The Age-Accompanied and Diet-Associated Remodeling of the Phospholipid, Amino Acid, and SCFA Metabolism of Healthy Centenarians from a Chinese Longevous Region: A Window into Exceptional Longevity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Dietary Assessment
2.3. Sample Collection and Preparation
2.4. Non-Targeted Metabolomics Analysis Based on UPLC-MS
2.5. Analysis of SCFAs in Feces
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Characteristics of the Participants
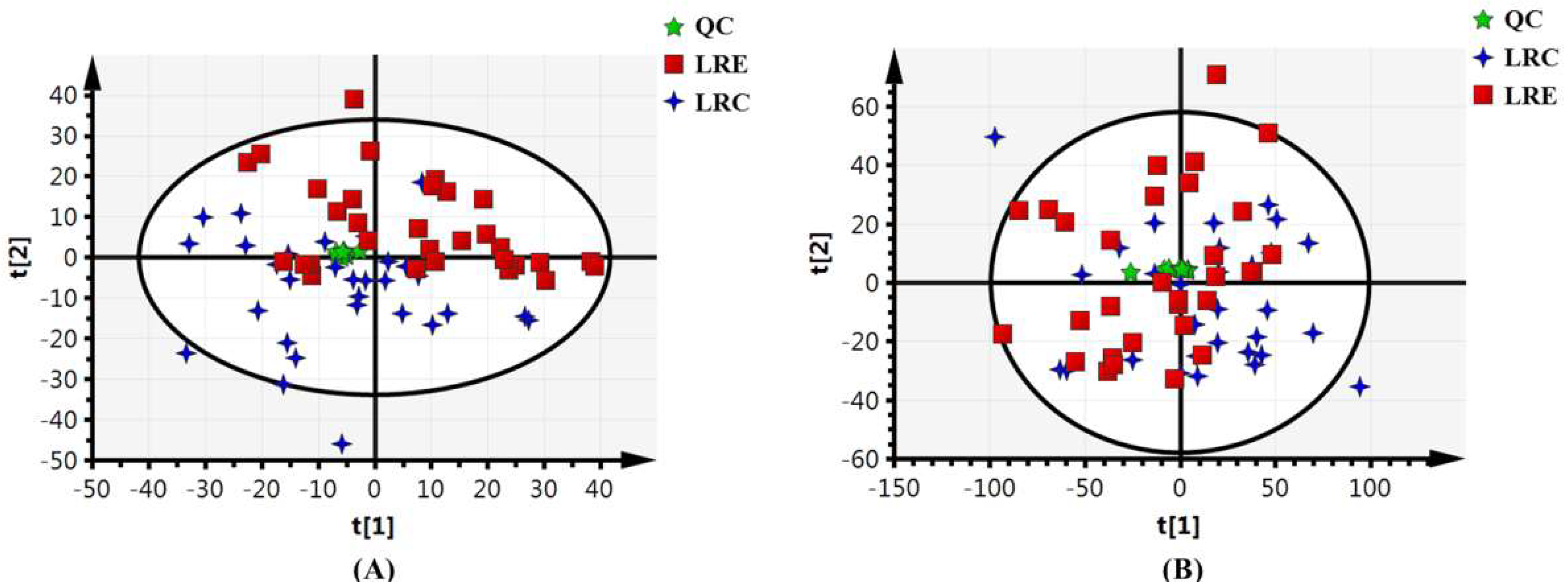
3.2. Validation of Stability and Repeatability of Metabolomics Analysis
3.3. Global Metabolic Profiling of Urine
3.4. Identification of Characteristic Metabolites of the Centenarians
3.5. Correlation Relationships of Differential Metabolites in Urine
3.6. Discovery of Metabolic Pathways Relevant to Healthy Aging
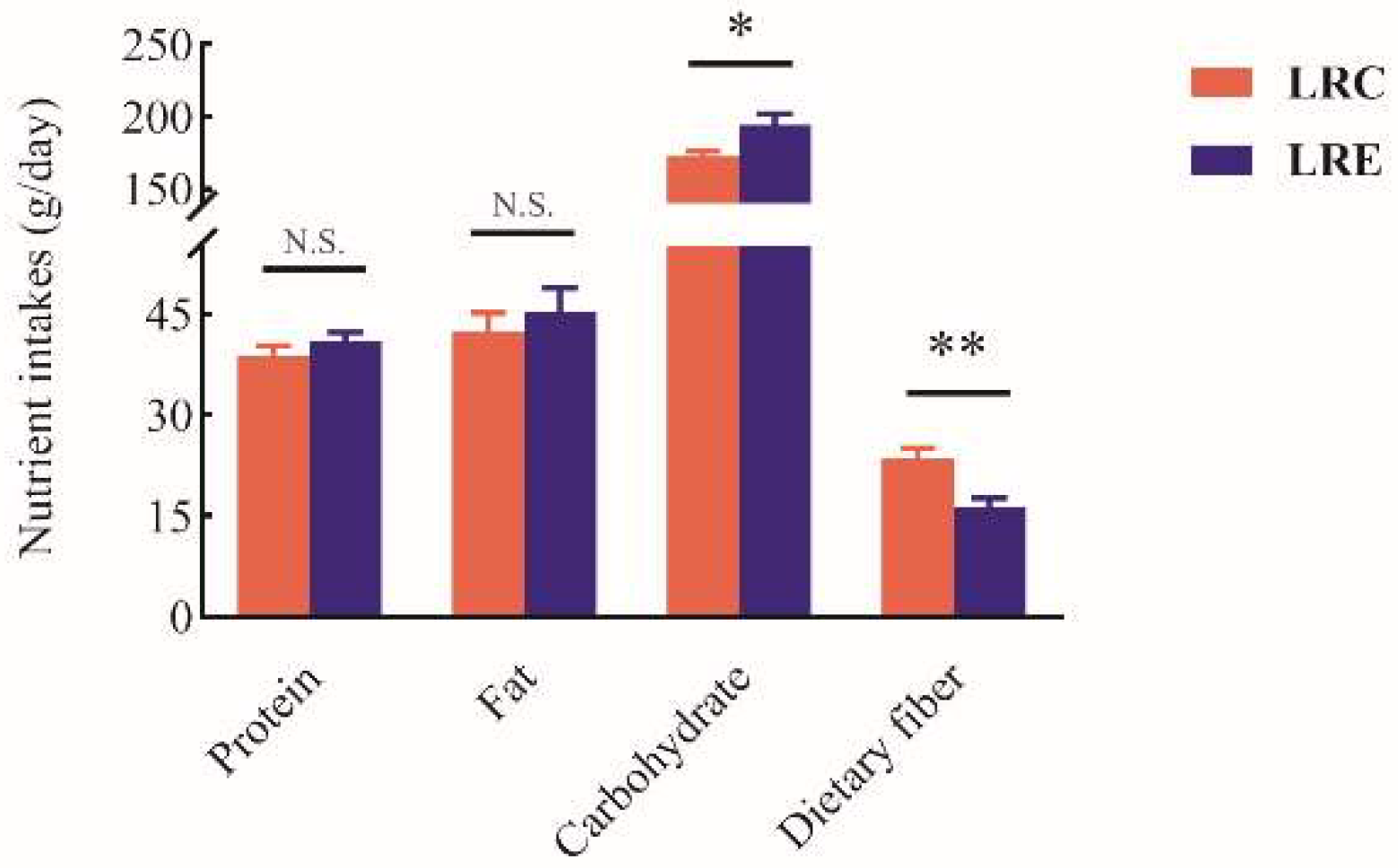
3.7. Diet-Associated Remodeling of SCFA Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collino, S.; Montoliu, I.; Martin, F.J.; Scherer, M.; Mari, D.; Salvioli, S.; Bucci, L.; Ostan, R.; Monti, D.; Biagi, E.; et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS ONE 2013, 8, e56564. [Google Scholar] [CrossRef]
- Wu, L.; Xie, X.; Liang, T.; Ma, J.; Yang, L.; Yang, J.; Li, L.; Xi, Y.; Li, H.; Zhang, J.; et al. Integrated Multi-Omics for Novel Aging Biomarkers and Antiaging Targets. Biomolecules 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Maté, I.; Naudí, A.; Mota-Martorell, N.; Portero-Otín, M.; Fuente, M.D.; Pamplona, R. Human Aging Is a Metabolome-related Matter of Gender. J. Gerontol. Biol. Sci. 2015, 71, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Chak, C.M.; Lacruz, M.E.; Adam, J.; Brandmaier, S.; Covic, M.; Huang, J.; Meisinger, C.; Tiller, D.; Prehn, C.; Adamski, J.; et al. Ageing Investigation Using Two-Time-Point Metabolomics Data from KORA and CARLA Studies. Metabolites 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Bunning, B.J.; Contrepois, K.; Lee-McMullen, B.; Dhondalay, G.K.R.; Zhang, W.; Tupa, D.; Raeber, O.; Desai, M.; Nadeau, K.C.; Snyder, M.P.; et al. Global metabolic profiling to model biological processes of aging in twins. Aging Cell 2020, 19, e13073. [Google Scholar] [CrossRef]
- Montoliu, I.; Scherer, M.; Beguelin, F.; DaSilva, L.; Mari, D.; Salvioli, S.; Martin, F.P.J.; Capri, M.; Bucci, L.; Ostan, R.; et al. Serum profiling of healthy aging identifies phosphor- and sphingolipid species as markers of human longevity. Aging 2014, 6, 9–25. [Google Scholar] [CrossRef]
- Interpretation of the Main Data Bulletin of the Seventh National Census in Bama Yao Autonomous County. Available online: http://www.bama.gov.cn/sjfb/tjgb/t9295862.shtml (accessed on 19 June 2021).
- Lv, J.; Wang, W.; Krafft, T.; Li, Y.; Zhang, F.; Yuan, F. Effects of several environmental factors on longevity and health of the human population of Zhongxiang, Hubei, China. Biol. Trace Elem. Res. 2011, 143, 702–716. [Google Scholar] [CrossRef]
- Cai, D.; Li, D.; Zhao, S.; Dou, X.; Wang, F.; Huang, G.; Zhao, M.; Li, Q. A Correlation between Diet and Longevity Characterization by Means of Element Profiles in Healthy People over 80 Years from a Chinese Longevous Region. Biol. Trace Elem. Res. 2015, 165, 18–29. [Google Scholar] [CrossRef]
- Cai, D.; Zhao, S.; Li, D.; Chang, F.; Tian, X.; Huang, G.; Zhu, Z.; Liu, D.; Dou, X.; Li, S.; et al. Nutrient Intake Is Associated with Longevity Characterization by Metabolites and Element Profiles of Healthy Centenarians. Nutrients 2016, 8, 564. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition; The Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Gika, H.G.; Theodoridis, G.A.; Wingate, J.E.; Wilson, I.D. Within-day reproducibility of an HPLC-MS-based method for metabonomic analysis: Application to human urine. J. Proteome Res. 2007, 6, 3291–3303. [Google Scholar] [CrossRef]
- Gika, H.G.; Macpherson, E.; Theodoridis, G.A.; Wilson, I.D. Evaluation of the repeatability of ultra-performance liquid chromatography-TOF-MS for global metabolic profiling of human urine samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhai, G.; Singmann, P.; He, Y.; Xu, T.; Prehn, C.; Römisch-Margl, W.; Lattka, E.; Gieger, C.; Soranzo, N.; et al. Human serum metabolic profiles are age dependent. Aging Cell 2012, 11, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.; Kim, H.S.; Jung, B.H. Metabolomic approaches to the normal aging process. Metabolomics 2014, 10, 1268–1292. [Google Scholar] [CrossRef]
- Pradas, I.; Jové, M.; Huynh, K.; Puig, J.; Ingles, M.; Borras, C.; Viña, J.; Meikle, P.J.; Pamplona, R. Exceptional human longevity is associated with a specific plasma phenotype of ether lipids. Redox Biol. 2019, 21, 101127. [Google Scholar] [CrossRef]
- Richter, Y.; Herzog, Y.; Lifshitz, Y.; Hayun, R.; Zchut, S. The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: A pilot study. Clin. Interv. Aging 2013, 8, 557–563. [Google Scholar]
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef]
- Zhao, Y.; Hasse, S.; Vaillancourt, M.; Zhao, C.; Davis, L.; Boilard, E.; Fortin, P.; Di Battista, J.; Poubelle, P.E.; Bourgoin, S.G. Phospholipase A1 Member A Activates Fibroblast-like Synoviocytes through the Autotaxin-Lysophosphatidic Acid Receptor Axis. Int. J. Mol. Sci. 2021, 22, 12685. [Google Scholar] [CrossRef]
- Weng, Z.; Chen, Y.; Lv, J.; Wang, M.; Chen, Z.; Zhou, W.; Shen, X.; Zhan, L.; Wang, F. A Review of Bile Acid Metabolism and Signaling in Cognitive Dysfunction-Related Diseases. Oxid. Med. Cell. Longev. 2022, 2020, 4289383. [Google Scholar] [CrossRef]
- Arlia-Ciommo, A.; Piano, A.; Svistkova, V.; Mohtashami, S.; Titorenko, V.I. Mechanisms Underlying the Anti-Aging and Anti-Tumor Effects of Lithocholic Bile Acid. Int. J. Mol. Sci. 2014, 15, 16522–16543. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, Q.; Zhu, Q.; Liu, J.; Dong, Y.; Li, Y.; Wang, R.; Tang, X.; Lv, X.; Li, X.; et al. Metabolomics Study of Isocaloric Different Dietary Patterns on the Life Span in Healthy Population. Clin. Interv. Aging 2021, 16, 2111–2123. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Aging, proteotoxicity, mitochondria, glycation, NAD and carnosine: Possible inter-relationships and resolution of the oxygen paradox. Front. Aging Neurosci. 2010, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Green, C.L.; Wang, G.; Yang, D.; Li, L.; Li, B.; Wang, L.; Li, M.; Li, J.; Xu, Y.; et al. Effects of dietary macronutrients on the hepatic transcriptome and serum metabolome in mice. Aging Cell 2022, 21, e13585. [Google Scholar] [CrossRef] [PubMed]
- Delic, V.; Griffin, J.W.D.; Zivkovic, S.; Zhang, Y.; Phan, T.A.; Gong, H.; Chaput, D.; Reynes, C.; Dinh, V.B.; Cruz, J.; et al. Individual amino acid supplementation can improve energy metabolism and decrease ROS production in neuronal cells overexpressing alpha-synuclein. NeuroMol. Med. 2017, 19, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Lu, L.; Deng, J.; Fan, W.; Li, T.; Yao, J. Effects of glutamate and aspartate on serum antioxidative enzyme, sex hormones, and genital inflammation in boars challenged with hydrogen peroxide mediators. Inflamm. 2016, 10, 4394695. [Google Scholar] [CrossRef]
- Darcy, J.; Fang, Y.; McFadden, S.; Lynes, M.D.; Leiria, L.O.; Dreyfuss, J.M.; Bussburg, V.; Tolstikov, V.; Greenwood, B.; Narain, N.R.; et al. Integrated metabolomics reveals altered lipid metabolism in adipose tissue in a model of extreme longevity. GeroScience 2020, 42, 1527–1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Herrup, K. Glutamine acts as a neuroprotectant against DNA damage, beta-amyloid and H2O2-induced stress. PLoS ONE 2012, 7, e33177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Kerbl-Knapp, J.; Zhang, F.; Korbelius, M.; Kuentzel, K.B.; Vujić, N.; Akhmetshina, A.; Hörl, G.; Paar, M.; Steyrer, E.; et al. Metabolomic Profiles of Mouse Tissues Reveal an Interplay between Aging and Energy Metabolism. Metabolites 2022, 12, 17. [Google Scholar] [CrossRef]
- Shi, D.; Xia, X.; Cui, A.; Xiong, Z.; Yan, Y.; Luo, J.; Chen, G.; Zeng, Y.; Cai, D.; Hou, L.; et al. The precursor of PI(3,4,5)P3 alleviates aging by activating daf-18(Pten) and independent of daf-16. Nat. Commun. 2020, 11, 4496. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, S.; Sanchez-Carrillo, S.; Ciordia, S.; Mena, M.C.; Méndez-García, C.; Rojo, D.; Bargiela, R.; Zubeldia-Varela, E.; Martínez-Martínez, M.; Barbas, C.; et al. Functional microbiome deficits associated with ageing: Chronological age threshold. Aging Cell 2020, 19, e13063. [Google Scholar] [CrossRef]
- Collet, T.; Sonoyama, T.; Henning, E.; Keogh, J.M.; Ingram, B.; Kelway, S.; Guo, L.; Farooqi, I.S. A Metabolomic Signature of Acute Caloric Restriction. J. Clin. Endocrinol. Metab. 2017, 102, 4486–4495. [Google Scholar] [CrossRef]
- Douris, N.; Melman, T.; Pecherer, J.M.; Pissios, P.; Flier, J.S.; Cantley, L.C.; Locasale, J.W.; Maratos-Flier, E. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochim. Biophys. Acta 2015, 1852, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Bernier, M.; Mitchell, S.J.; Germanio, C.D.; Mattison, J.A.; Ehrlich, M.R.; Colman, R.J.; Anderson, R.M.; Cabo, R. Untangling determinants of enhanced health and lifespan through a multi-omics approach in mice. Cell Metab. 2020, 32, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Vadder, F.D.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Yuan, Y.; Dong, F.X.; Liu, X.; Xiao, H.B.; Zhou, Z.G. Liquid Chromatograph-Mass Spectrometry-Based Non-targeted Metabolomics Discovery of Potential Endogenous Biomarkers Associated with Prostatitis Rats to Reveal the Effects of Magnoflorine. Front. Pharmacol. 2021, 12, 741378. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, F.; Zhu, J. Bridging Targeted and Untargeted Mass Spectrometry-Based Metabolomics via Hybrid Approaches. Metabolites 2020, 10, 348. [Google Scholar] [CrossRef]
- Li, H.; Ren, M.; Li, Q. 1H NMR-Based Metabolomics Reveals the Intrinsic Interaction of Age, Plasma Signature Metabolites, and Nutrient Intake in the Longevity Population in Guangxi, China. Nutrients 2022, 14, 2539. [Google Scholar] [CrossRef]
- Tokudome, Y.; Imaeda, N.; Nagaya, T.; Ikeda, M.; Fujiwara, N.; Sato, J.; Kuriki, K.; Kikuchi, S.; Maki, S.; Tokudome, S. Daily, weekly, seasonal, within- and between-individual variation in nutrient intake according to four season consecutive 7 day weighed diet records in Japanese female dietitians. J. Epidemiol. 2002, 12, 85–92. [Google Scholar] [CrossRef]
- Zhuang, M.; Yuan, Z.; Lin, L.; Hu, B.; Wang, X.; Yang, Y.; Chen, X.; Jin, L.; Lu, M.; Ye, W. Reproducibility and relative validity of a food frequency questionnaire developed for adults in Taizhou, China. PLoS ONE 2012, 7, e48341. [Google Scholar] [CrossRef]
- Davinelli, S.; Willcox, D.C.; Scapagnini, G. Extending healthy ageing: Nutrient sensitive pathway and centenarian population. Immun. Ageing 2012, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Boulesteix, A.L.; Strimmer, K. Partial least squares: A versatile tool for the analysis of high-dimensional genomic data. Brief. Bioinform. 2006, 8, 32–44. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | LRC Group | LRE Group |
|---|---|---|
| Age (year) | 103 ± 3 | 63 ± 3 |
| Sex (male/female) | 11/19 | 12/19 |
| Height (cm) | 145.9 ± 10.6 | 153.7 ± 7.2 |
| Weight (kg) | 43.1 ± 10.0 | 49.4 ± 9.4 |
| Body mass index (kg/m2) | 20.0 ± 2.8 | 20.8 ± 2.9 |
| Component | R2X (cum) | R2Y (cum) | Q2Y (cum) | |
|---|---|---|---|---|
| ESI+ mode | 4P+1O | 0.329 | 0.984 | 0.801 |
| ESI− mode | 5P+1O | 0.434 | 0.993 | 0.796 |
| Metabolites | Molecular Weight | Retention Time | VIP | p | FC | Change Trend |
|---|---|---|---|---|---|---|
| PS(22:4(7Z,10Z,13Z,16Z)/22:4(7Z,10Z,13Z,16Z)) | 887.5598 | 11.82 | 2.361 | <0.001 | 12.478 | ↑ |
| PS(20:0/19:0) | 833.6138 | 12.50 | 2.922 | <0.001 | 12.004 | ↑ |
| PS(O-18:0/19:0) | 791.6030 | 7.77 | 2.641 | <0.001 | 12.892 | ↑ |
| PS(22:0/18:3(6Z,9Z,12Z)) | 841.5833 | 8.48 | 3.275 | <0.001 | 4.824 | ↑ |
| LysoPE(0:0/18:1(11Z)) | 479.3020 | 8.02 | 3.044 | <0.001 | 1.154 | ↑ |
| LysoPE(0:0/22:5(7Z,10Z,13Z,16Z,19Z)) | 527.3031 | 7.83 | 3.062 | <0.001 | 2.630 | ↑ |
| LysoPE(0:0/22:4(7Z,10Z,13Z,16Z)) | 529.3183 | 8.12 | 3.255 | <0.001 | 2.189 | ↑ |
| LysoPE(0:0/20:4(5Z,8Z,11Z,14Z)) | 501.2858 | 7.31 | 3.005 | <0.001 | 1.066 | ↑ |
| LysoPE(0:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 525.2857 | 7.27 | 2.949 | <0.001 | 1.948 | ↑ |
| LysoPE(0:0/18:4(6Z,9Z,12Z,15Z)) | 473.2537 | 4.31 | 1.588 | 0.004 | 3.118 | ↑ |
| PI(20:2(11Z,14Z)/18:3(6Z,9Z,12Z)) | 884.5386 | 8.26 | 1.416 | 0.010 | 10.920 | ↑ |
| PC(16:0/17:1(9Z)) | 745.5593 | 9.96 | 1.612 | 0.003 | 9.817 | ↑ |
| Deoxycholic acid | 392.2937 | 5.76 | 1.673 | 0.002 | 3.204 | ↑ |
| Glycocholic acid | 465.3101 | 4.33 | 1.424 | 0.010 | 1.709 | ↑ |
| Cholic acid | 408.2888 | 4.74 | 1.447 | 0.009 | 2.910 | ↑ |
| Nutriacholic acid | 390.2742 | 4.74 | 1.397 | 0.006 | 1.987 | ↑ |
| PG(12:0/0:0) | 428.2236 | 6.35 | 1.444 | 0.009 | 2.348 | ↑ |
| MG(0:0/20:4(5Z,8Z,11Z,14Z)/0:0) | 378.2778 | 6.25 | 1.908 | <0.001 | 3.062 | ↑ |
| Niacin | 123.0325 | 1.24 | 2.013 | <0.001 | 1.059 | ↑ |
| Caffeic acid | 180.0418 | 1.86 | 3.143 | <0.001 | 1.134 | ↑ |
| Orotic acid | 156.0172 | 0.90 | 1.384 | 0.012 | 1.012 | ↑ |
| Urothion | 324.1676 | 3.45 | 1.655 | 0.001 | 1.770 | ↑ |
| Histamine | 111.0801 | 0.82 | 2.233 | <0.001 | −1.471 | ↓ |
| L-Histidine | 155.0702 | 0.77 | 2.340 | <0.001 | −1.227 | ↓ |
| Citrulline | 175.0965 | 0.86 | 1.869 | 0.001 | −1.449 | ↓ |
| L-Lysine | 146.1060 | 0.86 | 1.691 | 0.002 | −1.199 | ↓ |
| Hydroxylysine | 162.1011 | 0.75 | 1.348 | 0.015 | −1.419 | ↓ |
| Indole | 117.0543 | 0.86 | 2.338 | <0.001 | −1.057 | ↓ |
| Pathway Name | Total | Hits | Raw p | Holm Adjusted p | Impact |
|---|---|---|---|---|---|
| Alanine, aspartate and glutamate metabolism | 28 | 4 | 4.96 × 10−6 | 0.0002 | 0.42 |
| β-Alanine metabolism | 21 | 3 | 6.75 × 10−6 | 0.0003 | 0.40 |
| Histidine metabolism | 16 | 4 | 6.85 × 10−6 | 0.0003 | 0.53 |
| Tryptophan metabolism | 41 | 2 | 3.14 × 10−5 | 0.0010 | 0.27 |
| Ascorbate and aldarate metabolism | 8 | 3 | 0.0003 | 0.0089 | 0.50 |
| Arginine biosynthesis | 14 | 6 | 0.0003 | 0.0089 | 0.30 |
| Pyruvate metabolism | 22 | 1 | 0.0059 | 0.0828 | 0.21 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 4 | 1 | 0.0060 | 0.0828 | 0.50 |
| LRC Group | LRE Group | p | |
|---|---|---|---|
| Energy (Kcal) | 1220.30 ± 134.60 a | 1349.42 ± 97.67 b | <0.001 |
| Protein-calorie percent composition | 12.72% ± 1.56% a | 12.11% ± 1.99% a | 0.188 |
| Fat-calorie percent composition | 30.72% ± 7.97% a | 29.91% ± 11.37% a | 0.748 |
| Carbohydrate-calorie percent composition | 56.90% ± 6.33% a | 57.89% ± 12.54% a | 0.699 |
| Acetic Acid | Propionic Acid | Isobutyric Acid | Butyric Acid | Isovaleric Acid | Valeric Acid | Total SCFA | |
|---|---|---|---|---|---|---|---|
| R | 0.548 ** | 0.571 ** | 0.219 | 0.930 ** | 0.112 | 0.408 ** | 0.724 ** |
| p | <0.001 | <0.001 | 0.089 | <0.001 | 0.392 | 0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, D.; Zhao, Z.; Zhao, L.; Dong, Y.; Wang, L.; Zhao, S.; Li, Q. The Age-Accompanied and Diet-Associated Remodeling of the Phospholipid, Amino Acid, and SCFA Metabolism of Healthy Centenarians from a Chinese Longevous Region: A Window into Exceptional Longevity. Nutrients 2022, 14, 4420. https://doi.org/10.3390/nu14204420
Cai D, Zhao Z, Zhao L, Dong Y, Wang L, Zhao S, Li Q. The Age-Accompanied and Diet-Associated Remodeling of the Phospholipid, Amino Acid, and SCFA Metabolism of Healthy Centenarians from a Chinese Longevous Region: A Window into Exceptional Longevity. Nutrients. 2022; 14(20):4420. https://doi.org/10.3390/nu14204420
Chicago/Turabian StyleCai, Da, Zimo Zhao, Lingjun Zhao, Yanjie Dong, Lei Wang, Shancang Zhao, and Quanyang Li. 2022. "The Age-Accompanied and Diet-Associated Remodeling of the Phospholipid, Amino Acid, and SCFA Metabolism of Healthy Centenarians from a Chinese Longevous Region: A Window into Exceptional Longevity" Nutrients 14, no. 20: 4420. https://doi.org/10.3390/nu14204420
APA StyleCai, D., Zhao, Z., Zhao, L., Dong, Y., Wang, L., Zhao, S., & Li, Q. (2022). The Age-Accompanied and Diet-Associated Remodeling of the Phospholipid, Amino Acid, and SCFA Metabolism of Healthy Centenarians from a Chinese Longevous Region: A Window into Exceptional Longevity. Nutrients, 14(20), 4420. https://doi.org/10.3390/nu14204420




