic diet’: A UK and Ireland perspective. J. Hum. Nutr. Diet. 2019, 32, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mohorko, N.; Černelič-Bizjak, M.; Poklar-Vatovec, T.; Grom, G.; Kenig, S.; Petelin, A.; Jenko-Pražnikar, Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr. Res. 2018, 62, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Roehl, K.; Falco-Walter, J.; Ouyang, B.; Balabanov, A. Modified ketogenic diets in adults with refractory epilepsy: Efficacious improvements in seizure frequency, seizure severity, and quality of life. Epilepsy Behav. 2019, 93, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E.; Rho, J.M. The Ketogenic Diet as a Treatment Paradigm for Diverse Neurological Disorders. Front. Pharmacol. 2012, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Tondt, J.; Maguire, E.; Yancy, W.S., Jr. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev. Endocrinol. Metab. 2018, 13, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Włodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Stumvoll, M.; Kramer, W.; Kempf, K.; Martin, S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018, 16, 232. [Google Scholar] [CrossRef]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes/Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Silva, B.; Mantha, O.L.; Schor, J.; Pascual, A.; Plaçais, P.-Y.; Pavlowsky, A.; Preat, T. Glia fuel neurons with locally synthesized ketone bodies to sustain memory under starvation. Nat. Metab. 2022, 4, 213–224. [Google Scholar] [CrossRef]
- Weis, E.; Puchalska, P.; Nelson, A.B.; Taylor, J.; Moll, I.; Hasan, S.S.; Dewenter, M.; Hagenmüller, M.; Fleming, T.; Poschet, G.; et al. Ketone body oxidation increases cardiac endothelial cell proliferation. EMBO Mol. Med. 2022, 14, e14753. [Google Scholar] [CrossRef]
- Cameron, D.; Soto-Mota, A.; Willis, D.R.; Ellis, J.; Procter, N.E.K.; Greenwood, R.; Saunders, N.; Schulte, R.F.; Vassiliou, V.S.; Tyler, D.J.; et al. Evaluation of Acute Supplementation With the Ketone Ester (R)-3-Hydroxybutyl-(R)-3-Hydroxybutyrate (deltaG) in Healthy Volunteers by Cardiac and Skeletal Muscle 31P Magnetic Resonance Spectroscopy. Front. Physiol. 2022, 13, 793987. [Google Scholar] [CrossRef] [PubMed]
- Cuenoud, B.; Hartweg, M.; Godin, J.-P.; Croteau, E.; Maltais, M.; Castellano, C.-A.; Carpentier, A.C.; Cunnane, S.C. Metabolism of Exogenous D-Beta-Hydroxybutyrate, an Energy Substrate Avidly Consumed by the Heart and Kidney. Front. Nutr. 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Bak, L.K.; Walls, A.B.; Schousboe, A.; Waagepetersen, H.S. Astrocytic glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 2018, 293, 7108–7116. [Google Scholar] [CrossRef] [PubMed]
- Bordone, M.; Salman, M.M.; Titus, H.E.; Amini, E.; Andersen, J.V.; Chakraborti, B.; Diuba, A.V.; Dubouskaya, T.G.; Ehrke, E.; De Freitas, A.E.; et al. The energetic brain—A review from students to students. J. Neurochem. 2019, 151, 139–165. [Google Scholar] [CrossRef] [PubMed]
- Courchesne-Loyer, A.; Croteau, E.; Castellano, C.-A.; St-Pierre, V.; Hennebelle, M.; Cunnane, S.C. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: A dual tracer quantitative positron emission tomography study. J. Cereb. Blood Flow Metab. 2016, 37, 2485–2493. [Google Scholar] [CrossRef]
- Jensen, N.J.; Wodschow, H.Z.; Nilsson, M.; Rungby, J. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8767. [Google Scholar] [CrossRef] [PubMed]
- Oyarzabal, A.; Marin-Valencia, I. Synaptic energy metabolism and neuronal excitability, in sickness and health. J. Inherit. Metab. Dis. 2019, 42, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Andreux, P.A.; Van Diemen, M.P.J.; Heezen, M.R.; Auwerx, J.; Rinsch, C.; Groeneveld, G.J.; Singh, A. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci. Rep. 2018, 8, 8548. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, J.C.; Visser, G.; Clarke, K.; Ferdinandusse, S.; de Haan, F.H.; Houtkooper, R.H.; Ijlst, L.; Kok, I.L.; Langeveld, M.; van der Pol, W.L.; et al. Nutritional ketosis improves exercise metabolism in patients with very long-chain acyl-CoA dehydrogenase deficiency. J. Inherit. Metab. Dis. 2020, 43, 787–799. [Google Scholar] [CrossRef]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2016, 595, 2857–2871. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Metabolic and Signaling Roles of Ketone Bodies in Health and Disease. Annu. Rev. Nutr. 2021, 41, 49–77. [Google Scholar] [CrossRef]
- Abdurrachim, D.; Woo, C.C.; Teo, X.Q.; Chan, W.X.; Radda, G.K.; Lee, P.T.H. A new hyperpolarized 13C ketone body probe reveals an increase in acetoacetate utilization in the diabetic rat heart. Sci. Rep. 2019, 9, 5532. [Google Scholar] [CrossRef]
- Al-Zaid, N.S.; Dashti, H.M.; Mathew, T.C.; Juggi, J.S. Low carbohydrate ketogenic diet enhances cardiac tolerance to global ischaemia. Acta Cardiol. 2007, 62, 381–389. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, C.; Xie, M. Beta-Hydroxybutyrate, Friend or Foe for Stressed Hearts. Front. Aging 2021, 2, 681513. [Google Scholar] [CrossRef]
- Han, Y.-M.; Ramprasath, T.; Zou, M.-H. β-hydroxybutyrate and its metabolic effects on age-associated pathology. Exp. Mol. Med. 2020, 52, 548–555. [Google Scholar] [CrossRef]
- Dhillon, K.K.; Gupta, S. Biochemistry, Ketogenesis; [Updated 2022 February 10]; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Brahma, M.K.; Ha, C.; Pepin, M.E.; Mia, S.; Sun, Z.; Chatham, J.C.; Habegger, K.M.; Abel, E.D.; Paterson, A.J.; Young, M.E.; et al. Increased Glucose Availability Attenuates Myocardial Ketone Body Utilization. J. Am. Heart Assoc. 2020, 9, e013039. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F., Jr. Fuel Metabolism in Starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.; Payne, E.T.; Wong-Kisiel, L.; Nickels, K.C.; Eckert, S.; Wirrell, E.C. The Ketogenic and Modified Atkins Diet Therapy for Children With Refractory Epilepsy of Genetic Etiology. Pediatr. Neurol. 2018, 94, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2013, 25, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Poff, A.M.; Moss, S.; Soliven, M.; D’Agostino, D.P. Ketone Supplementation: Meeting the Needs of the Brain in an Energy Crisis. Front. Nutr. 2021, 8, 783659. [Google Scholar] [CrossRef]
- Poplawski, M.M.; Mastaitis, J.W.; Isoda, F.; Grosjean, F.; Zheng, F.; Mobbs, C.V. Reversal of Diabetic Nephropathy by a Ketogenic Diet. PLoS ONE 2011, 6, e18604. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Pedraza-Chaverri, J.; Tapia, E. Ketone bodies, stress response, and redox homeostasis. Redox Biol. 2019, 29, 101395. [Google Scholar] [CrossRef]
- Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef]
- Waldman, H.S.; McAllister, M.J. Exogenous Ketones as Therapeutic Signaling Molecules in High-Stress Occupations: Implications for Mitigating Oxidative Stress and Mitochondrial Dysfunction in Future Research. Nutr. Metab. Insights 2020, 13, 1178638820979029. [Google Scholar] [CrossRef]
- Walsh, J.J.; Caldwell, H.G.; Neudorf, H.; Ainslie, P.N.; Little, J.P. Short-term ketone monoester supplementation improves cerebral blood flow and cognition in obesity: A randomized cross-over trial. J. Physiol. 2021, 599, 4763–4778. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.J.; Kirk, T.; Ashmore, T.; Willerton, K.; Evans, R.; Smith, A.; Murray, A.J.; Stubbs, B.; West, J.; McLure, S.W.; et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab. 2016, 24, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.L.; Holcomb, L.E.; Kolwicz, S.C., Jr. Ketogenic diets and exercise performance. Nutrients 2019, 11, 2296. [Google Scholar] [CrossRef] [PubMed]
- CIR. Safety Assessment of Glycerin as Used in Cosmetics. Available online: https://www.cir-safety.org/sites/default/files/glycer_122014_FR.pdf (accessed on 1 August 2022).
- Stoewsand, G.S.; Dymsza, H.A. Synthetic sources of calories in the diets of rats and dogs. Proc. Seventh Int. Congr. Nutr. 1966, 4, 1082–1087. [Google Scholar]
- UK. SIDS Initial Assessment Report for SIAM 14: Glycerol, Sponsored by the United Kingdom for OECD SIDS Chemical Program. Available online: https://hpvchemicals.oecd.org/ui/handler.axd?id=4b0a2d87-3183-40d4-84f5-0e118c647b19 (accessed on 1 August 2022).
- Shivva, V.; Cox, P.J.; Clarke, K.; Veech, R.L.; Tucker, I.G.; Duffull, S.B. The Population Pharmacokinetics of d-β-hydroxybutyrate Following Administration of (R)-3-Hydroxybutyl (R)-3-Hydroxybutyrate. AAPS J. 2016, 18, 678–688. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Blatter, L.A. Role of β-hydroxybutyrate, its polymer poly-β-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease. Front. Physiol. 2014, 5, 260. [Google Scholar] [CrossRef]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; Knight, N.S.; Murray, A.J.; Cochlin, L.E.; King, M.T.; Wong, A.W.; Roberts, A.; et al. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul. Toxicol. Pharmacol. 2012, 63, 196–208. [Google Scholar] [CrossRef]
- WHO. Chemical-Specific Adjustment Factors for Interspecies Differences and Human Variability: Guidance Document for Use of Data in Dose/Concentration-Response Assessment. Available online: https://apps.who.int/iris/bitstream/handle/10665/43294/9241546786_eng.pdf;sequence=1 (accessed on 15 September 2022).
- Borzelleca, J.F. Macronutrient substitutes: Safety evaluation. Regul. Toxicol. Pharmacol. 1992, 16, 253–264. [Google Scholar] [CrossRef]
- FDA. GRAS Notice (GRN) 515, D-Beta-Hydroxybutyrate Ester. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=515&sort=GRN_No&order=DESC&startrow=1&type=basic&search=515 (accessed on 15 September 2022).
- FDA. Gras Notice (GRN) No. 1032, D-β-Hydroxybutyrate. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=1032&sort=GRN_No&order=DESC&startrow=1&type=basic&search=butyrate (accessed on 15 September 2022).
- Stefan, M.; Sharp, M.; Gheith, R.; Lowery, R.; Wilson, J. The Effects of Exogenous Beta-Hydroxybutyrate Supplementation on Metrics of Safety and Health. Int. J. Nutr. Food Sci. 2020, 9, 154–162. [Google Scholar] [CrossRef]
| Concentration (µg/Plate) | TA98 | TA100 | TA1535 | TA1537 | WP2uvrA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | |
| Experiment 1 | ||||||||||
| 0 a | 21 | 29 | 116 | 120 | 11 | 13 | 11 | 15 | 46 | 53 |
| 1.58 | 25 | 29 | 120 | 116 | 14 | 11 | 9 | 15 | 48 | 53 |
| 5 | 22 | 23 | 122 | 119 | 13 | 12 | 13 | 11 | 53 | 56 |
| 15.8 | 21 | 29 | 116 | 123 | 11 | 13 | 11 | 17 | 43 | 57 |
| 50 | 22 | 31 | 112 | 111 | 10 | 10 | 16 | 15 | 44 | 56 |
| 158 | 25 | 29 | 117 | 125 | 11 | 15 | 15 | 10 | 46 | 52 |
| 500 | 20 | 30 | 110 | 120 | 10 | 12 | 9 | 12 | 47 | 53 |
| 1580 | 22 | 29 | 119 | 122 | 12 | 8 | 9 | 16 | 54 | 50 |
| 5000 | 24 | 30 | 112 | 109 | 13 | 14 | 13 | 10 | 55 | 50 |
| Positive control | 194 b | 159 c | 519 d | 746 c | 547 d | 492 e | 247 f | 254 e | 612 g | 180 e |
| Historical negative control (−S9, range) | 15–35 | 72–129 | 6–20 | 5–17 | 30–66 | |||||
| Historical negative control (+S9, range) | 18–32 | 78–123 | 8–18 | 8–20 | 32–68 | |||||
| Experiment 2 | ||||||||||
| 0 a | 21 | 27 | 119 | 120 | 12 | 12 | 9 | 10 | 46 | 54 |
| 1.58 | 20 | 24 | 109 | 118 | 13 | 12 | 8 | 8 | 50 | 53 |
| 5 | 18 | 25 | 111 | 117 | 13 | 15 | 8 | 11 | 45 | 59 |
| 15.8 | 21 | 23 | 114 | 120 | 10 | 9 | 9 | 12 | 43 | 62 |
| 50 | 25 | 25 | 105 | 102 | 10 | 9 | 10 | 11 | 44 | 52 |
| 158 | 20 | 25 | 111 | 113 | 13 | 11 | 8 | 12 | 42 | 59 |
| 500 | 21 | 25 | 113 | 107 | 17 | 11 | 10 | 13 | 48 | 50 |
| 1580 | 22 | 21 | 102 | 119 | 11 | 11 | 9 | 13 | 49 | 53 |
| 5000 | 24 | 21 | 110 | 118 | 11 | 9 | 10 | 10 | 47 | 53 |
| Positive control | 176 b | 168 c | 510 d | 622 c | 521 d | 391 e | 452 f | 248 e | 702 g | 178 e |
| Historical negative control (−S9, range) | 17–38 | 80–138 | 6–22 | 6–16 | 26–59 | |||||
| Historical negative control (+S9, range) | 17–38 | 82–134 | 6–17 | 6–14 | 32–62 | |||||
| Concentration (µg/mL) | % A/N | Nuclei: Beads | % Relative Survival | % MN | p-Value | RICC |
|---|---|---|---|---|---|---|
| Four hour incubation without S9 | ||||||
| 0 (water) | 1.8 | 9.0 | 100 | 0.5 | NR | 100.0 |
| 55 | 2.2 | 8.5 | 94.0 | 0.5 | 0.9398 | 101.7 |
| 111 | 1.6 | 8.8 | 97.9 | 0.6 | 0.8308 | 103.9 |
| 223 | 2.1 | 9.1 | 100.8 | 0.4 | 0.9986 | 95.8 |
| 445 | 2.0 | 8.5 | 94.2 | 0.7 | 0.1550 | 98.1 |
| 890 | 1.6 | 9.0 | 99.9 | 0.6 | 0.6813 | 95.5 |
| 1780 | 1.8 | 8.7 | 95.9 | 0.5 | 0.9270 | 93.7 |
| Trend | 0.4361 | |||||
| VIN | 15.6 | 5.1 | 56.5 | 14.4 * | <0.0001 | 98.1 |
| Four hour incubation with S9 | ||||||
| 0 (water) | 1.7 | 12.6 | 100 | 0.5 | NR | 100 |
| 55 | 1.3 | 12.8 | 101.5 | 0.4 | 0.9995 | 96.7 |
| 111 | 1.4 | 13.5 | 107.0 | 0.5 | 0.9120 | 82.2 |
| 223 | 1.4 | 15.1 | 120.1 | 0.4 | 0.9995 | 92.9 |
| 445 | 1.3 | 14.1 | 112.0 | 0.6 | 0.7759 | 95.1 |
| 890 | 1.1 | 13.2 | 105.0 | 0.6 | 0.6593 | 93.9 |
| 1780 | 1.5 | 15.1 | 120.2 | 0.6 | 0.4419 | 82.2 |
| Trend | 0.0553 | |||||
| CP | 7.5 | 8.1 | 64.2 | 4.9 * | 0.0005 | 39.7 |
| Twenty four hour incubation without S9 | ||||||
| 0 (water) | 1.8 | 11.7 | 100.0 | 0.6 | NR | 100 |
| 55 | 1.6 | 11.6 | 98.9 | 0.5 | 0.9999 | 101.3 |
| 111 | 1.8 | 11.8 | 101.0 | 0.4 | 1.0000 | 107.8 |
| 223 | 1.6 | 12.7 | 108.3 | 0.4 | 1.0000 | 102.5 |
| 445 | 1.5 | 11.3 | 96.3 | 0.4 | 1.0000 | 81.9 |
| 890 | 1.5 | 11.3 | 96.3 | 0.6 | 0.9866 | 100.1 |
| 1780 | 1.4 | 13.2 | 112.4 | 0.3 | 1.0000 | 109.5 |
| Trend | 0.0902 | |||||
| VIN | 11.8 | 7.2 | 61.2 | 12.0 * | <0.0001 | 59.9 |
| Parameter | Control | 75,000 ppm | 125,000 ppm | 200,000 ppm | Historical Control |
|---|---|---|---|---|---|
| Males | |||||
| AST (U/L) | 79.5 ± 22.3 | 80.5 ± 14.8 | 77.6 ± 18.9 | 72.3 ± 10.4 | 63–175 |
| ALT (U/L) | 33.1 ± 7.7 | 35.1 ± 8.0 | 33.2 ± 7.7 | 24.1 ± 3.1 ** | 19–48 |
| ALKP (U/L) | 52.2 ± 10.1 | 48.0 ± 6.7 | 49.6 ± 8.9 | 47.4 ± 10.3 | 36–141 |
| BILI (mg/dL) | 0.068 ± 0.019 | 0.065 ± 0.012 | 0.058 ± 0.018 | 0.053 ± 0.0016 | 0.04–0.2 |
| BUN (mg/dL) | 19.9 ± 3.4 | 18.4 ± 1.7 | 18.8 ± 2.9 | 17.9 ± 3.3 | 10.7–20.0 |
| CREA (mg/dL) | 0.249 ± 0.039 | 0.224 ± 0.033 | 0.263 ± 0.050 | 0.233 ± 0.056 | 0.3–0.5 |
| CHOL (mg/dL) | 70.9 ± 11.3 | 72.8 ± 15.1 | 65.1 ± 15.5 | 64.7 ± 7.5 | 37–95 |
| LDL (mmol/L) | 0.375 ± 0.073 | 0.342 ± 0.126 | 0.313 ± 0.120 | 0.268 ± 0.039 | ND |
| HDL (mmol/L) | 1.221 ± 0.258 | 1.291 ± 0.298 | 1.145 ± 0.302 | 1.108 ± 0.146 | ND |
| TRIG (mg/dL) | 63.7 ± 18.4 | 99.8 ± 28.4 ** | 85.8 ± 25.2 | 101.2 ± 35.7 ** | 27–160 |
| SDH (U/L) | 15.29 ± 4.31 | 20.50 ± 8.84 | 18.70 ± 5.78 | 14.94 ± 5.19 | ND |
| GLUC (mg/dL) | 230.6 ± 48.0 | 237.3 ± 50.8 | 259.7 ± 47.7 | 227.7 ± 49.5 | 106–184 |
| TP (g/dL) | 5.46 ± 0.26 | 5.56 ± 0.35 | 5.73 ± 0.29 | 5.62 ± 0.15 | 5.6–7.6 |
| ALB (g/dL) | 3.90 ± 0.16 | 3.96 ± 0.23 | 4.05 ± 0.18 | 3.94 ± 0.17 | 3.6–4.7 |
| GLOB (g/dL) | 1.56 ± 0.19 | 1.60 ± 0.23 | 1.68 ± 0.23 | 1.68 ± 0.14 | 1.8–2.5 |
| Ca (mg/dL) | 12.02 ± 0.66 | 11.92 ± 0.72 | 12.52 ± 0.78 | 11.78 ± 0.32 | 9.1–11.9 |
| P (mg/dL) | 9.24 ± 0.69 | 9.27 ± 1.02 | 10.04 ± 0.80 | 8.81 ± 0.52 | 3.64–8.4 |
| Na (mmol/L) | 142.2 ± 2.7 | 141.0 ± 1.9 | 141.2 ± 2.1 | 142.0 ± 3.2 | 137–147 |
| K (mmol/L) | 7.16 ± 0.90 | 7.80 ± 0.97 | 8.31 ± 1.24 * | 6.79 ± 0.82 | 3.88–6.11 |
| Cl (mmol/L) | 103.62 ± 1.70 | 103.52 ± 2.11 | 103.20 ± 1.84 | 103.25 ± 1.59 | 98–106 |
| TSH (ng/mL) | 3.245 ± 0.212 | 2.827 ± 0.293 * | 3.930 ± 0.444 ** | 3.590 ± 0.595 | ND |
| T3 (ng/mL) | 1.365 ± 0.052 | 1.471 ± 0.097 * | 1.684 ± 0.122 *** | 1.768 ± 0.085 *** | ND |
| T4 (ng/mL) | 62.818 ± 5.247 | 57.874 ± 5.593 | 51.336 ± 4.332 *** | 53.748 ± 3.091 *** | ND |
| Females | |||||
| AST (U/L) | 108.8 ± 17.0 | 86.4 ± 26.6 * | 88.7 ± 18.3 | 69.0 ± 11.6 *** | 64–222 |
| ALT (U/L) | 31.4 ± 8.3 | 23.5 ± 7.3 * | 24.5 ± 4.7 | 20.1 ± 4.1 *** | 14–64 |
| ALKP (U/L) | 30.4 ± 10.2 | 19.6 ± 2.3 ** | 22.4 ± 6.4 * | 18.5 ± 3.9 *** | 18–62 |
| BILI (mg/dL) | 0.067 ± 0.020 | 0.070 ± 0.018 | 0.057 ± 0.016 | 0.062 ± 0.018 | 0.07–0.2 |
| BUN (mg/dL) | 23.8 ± 3.9 | 23.0 ± 4.1 | 22.2 ± 4.7 | 21.2 ± 4.5 | 11.7–25.0 |
| CREA (mg/dL) | 0.313 ± 0.050 | 0.289 ± 0.056 | 0.268 ± 0.046 | 0.237 ± 0.035 ** | 0.3–0.6 |
| CHOL (mg/dL) | 46.5 ± 12.0 | 61.7 ± 11.0 * | 63.7 ± 12.0 ** | 68.7 ± 9.9 *** | 23–97 |
| LDL (mmol/L) | 0.120 ± 0.036 | 0.144 ± 0.044 | 0.146 ± 0.040 | 0.160 ± 0.043 | ND |
| HDL (mmol/L) | 1.023 ± 0.266 | 1.358 ± 0.217 * | 1.374 ± 0.255 ** | 1.477 ± 0.261 *** | ND |
| TRIG (mg/dL) | 40.9 ± 11.9 | 50.3 ± 9.8 | 51.2 ± 10.4 | 55.6 ± 16.5 | 16–175 |
| SDH (U/L) | 13.75 ± 6.31 | 15.79 ± 7.21 | 11.36 ± 5.10 | 11.60 ± 3.76 | ND |
| GLUC (mg/dL) | 174.0 ± 50.0 | 175.5 ± 51.9 | 190.4 ± 63.8 | 195.2 ± 28.0 | 89–163 |
| TP (g/dL) | 5.24 ± 0.43 | 5.78 ± 0.26 * | 5.59 ± 0.33 | 5.73 ± 0.63 * | 5.7–8.3 |
| ALB (g/dL) | 4.10 ± 0.42 | 4.57 ± 0.17 | 4.32 ± 0.34 | 4.59 ± 0.67 | 3.7–5.8 |
| GLOB (g/dL) | 1.14 ± 0.18 | 1.21 ± 0.17 | 1.27 ± 0.09 | 1.14 ± 0.16 | 1.6–2.3 |
| Ca (mg/dL) | 11.20 ± 0.54 | 11.98 ± 0.67 | 12.55 ± 1.25 ** | 12.65 ± 1.06 ** | 9.5–12.1 |
| P (mg/dL) | 9.12 ± 1.38 | 9.13 ± 1.00 | 9.35 ± 1.42 | 9.23 ± 1.13 | 4.53–9.51 |
| Na (mmol/L) | 138.1 ± 3.7 | 140.9 ± 1.9 | 142.0 ± 3.0 * | 139.8 ± 2.7 | 135–146 |
| K (mmol/L) | 7.67 ± 1.70 | 7.33 ± 1.27 | 6.93 ± 0.91 | 7.40 ± 1.00 | 3.37–5.11 |
| Cl (mmol/L) | 104.72 ± 2.74 | 105.67 ± 2.25 | 107.06 ± 2.58 | 104.98 ± 1.81 | 97–106 |
| TSH (ng/mL) | 3.316 ± 0.424 | 2.955 ± 0.191 | 3.956 ± 0.500 ** | 3.564 ± 0.463 | ND |
| T3 (ng/mL) | 1.579 ± 0.139 | 1.710 ± 0.299 | 1.827 ± 0.069 ** | 1.844 ± 0.177 ** | ND |
| T4 (ng/mL) | 59.631 ± 8.321 | 57.190 ± 9.115 | 57.918 ± 6.676 | 53.628 ± 5.312 | ND |
| Parameter | Control | 75,000 ppm | 125,000 ppm | 200,000 ppm |
|---|---|---|---|---|
| Males | ||||
| Terminal body weight (g) | 483.6 ± 31.3 | 500.3 ± 32.3 | 510.2 ± 35.7 | 496.3 ± 24.7 |
| Adrenals (g) | 0.078 ± 0.011 | 0.075 ± 0.013 | 0.075 ± 0.011 | 0.076 ± 0.018 |
| Adrenals/TBW | 0.163 ± 0.027 | 0.149 ± 0.021 | 0.146 ± 0.017 | 0.152 ± 0.032 |
| Brain (g) | 2.194 ± 0.073 | 2.188 ± 0.083 | 2.159 ± 0.263 | 2.178 ± 0.115 |
| Brain/TBW | 4.551 ± 0.276 | 4.385 ± 0.259 | 4.235 ± 0.490 | 4.397 ± 0.300 |
| Epididymides (g) | 1.546 ± 0.109 | 1.640 ± 0.125 | 1.666 ± 0.146 | 1.578 ± 0.198 |
| Epididymides/TBW | 3.204 ± 0.258 | 3.286 ± 0.272 | 3.283 ± 0.408 | 3.180 ± 0.366 |
| Heart (g) | 1.323 ± 0.099 | 1.335 ± 0.074 | 1.398 ± 0.066 | 1.280 ± 0.076 |
| Heart/TBW | 2.736 ± 0.125 | 2.673 ± 0.140 | 2.750 ± 0.209 | 2.581 ± 0.124 |
| Kidneys (g) | 3.403 ± 0.304 | 3.418 ± 0.308 | 3.560 ± 0.214 | 3.460 ± 0.229 |
| Kidneys/TBW | 7.052 ± 0.659 | 6.827 ± 0.320 | 7.005 ± 0.599 | 6.972 ± 0.315 |
| Liver (g) | 12.803 ± 1.164 | 13.572 ± 1.488 | 14.248 ± 0.960 | 13.590 ± 0.673 |
| Liver/TBW | 26.441 ± 1.044 | 27.094 ± 1.962 | 27.964 ± 1.530 | 27.415 ± 1.412 |
| Pituitary (g) | 0.016 ± 0.006 | 0.016 ± 0.004 | 0.017 ± 0.006 | 0.021 ± 0.004 |
| Pituitary/TBW | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.004 ± 0.001 |
| Prostate + SV + CG (g) | 3.580 ± 0.412 | 3.329 ± 0.665 | 3.525 ± 0.524 | 3.242 ± 0.337 |
| Prostate + SV + CG/TBW | 0.007 ± 0.001 | 0.007 ± 0.001 | 0.007 ± 0.001 | 0.007 ± 0.001 |
| Spleen (g) | 0.897 ± 0.154 | 0.940 ± 0.142 | 1.021 ± 0.114 | 0.937 ± 0.102 |
| Spleen/TBW | 1.853 ± 0.275 | 1.885 ± 0.310 | 2.003 ± 0.198 | 1.892 ± 0.227 |
| Testes (g) | 3.882 ± 0.192 | 3.856 ± 0.193 | 3.905 ± 0.297 | 3.761 ± 0.168 |
| Testes/TBW | 8.044 ± 0.437 | 7.726 ± 0.467 | 7.705 ± 0.947 | 7.586 ± 0.342 |
| Thymus (g) | 0.324 ± 0.073 | 0.333 ± 0.093 | 0.315 ± 0.060 | 0.353 ± 0.061 |
| Thymus/TBW | 0.667 ± 0.134 | 0.668 ± 0.188 | 0.616 ± 0.108 | 0.709 ± 0.109 |
| Thyroid + PT | 0.040 ± 0.010 | 0.043 ± 0.009 | 0.043 ± 0.009 | 0.045 ± 0.013 |
| Thyroid + PT/TBW | 0.824 ± 0.236 | 0.855 ± 0.182 | 0.843 ± 0.169 | 0.914 ± 0.268 |
| Thyroid + PT/TBrW | 0.0180 ± 0.004 | 0.0195 ± 0.004 | 0.020 ± 0.004 | 0.021 ± 0.006 |
| Females | ||||
| Terminal body weight (g) | 249.6 ± 20.4 | 263.7 ± 27.7 | 270.9 ± 19.2 | 267.5 ± 20.5 |
| Adrenals (g) | 0.100 ± 0.010 | 0.093 ± 0.008 | 0.094 ± 0.011 | 0.101 ± 0.012 |
| Adrenals/TBW | 0.401 ± 0.050 | 0.356 ± 0.042 | 0.351 ± 0.048 | 0.381 ± 0.061 |
| Brain (g) | 1.996 ± 0.112 | 1.975 ± 0.065 | 2.001 ± 0.109 | 2.032 ± 0.101 |
| Brain/TBW | 8.026 ± 0.537 | 7.571 ± 0.900 | 7.414 ± 0.589 | 7.625 ± 0.533 |
| Heart (g) | 0.873 ± 0.090 | 0.875 ± 0.075 | 0.889 ± 0.059 | 0.919 ± 0.085 |
| Heart/TBW | 3.503 ± 0.286 | 3.330 ± 0.221 | 3.285 ± 0.141 | 3.441 ± 0.252 |
| Kidneys (g) | 1.827 ± 0.148 | 1.925 ± 0.190 | 2.017 ± 0.177 | 2.269 ± 0.302 *** |
| Kidneys/TBW | 7.347 ± 0.662 | 7.334 ± 0.717 | 7.451 ± 0.486 | 8.482 ± 0.866 ** |
| Kidneys/TBrW | 0.915 ± 0.050 | 0.975 ± 0.096 | 1.009 ± 0.084 | 1.114 ± 0.111 *** |
| Liver (g) | 7.037 ± 0.903 | 7.519 ± 0.818 | 7.614 ± 0.610 | 7.961 ± 0.870 |
| Liver/TBW | 28.132 ± 2.089 | 28.534 ± 1.538 | 28.153 ± 1.989 | 29.794 ± 2.744 |
| Ovaries (g) | 0.148 ± 0.030 | 0.137 ± 0.016 | 0.134 ± 0.020 | 0.135 ± 0.023 |
| Ovaries/TBW | 0.595 ± 0.128 | 0.521 ± 0.055 | 0.500 ± 0.093 | 0.502 ± 0.066 |
| Pituitary (g) | 0.020 ± 0.006 | 0.018 ± 0.003 | 0.018 ± 0.004 | 0.020 ± 0.005 |
| Pituitary/TBW | 0.008 ± 0.002 | 0.007 ± 0.001 | 0.007 ± 0.002 | 0.007 ± 0.002 |
| Spleen (g) | 0.560 ± 0.075 | 0.574 ± 0.091 | 0.656 ± 0.118 | 0.597 ± 0.067 |
| Spleen/TBW | 2.245 ± 0.252 | 2.172 ± 0.198 | 2.42 ± 0.381 | 2.231 ± 0.187 |
| Thymus (g) | 0.249 ± 0.075 | 0.218 ± 0.044 | 0.237 ± 0.071 | 0.229 ± 0.078 |
| Thymus/TBW | 0.987 ± 0.241 | 0.827 ± 0.136 | 0.878 ± 0.252 | 0.853 ± 0.264 |
| Thyroid+ PT | 0.048 ± 0.014 | 0.033 ± 0.008 ** | 0.036 ± 0.010 * | 0.033 ± 0.007 ** |
| Thyroid + PT/TBW | 1.946 ± 0.597 | 1.245 ± 0.260 ** | 1.349 ± 0.424 ** | 1.225 ± 0.286 ** |
| Thyroid + PT/TBrW | 0.024 ± 0.007 | 0.017 ± 0.004 ** | 0.018 ± 0.005 * | 0.016 ± 0.003 ** |
| Uterus (g) | 0.757 ± 0.363 | 0.632 ± 0.232 | 0.648 ± 0.285 | 0.647 ± 0.254 |
| Uterus/TBW | 2.988 ± 1.235 | 2.408 ± 0.874 | 2.403 ± 1.066 | 2.397 ± 0.833 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolan, L.C.; Karikachery, A.R.; Thipe, V.C.; Arceneaux, B.G.; Katti, K.K.; Katti, K.V.; Chesne, A.M. Toxicity Investigations of (R)-3-Hydroxybutyrate Glycerides In Vitro and in Male and Female Rats. Nutrients 2022, 14, 4426. https://doi.org/10.3390/nu14204426
Dolan LC, Karikachery AR, Thipe VC, Arceneaux BG, Katti KK, Katti KV, Chesne AM. Toxicity Investigations of (R)-3-Hydroxybutyrate Glycerides In Vitro and in Male and Female Rats. Nutrients. 2022; 14(20):4426. https://doi.org/10.3390/nu14204426
Chicago/Turabian StyleDolan, Laurie C., Alice Raphael Karikachery, Velaphi C. Thipe, Benjamin G. Arceneaux, Kavita K. Katti, Kattesh V. Katti, and Alton M. Chesne. 2022. "Toxicity Investigations of (R)-3-Hydroxybutyrate Glycerides In Vitro and in Male and Female Rats" Nutrients 14, no. 20: 4426. https://doi.org/10.3390/nu14204426
APA StyleDolan, L. C., Karikachery, A. R., Thipe, V. C., Arceneaux, B. G., Katti, K. K., Katti, K. V., & Chesne, A. M. (2022). Toxicity Investigations of (R)-3-Hydroxybutyrate Glycerides In Vitro and in Male and Female Rats. Nutrients, 14(20), 4426. https://doi.org/10.3390/nu14204426






