Association between Branched-Chain Amino Acid Intake and Physical Function among Chinese Community-Dwelling Elderly Residents
Abstract
1. Introduction
2. Materials and Methods
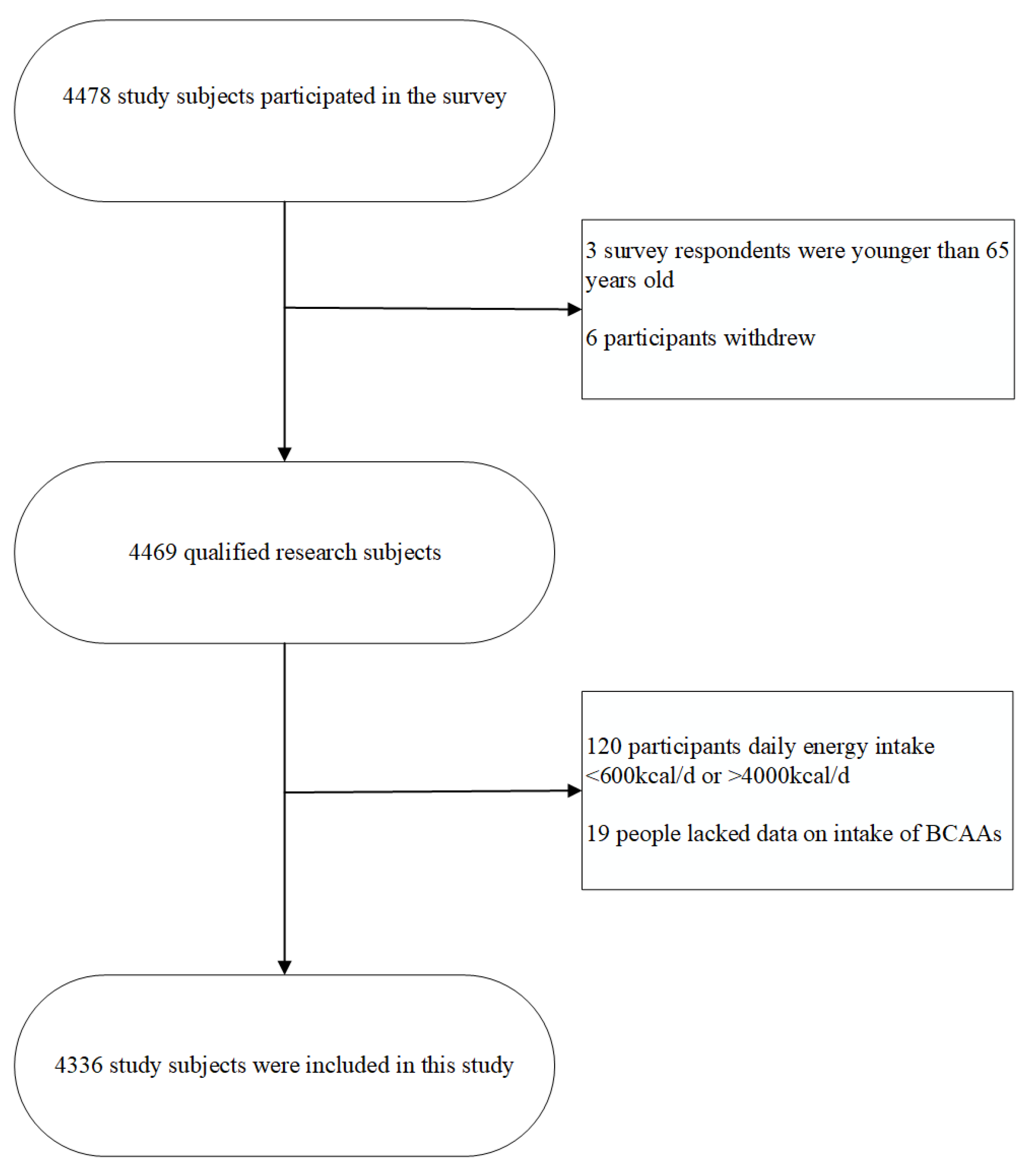
2.1. Participants
2.2. Covariate Collection
2.3. Dietary Assessment
2.4. Muscle Strength and Functional Performance Measures
2.5. Criteria for Physical Function Decline
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Addressing the Challenges of Population Ageing in Asia and the Pacific, Implementation of the Madrid International Plan of Action on Ageing. Available online: https://www.unescap.org/sites/default/files/publications/Addressing%20the%20Challenges%20of%20Population%20Ageing%20in%20Asia%20and%20the%20Pacific.pdf (accessed on 13 September 2017).
- Farsijani, S.; Payette, H.; Morais, J.A.; Shatenstein, B.; Gaudreau, P.; Chevalier, S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: The Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study). Am. J. Clin. Nutr. 2017, 106, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Increased healthcare costs associated with frailty among community-dwelling older people: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2019, 84, 103898. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, C.; Charnley, J.M.; Evans, W.J.; Crim, M.C. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am. J. Clin. Nutr. 1995, 62, 30–39. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Cifelli, A.M.; Kostas, G.; Kim, I.Y. Optimizing Protein Intake in Adults: Interpretation and Application of the Recommended Dietary Allowance Compared with the Acceptable Macronutrient Distribution Range. Adv. Nutr. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Wallace, T.C.; Frankenfeld, C.L. Dietary protein intake above the current RDA and bone health: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2017, 36, 481–496. [Google Scholar] [CrossRef]
- Rizzoli, R.; Biver, E.; Bonjour, J.P.; Coxam, V.; Goltzman, D.; Kanis, J.A.; Lappe, J.; Rejnmark, L.; Sahni, S.; Weaver, C.; et al. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos Int. 2018, 29, 1933–1948. [Google Scholar] [CrossRef]
- Fung, T.T.; Meyer, H.E.; Willett, W.C.; Feskanich, D. Protein intake and risk of hip fractures in postmenopausal women and men age 50 and older. Osteoporos Int. 2017, 28, 1401–1411. [Google Scholar] [CrossRef]
- Mustafa, J.; Ellison, R.C.; Singer, M.R.; Bradlee, M.L.; Kalesan, B.; Holick, M.F.; Moore, L.L. Dietary Protein and Preservation of Physical Functioning Among Middle-Aged and Older Adults in the Framingham Offspring Study. Am. J. Epidemiol. 2018, 187, 1411–1419. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Caldo-Silva, A.; Furtado, G.E.; Chupel, M.U.; Letieri, R.V.; Valente, P.A.; Farhang, M.; Barros, M.P.; Bachi, A.L.L.; Marzetti, E.; Teixeira, A.M.; et al. Effect of a 40-weeks multicomponent exercise program and branched chain amino acids supplementation on functional fitness and mental health in frail older persons. Exp. Gerontol. 2021, 155, 111592. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Antonio, L.; Dedeyne, L.; O’Neill, T.W.; Vanderschueren, D.; Rastrelli, G.; Maggi, M.; Bártfai, G.; Casanueva, F.F.; Giwercman, A.; et al. Inflammatory markers are associated with quality of life, physical activity, and gait speed but not sarcopenia in aged men (40–79 years). J. Cachexia Sarcopenia Muscle 2021, 12, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.; Luiking, Y.C.; van Helvoort, A.; Boirie, Y.; Schols, J.; de Groot, C. Low Levels of Branched Chain Amino Acids, Eicosapentaenoic Acid and Micronutrients Are Associated with Low Muscle Mass, Strength and Function in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 27–34. [Google Scholar] [CrossRef]
- Moriwaki, M.; Wakabayashi, H.; Sakata, K.; Domen, K. The Effect of Branched Chain Amino Acids-Enriched Nutritional Supplements on Activities of Daily Living and Muscle Mass in Inpatients with Gait Impairments: A Randomized Controlled Trial. J. Nutr. Health Aging 2019, 23, 348–353. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef]
- Singh Tejavath, A.; Mathur, A.; Nathiya, D.; Singh, P.; Raj, P.; Suman, S.; Mundada, P.R.; Atif, S.; Rai, R.R.; Tomar, B.S. Impact of Branched Chain Amino Acid on Muscle Mass, Muscle Strength, Physical Performance, Combined Survival, and Maintenance of Liver Function Changes in Laboratory and Prognostic Markers on Sarcopenic Patients With Liver Cirrhosis (BCAAS Study): A Randomized Clinical Trial. Front. Nutr. 2021, 8, 715795. [Google Scholar] [CrossRef]
- Zhang, C.X.; Ho, S.C. Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac. J. Clin. Nutr. 2009, 18, 240–250. [Google Scholar]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition; Peking University Medical Press: Beijing, China, 2009; Volume 42, pp. 795–799. [Google Scholar]
- Garg, P.K.; Liu, K.; Tian, L.; Guralnik, J.M.; Ferrucci, L.; Criqui, M.H.; Tan, J.; McDermott, M.M. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation 2009, 119, 251–260. [Google Scholar] [CrossRef]
- Landi, F.; Russo, A.; Liperoti, R.; Tosato, M.; Barillaro, C.; Pahor, M.; Bernabei, R.; Onder, G. Anorexia, physical function, and incident disability among the frail elderly population: Results from the ilSIRENTE study. J. Am. Med. Dir. Assoc. 2010, 11, 268–274. [Google Scholar] [CrossRef]
- Master, H.; Neogi, T.; Callahan, L.F.; Nelson, A.E.; LaValley, M.; Cleveland, R.J.; Golightly, Y.M.; Thoma, L.M.; Zhang, Y.; Voinier, D.; et al. The association between walking speed from short- and standard-distance tests with the risk of all-cause mortality among adults with radiographic knee osteoarthritis: Data from three large United States cohort studies. Osteoarthr. Cartil. 2020, 28, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Park, H.S.; Park, K. Association between dietary branched-chain amino acid intake and skeletal muscle mass index among Korean adults: Interaction with obesity. Nutr. Res. Pract. 2021, 15, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Asoudeh, F.; Salari-Moghaddam, A.; Keshteli, A.H.; Esmaillzadeh, A.; Adibi, P. Dietary intake of branched-chain amino acids in relation to general and abdominal obesity. Eat. Weight. Disord. 2021, 27, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Park, H.; Park, K. Estimation of Dietary Amino Acid Intake and Independent Correlates of Skeletal Muscle Mass Index among Korean Adults. Nutrients 2020, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chae, M.; Park, H.; Park, K. Higher Branched-Chain Amino Acid Intake Is Associated with Handgrip Strength among Korean Older Adults. Nutrients 2021, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Cereda, E.; Klersy, C.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Gasparri, C.; Iannello, G.; Spadaccini, D.; Infantino, V.; et al. Improving rehabilitation in sarcopenia: A randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J. Cachexia Sarcopenia Muscle 2020, 11, 1535–1547. [Google Scholar] [CrossRef]
- Ko, C.H.; Wu, S.J.; Wang, S.T.; Chang, Y.F.; Chang, C.S.; Kuan, T.S.; Chuang, H.Y.; Chang, C.M.; Chou, W.; Wu, C.H. Effects of enriched branched-chain amino acid supplementation on sarcopenia. Aging-Us 2020, 12, 15091–15103. [Google Scholar] [CrossRef]
- Guarente, L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell 2008, 132, 171–176. [Google Scholar] [CrossRef]
- López-Lluch, G.; Irusta, P.M.; Navas, P.; de Cabo, R. Mitochondrial biogenesis and healthy aging. Exp. Gerontol. 2008, 43, 813–819. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Newgard, C.B. Branched-chain amino acids in disease. Science 2019, 363, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Moberg, M.; Apró, W.; Ekblom, B.; van Hall, G.; Holmberg, H.C.; Blomstrand, E. Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Am. J. Physiol. Cell Physiol 2016, 310, C874–C884. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides in Combination with Leucine and Vitamin D Increase Muscle Strength and Function in Frail Elderly Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 1017–1026. [Google Scholar] [CrossRef]
- Buondonno, I.; Sassi, F.; Carignano, G.; Dutto, F.; Ferreri, C.; Pili, F.G.; Massaia, M.; Nisoli, E.; Ruocco, C.; Porrino, P.; et al. From mitochondria to healthy aging: The role of branched-chain amino acids treatment: MATeR a randomized study. Clin. Nutr. 2020, 39, 2080–2091. [Google Scholar] [CrossRef]
- Barichella, M.; Cereda, E.; Pinelli, G.; Iorio, L.; Caroli, D.; Masiero, I.; Ferri, V.; Cassani, E.; Bolliri, C.; Caronni, S.; et al. Muscle-targeted nutritional support for rehabilitation in patients with parkinsonian syndrome. Neurology 2019, 93, e485–e496. [Google Scholar] [CrossRef]
- McDermott, M.M.; Liu, K.; Greenland, P.; Guralnik, J.M.; Criqui, M.H.; Chan, C.; Pearce, W.H.; Schneider, J.R.; Ferrucci, L.; Celic, L.; et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA 2004, 292, 453–461. [Google Scholar] [CrossRef]
- Burd, N.A.; West, D.W.; Moore, D.R.; Atherton, P.J.; Staples, A.W.; Prior, T.; Tang, J.E.; Rennie, M.J.; Baker, S.K.; Phillips, S.M. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J. Nutr. 2011, 141, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Lixandrão, M.E.; Longobardi, I.; Leitão, A.E.; Morais, J.V.M.; Swinton, P.A.; Aihara, A.Y.; Goes, P.C.K.; Ugrinowitsch, C.; Candow, D.G.; Gualano, B.; et al. Daily Leucine Intake Is Positively Associated with Lower Limb Skeletal Muscle Mass and Strength in the Elderly. Nutrients 2021, 13, 3536. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Calvani, R.; Picca, A.; Goncalves, I.O.; Landi, F.; Bernabei, R.; Cesari, M.; Uchida, M.C.; Marzetti, E. Protein-Related Dietary Parameters and Frailty Status in Older Community-Dwellers across Different Frailty Instruments. Nutrients 2020, 12, 508. [Google Scholar] [CrossRef]
- Elmadfa, I.; Meyer, A.L. Animal Proteins as Important Contributors to a Healthy Human Diet. Annu. Rev. Anim. Biosci. 2017, 5, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Sukhato, K.; Akksilp, K.; Dellow, A.; Vathesatogkit, P.; Anothaisintawee, T. Efficacy of different dietary patterns on lowering of blood pressure level: An umbrella review. Am. J. Clin. Nutr. 2020, 112, 1584–1598. [Google Scholar] [CrossRef]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; Silva, D.D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 1–38. [Google Scholar] [CrossRef]
| Quartiles of BCAAs Intake a | ||||||
|---|---|---|---|---|---|---|
| Q1 (n = 1084) | Q2 (n = 1084) | Q3 (n = 1084) | Q4 (n = 1084) | p-Value b | p-Trend c | |
| Age (years), mean (sd) | 72.49 (5.44) | 72.77 (5.54) | 73.07 (5.52) | 72.57 (5.41) | 0.070 | 0.475 |
| Sex (male), n (%) | 386.00 (35.6) | 446.00 (41.1) | 482.00 (44.5) | 549.00 (50.6) | <0.001 | <0.001 |
| Household registration, n (%) | <0.001 | <0.001 | ||||
| Large cities | 500.00 (46.1) | 591.00 (54.5) | 748.00 (69.0) | 753.00 (69.5) | ||
| Small–medium cities | 138.00 (12.7) | 147.00 (13.6) | 92.00 (8.5) | 104.00 (9.6) | ||
| Rural counties | 446.00 (41.2) | 346.00 (31.9) | 244.00 (25.5) | 227.00 (20.9) | ||
| BMI (kg/m2) | 0.199 | 0.598 | ||||
| <18.5 | 27.00 (27.84%) | 20.00 (20.62%) | 29.00 (29.90%) | 21.00 (21.65%) | ||
| 18.5–23.9 | 430.00 (25.78%) | 420.00 (25.18%) | 403.00 (24.16%) | 415.00 (24.88%) | ||
| 24.0–27.9 | 323.00 (21.96%) | 375.00 (25.49%) | 398.00 (27.06%) | 375.00 (25.49%) | ||
| ≥28.0 | 107.00 (25.06%) | 116.00 (27.17%) | 96.00 (22.48%) | 108.00 (25.29%) | ||
| Smoking status, n (%) | 0.154 | 0.058 | ||||
| Yes | 200.00 (18.5) | 232.00 (21.4) | 222.00 (20.5) | 241.00 (22.2) | ||
| No | 884.00 (81.5) | 852.00 (78.6) | 862.00 (79.5) | 843.00 (77.8) | ||
| Drinking status, n (%) | 0.002 | <0.001 | ||||
| Yes | 137.00 (12.6) | 148.00 (13.7) | 175.00 (16.1) | 195.00 (18.0) | ||
| No | 947.00 (87.4) | 936.00 (86.3) | 909.00 (83.9) | 889.00 (82.0) | ||
| Diabetes, n (%) | 219.00 (20.2) | 257.00 (23.7) | 216.00 (19.9) | 237.00 (21.9) | 0.117 | 0.830 |
| Hypertension, n (%) | 515.00 (47.5) | 508.00 (46.9) | 479.00 (44.2) | 465.00 (42.9) | 0.099 | 0.015 |
| Dyslipidemia, n (%) | 158.00 (14.6) | 168.00 (15.5) | 146.00 (13.5) | 158.00 (14.6) | 0.614 | 0.672 |
| GLU (mmol/L) mean (sd) | 5.66 (1.64) | 5.66 (1.63) | 5.55 (1.54) | 5.65 (1.6) | 0.339 | 0.517 |
| TC (mmol/L) mean (sd) | 5.12 (1.26) | 5.08 (1.12) | 5.08 (1.14) | 5.07 (1.52) | 0.819 | 0.404 |
| TG (mmol/L) mean (sd) | 1.54 (1.23) | 1.53 (0.9) | 1.51 (0.93) | 1.45 (1.1) | 0.242 | 0.056 |
| HDL-C (mmol/L) mean (sd) | 1.55 (3.94) | 1.38 (0.45) | 1.4 (0.51) | 1.39 (0.42) | 0.117 | 0.207 |
| LDL-C (mmol/L) mean (sd) | 3.23 (0.89) | 3.21 (0.92) | 3.2 (0.88) | 3.17 (0.85) | 0.143 | 0.539 |
| Handgrip strength, mean (sd) | 21.33 (7.87) | 22.77 (8.66) | 23.50 (8.56) | 24.31 (8.25) | <0.001 | <0.001 |
| 4-m usual walking speed (m/s), mean (sd) | 3.99 (1.36) | 4.01 (1.78) | 4.03 (4.49) | 3.75 (1.60) | 0.054 | 0.055 |
| 4-m fast walking speed (m/s), mean (sd) | 3.01 (0.95) | 2.99 (1.33) | 2.87 (0.88) | 2.77 (1.05) | <0.001 | <0.001 |
| Repeated chair rises (s), mean (sd) | 11.74 (3.93) | 11.43 (3.82) | 11.11 (3.68) | 10.84 (3.75) | <0.001 | <0.001 |
| Quartiles of BCAA Intake a | p-Value b | p-Trend c | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Energy intake (kcal/day) | 1159.28 ± 446.25 | 1341.52 ± 394.68 | 1477.41 ± 389.17 | 1702.02 ± 561.19 | <0.001 | <0.001 |
| Fat (g/day) | 129.84 ± 118.67 | 117.00 ± 89.61 | 114.89 ± 100.02 | 117.49 ± 92.65 | 0.001 | 0.004 |
| Protein (g/day) | 53.14 ± 14.46 | 54.35 ± 15.68 | 53.96 ± 14.01 | 54.14 ± 15.9 | 0.261 | 0.201 |
| Carbohydrate (g/day) | 104.23 ± 91.13 | 112.08 ± 90.9 | 117.92 ± 89.99 | 113.86 ± 91.10 | 0.004 | 0.005 |
| Dietary soluble fiber (g/day) | 6.46 ± 8.44 | 6.74 ± 7.83 | 7.09 ± 7.93 | 6.66 ± 6.93 | 0.297 | 0.377 |
| Vitamin D (µg/day) | 147.25 ± 213.07 | 123.17 ± 157.57 | 121.84 ± 202.51 | 128.58 ± 169.44 | 0.005 | 0.025 |
| Folate (µg/day) | 173.85 ± 131.18 | 177.56 ± 120.1 | 192.11 ± 130.96 | 185.63 ± 132.82 | 0.005 | 0.005 |
| Quartiles of BCAAs Intake a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | MD b | p-Trend c | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| Handgrip strength (kg) | ||||||||||
| Crude | 21.4 | 0.25 | 22.8 | 0.25 | 23.6 | 0.25 | 24.4 | 0.25 | 2.97 | <0.001 |
| Model 1 | 22.6 | 0.22 | 23.6 | 0.21 | 24.2 | 0.22 | 24.3 | 0.21 | 1.65 | <0.001 |
| Model 2 | 22.6 | 0.34 | 23.5 | 0.33 | 24.1 | 0.33 | 24.3 | 0.33 | 1.64 | <0.001 |
| Model 3 | 21.4 | 0.34 | 23.4 | 0.33 | 24.0 | 0.33 | 24.4 | 0.33 | 1.74 | <0.001 |
| 4-m usual walking speed (m/s) | ||||||||||
| Crude | 4.1 | 0.08 | 4.1 | 0.08 | 4.1 | 0.08 | 3.8 | 0.08 | −0.29 | 0.034 |
| Model 1 | 4.1 | 0.08 | 4.1 | 0.08 | 4.0 | 0.08 | 3.8 | 0.08 | −0.25 | 0.086 |
| Model 2 | 4.1 | 0.11 | 4.1 | 0.11 | 4.2 | 0.11 | 3.9 | 0.11 | −0.23 | 0.128 |
| Model 3 | 4.1 | 0.12 | 4.1 | 0.11 | 4.2 | 0.11 | 3.9 | 0.11 | −0.22 | 0.122 |
| 4-m fast walking speed (m/s) | ||||||||||
| Crude | 3.1 | 0.03 | 3.0 | 0.03 | 2.9 | 0.03 | 2.8 | 0.03 | −0.28 | <0.001 |
| Model 1 | 3.1 | 0.03 | 3.0 | 0.03 | 2.9 | 0.03 | 2.8 | 0.03 | −0.24 | <0.001 |
| Model 2 | 3.1 | 0.05 | 3.0 | 0.05 | 2.9 | 0.05 | 2.9 | 0.05 | −0.25 | <0.001 |
| Model 3 | 3.1 | 0.05 | 3.0 | 0.05 | 2.9 | 0.05 | 2.9 | 0.04 | −0.26 | <0.001 |
| Repeated chair rises (s) | ||||||||||
| Crude | 11.8 | 0.12 | 11.5 | 0.12 | 11.1 | 0.12 | 10.5 | 0.12 | −0.93 | <0.001 |
| Model 1 | 11.8 | 0.12 | 11.5 | 0.12 | 11.1 | 0.12 | 10.9 | 0.71 | −0.83 | <0.001 |
| Model 2 | 12.0 | 0.15 | 11.5 | 0.15 | 11.3 | 0.15 | 11.1 | 0.15 | −1.11 | <0.001 |
| Model 3 | 12.0 | 0.15 | 11.5 | 0.15 | 11.3 | 0.15 | 11.1 | 0.15 | −0.90 | <0.001 |
| Quartiles of BCAAs Intake a | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Weak muscle strength | ||||
| Hang grip (<18 kg for female) | ||||
| Crude | 1 (Ref) | 0.77 (0.62–0.96) * | 0.67 (0.54–0.84) * | 0.55 (0.43–0.69) * |
| Model 1 | 1 (Ref) | 0.74 (0.59–0.93) * | 0.64 (0.51–0.81) * | 0.54 (0.42–0.68) * |
| Model 2 | 1 (Ref) | 0.73 (0.57–0.94) * | 0.64 (0.50–0.82) * | 0.51 (0.39–0.67) * |
| Model 3 | 1 (Ref) | 0.72 (0.56–0.92) * | 0.62 (0.48–0.80) * | 0.50 (0.38–0.65) * |
| Hang grip (<28 kg for male) | ||||
| Crude | 1 (Ref) | 0.68 (0.52–0.90) * | 0.80 (0.61–1.05) | 0.65 (0.50–0.85) * |
| Model 1 | 1 (Ref) | 0.67 (0.50–0.89) * | 0.77 (0.58–1.02) | 0.65 (0.49–0.85) * |
| Model 2 | 1 (Ref) | 0.68 (0.50–0.92) * | 0.80 (0.59–1.08) | 0.68 (0.51–0.92) * |
| Model 3 | 1 (Ref) | 0.67 (0.49–0.91) * | 0.79 (0.58–1.07) | 0.67 (0.50–0.91) * |
| Physical performance decline | ||||
| Slow 4-m usual walking speed (<0.8 m/s) | ||||
| Crude | 1 (Ref) | 0.90 (0.71–1.15) | 0.83 (0.65–1.06) | 0.60 (0.46–0.79) * |
| Model 1 | 1 (Ref) | 0.88 (0.68–1.14) | 0.80 (0.62–1.04) | 0.62 (0.47–0.82) * |
| Model 2 | 1 (Ref) | 0.93 (0.70–1.23) | 0.90 (0.68–1.19) | 0.68 (0.50–0.92) * |
| Model 3 | 1 (Ref) | 0.93 (0.70–1.24) | 0.91 (0.68–1.21) | 0.68 (0.50–0.93) * |
| Slow repeated chair rises (≥12 m/s) | ||||
| Crude | 1 (Ref) | 0.86 (0.72–1.03) | 0.79 (0.66–0.95) * | 0.65 (0.54–0.78) * |
| Model 1 | 1 (Ref) | 0.86 (0.71–1.03) | 0.77 (0.64–0.93) * | 0.66 (0.55–0.80) * |
| Model 2 | 1 (Ref) | 0.79 (0.65–0.97) * | 0.77 (0.63–0.94) * | 0.66 (0.54–0.81) * |
| Model 3 | 1 (Ref) | 0.79 (0.64–0.96) * | 0.77 (0.63–0.94) * | 0.66 (0.54–0.81) * |
| Isoleucine | Leucine | Valine | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 a | Q4 a | p-Trend b | Q1 a | Q4 a | p-Trend b | Q1 a | Q4 a | p-Trend b | |||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||||
| Handgrip strength (kg) | |||||||||||||||
| Crude | 23.1 | 0.26 | 23.2 | 0.26 | 0.784 | 23.0 | 0.26 | 23.0 | 0.26 | 0.938 | 23.0 | 0.26 | 23.0 | 0.26 | 0.938 |
| Model 1 | 23.7 | 0.22 | 23.7 | 0.22 | 0.978 | 23.7 | 0.22 | 23.7 | 0.22 | 0.999 | 23.6 | 0.22 | 23.6 | 0.22 | 0.927 |
| Model 2 | 23.8 | 0.29 | 23.7 | 0.28 | 0.781 | 23.8 | 0.29 | 23.7 | 0.28 | 0.816 | 23.7 | 0.29 | 23.6 | 0.28 | 0.709 |
| Model 3 | 23.7 | 0.29 | 23.7 | 0.28 | 0.927 | 23.7 | 0.29 | 23.7 | 0.28 | 0.548 | 23.6 | 0.29 | 23.6 | 0.28 | 0.596 |
| 4-m usual walking speed | |||||||||||||||
| Crude | 4.0 | 0.08 | 3.9 | 0.08 | 0.701 | 4.0 | 0.08 | 4.0 | 0.08 | 0.762 | 4.0 | 0.08 | 4.0 | 0.08 | 0.774 |
| Model 1 | 4.0 | 0.08 | 4.0 | 0.08 | 0.836 | 4.0 | 0.08 | 4.0 | 0.08 | 0.814 | 4.0 | 0.08 | 4.0 | 0.08 | 0.870 |
| Model 2 | 4.1 | 0.11 | 4.0 | 0.11 | 0.885 | 4.1 | 0.11 | 4.0 | 0.11 | 0.859 | 4.0 | 0.11 | 4.0 | 0.11 | 0.923 |
| Model 3 | 4.0 | 0.11 | 4.0 | 0.11 | 0.985 | 4.0 | 0.11 | 4.0 | 0.11 | 0.949 | 4.0 | 0.11 | 4.0 | 0.11 | 0.996 |
| 4-m fast walking speed | |||||||||||||||
| Crude | 3.0 | 0.03 | 2.9 | 0.03 | 0.308 | 3.0 | 0.03 | 2.9 | 0.03 | 0.397 | 3.0 | 0.03 | 2.9 | 0.03 | 0.538 |
| Model 1 | 3.0 | 0.03 | 2.9 | 0.03 | 0.429 | 3.0 | 0.03 | 2.9 | 0.03 | 0.537 | 3.0 | 0.03 | 2.9 | 0.03 | 0.646 |
| Model 2 | 3.0 | 0.04 | 2.9 | 0.04 | 0.540 | 3.0 | 0.04 | 2.9 | 0.04 | 0.654 | 2.9 | 0.04 | 2.9 | 0.04 | 0.766 |
| Model 3 | 3.0 | 0.04 | 2.9 | 0.04 | 0.544 | 3.0 | 0.04 | 2.9 | 0.04 | 0.664 | 2.9 | 0.04 | 2.9 | 0.04 | 0.775 |
| Repeated chair rises | |||||||||||||||
| Crude | 11.4 | 0.12 | 11.1 | 0.12 | 0.259 | 11.4 | 0.12 | 11.2 | 0.12 | 0.141 | 11.4 | 0.12 | 11.2 | 0.12 | 0.134 |
| Model 1 | 11.4 | 0.12 | 11.2 | 0.12 | 0.117 | 11.4 | 0.12 | 11.2 | 0.12 | 0.165 | 11.4 | 0.12 | 11.2 | 0.12 | 0.184 |
| Model 2 | 11.5 | 0.15 | 11.4 | 0.15 | 0.312 | 11.5 | 0.15 | 11.4 | 0.40 | 0.852 | 11.5 | 0.15 | 11.4 | 0.15 | 0.757 |
| Model 3 | 11.5 | 0.15 | 11.4 | 0.15 | 0.473 | 11.5 | 0.15 | 11.4 | 0.15 | 0.327 | 11.5 | 0.15 | 11.4 | 0.15 | 0.645 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, M.; Mu, Y.; Su, X.; Zheng, L.; Zhang, S.; Chen, H.; Xu, S.; Ma, J.; Ouyang, R.; Li, W.; et al. Association between Branched-Chain Amino Acid Intake and Physical Function among Chinese Community-Dwelling Elderly Residents. Nutrients 2022, 14, 4367. https://doi.org/10.3390/nu14204367
Liao M, Mu Y, Su X, Zheng L, Zhang S, Chen H, Xu S, Ma J, Ouyang R, Li W, et al. Association between Branched-Chain Amino Acid Intake and Physical Function among Chinese Community-Dwelling Elderly Residents. Nutrients. 2022; 14(20):4367. https://doi.org/10.3390/nu14204367
Chicago/Turabian StyleLiao, Minqi, Yingjun Mu, Xin Su, Lu Zheng, Shiwen Zhang, Hongen Chen, Shan Xu, Junrong Ma, Ruiqing Ouyang, Wanlin Li, and et al. 2022. "Association between Branched-Chain Amino Acid Intake and Physical Function among Chinese Community-Dwelling Elderly Residents" Nutrients 14, no. 20: 4367. https://doi.org/10.3390/nu14204367
APA StyleLiao, M., Mu, Y., Su, X., Zheng, L., Zhang, S., Chen, H., Xu, S., Ma, J., Ouyang, R., Li, W., Cheng, C., Cai, J., Chen, Y., Wang, C., & Zeng, F. (2022). Association between Branched-Chain Amino Acid Intake and Physical Function among Chinese Community-Dwelling Elderly Residents. Nutrients, 14(20), 4367. https://doi.org/10.3390/nu14204367







