Allergic Disorders and Risk of Anemia in Japanese Children: Findings from the Japan Environment and Children’s Study
Abstract
1. Introduction
2. Materials and Methods
2.1. JECS and Study Design
2.2. Allergic Diseases at the Age of 2 Years
2.3. Anemia at the Age of 3 Years and Confounding Variables
2.4. Statistical Methods
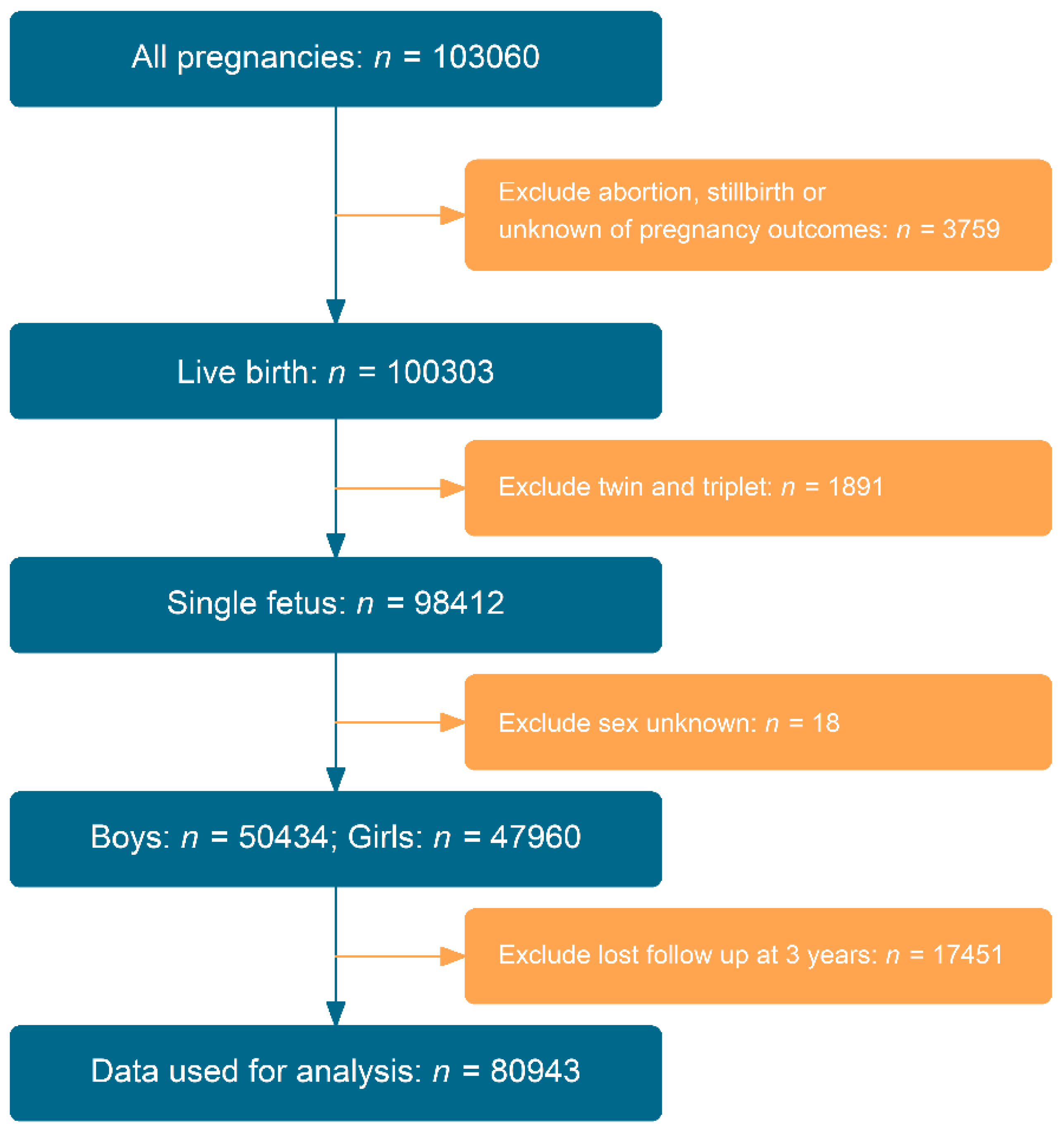
3. Results
3.1. Baseline Characteristics
3.2. Relationship between Allergic Disorders and Anemia
3.3. Propensity Score Analysis
3.4. Sensitivity Analysis and Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meena, K.; Tayal, D.K.; Gupta, V.; Fatima, A. Using classification techniques for statistical analysis of Anemia. Artif. Intell. Med. 2019, 94, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Rodak, B.F.; Keohane, E.M.; Fritsma, G.A. Hematology-E-Book: Clinical Principles and Applications; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Iglesias Vazquez, L.; Valera, E.; Villalobos, M.; Tous, M.; Arija, V. Prevalence of Anemia in Children from Latin America and the Caribbean and Effectiveness of Nutritional Interventions: Systematic Review and Meta(-)Analysis. Nutrients 2019, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Pena-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study, G. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- Janus, J.; Moerschel, S.K. Evaluation of anemia in children. Am. Fam. Physician 2010, 81, 1462–1471. [Google Scholar] [PubMed]
- Kleinman, E.R. World Health Organization, Global Health Observatory Data Repository/World Health Statistics. Prevalence of Anemia among Children (% of Children Ages 6–59 Months). Available online: https://data.worldbank.org/indicator/SH.ANM.CHLD.ZS?end=2019&locations=JP&most_recent_value_desc=false&start=2000&view=chart (accessed on 10 December 2021).
- Upton, M.N.; McConnachie, A.; McSharry, C.; Hart, C.L.; Smith, G.D.; Gillis, C.R.; Watt, G.C. Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: The Midspan family study surveys of parents and offspring. BMJ 2000, 321, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Marks, G.B.; Sporik, R.; Belosouva, E.G.; Car, N.G.; Peat, J.K. Continued increase in the prevalence of asthma and atopy. Arch. Dis. Child. 2001, 84, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Marklund, B.; Ahlstedt, S.; Nordstrom, G. Health-related quality of life among adolescents with allergy-like conditions-with emphasis on food hypersensitivity. Health Qual. Life Outcomes 2004, 2, 65. [Google Scholar] [CrossRef]
- Chang, J.E.; Lee, H.M.; Kim, J.; Rhew, K. Prevalence of Anemia in Pediatric Patients According to Asthma Control: Propensity Score Analysis. J. Asthma Allergy 2021, 14, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R. Nutritional disorders resulting from food allergy in children. Pediatr. Allergy Immunol. 2018, 29, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Adachi, Y.; Murakami, S.; Ito, Y.; Itazawa, T.; Tsuchida, A.; Matsumura, K.; Hamazaki, K.; Inadera, H.; Japan, E.; et al. Maternal exposure to smoking and infant’s wheeze and asthma: Japan Environment and Children’s Study. Allergol. Int. 2021, 70, 445–451. [Google Scholar] [CrossRef]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takami, M.; Misumi, T.; Kawakami, C.; Miyagi, E.; Ito, S.; Aoki, S.; Japan, E.; Children’s Study, G. Effects of breastfeeding on postpartum weight change in Japanese women: The Japan Environment and Children’s Study (JECS). PLoS ONE 2022, 17, e0268046. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Weiland, S.K.; Bjorksten, B.; Brunekreef, B.; Cookson, W.O.; von Mutius, E.; Strachan, D.P.; International Study of Asthma and Allergies in Childhood Phase II Study Group. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): Rationale and methods. Eur. Respir. J. 2004, 24, 406–412. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; Beasley, R.; Clayton, T.O.; Stewart, A.W.; Committee, I.S. The international study of asthma and allergies in childhood (ISAAC): Phase three rationale and methods. Int. J. Tuberc. Lung Dis. 2005, 9, 10–16. [Google Scholar]
- Kato, N.; Takimoto, H.; Sudo, N. The Cubic Functions for Spline Smoothed L, S and M Values for BMI Reference Data of Japanese Children. Clin. Pediatr. Endocrinol. 2011, 20, 47–49. [Google Scholar] [CrossRef]
- Isojima, T.; Kato, N.; Ito, Y.; Kanzaki, S.; Murata, M. Growth standard charts for Japanese children with mean and standard deviation (SD) values based on the year 2000 national survey. Clin. Pediatr. Endocrinol. 2016, 25, 71–76. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Suzuki, Y.; Yang, L.; Saito-Abe, M.; Sato, M.; Mezawa, H.; Nishizato, M.; Kato, N.; Ito, Y.; Hashimoto, K.; et al. Persistent eczema leads to both impaired growth and food allergy: JECS birth cohort. PLoS ONE 2021, 16, e0260447. [Google Scholar] [CrossRef]
- Cole, T.J. The LMS method for constructing normalized growth standards. Eur. J. Clin. Nutr. 1990, 44, 45–60. [Google Scholar]
- Drury, K.E.; Schaeffer, M.; Silverberg, J.I. Association Between Atopic Disease and Anemia in US Children. JAMA Pediatr. 2016, 170, 29–34. [Google Scholar] [CrossRef]
- Rhew, K.; Oh, J.M. Association between atopic disease and anemia in pediatrics: A cross-sectional study. BMC Pediatr. 2019, 19, 455. [Google Scholar] [CrossRef] [PubMed]
- Rhew, K.; Brown, J.D.; Oh, J.M. Atopic Disease and Anemia in Korean Patients: Cross-Sectional Study with Propensity Score Analysis. Int. J. Environ. Res. Public Health 2020, 17, 1978. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef]
- Narum, S.; Westergren, T.; Klemp, M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open 2014, 4, e004587. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.; Venter, C. The ins and outs of managing avoidance diets for food allergies. Curr. Opin. Pediatr. 2016, 28, 567–572. [Google Scholar] [CrossRef]
- Nowak, S.; Wang, H.; Schmidt, B.; Jarvinen, K.M. Vitamin D and iron status in children with food allergy. Ann. Allergy Asthma Immunol. 2021, 127, 57–63. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Boston, R.; Farrar, J.T.; Strom, B.L. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am. J. Epidemiol. 2003, 158, 280–287. [Google Scholar] [CrossRef]
| Model 1 # | Model 2 $ | ||||||
|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||
| Outcome Events | ORs | Lower | Upper | ORs | Lower | Upper | |
| Asthma | Yes vs. No | 1.85 | 1.32 | 2.60 | 1.91 | 1.36 | 2.69 |
| Atopic dermatitis | Yes vs. No | 2.18 | 1.66 | 2.85 | 2.17 | 1.65 | 2.84 |
| Rhinitis | Yes vs. No | 1.35 | 1.05 | 1.74 | 1.36 | 1.06 | 1.75 |
| Allergic rhinoconjunctivitis | Yes vs. No | 2.95 | 1.91 | 4.54 | 2.94 | 1.91 | 4.53 |
| Food allergy | Yes vs. No | 1.92 | 1.44 | 2.56 | 1.90 | 1.42 | 2.53 |
| Any allergy | Yes vs. No | 1.80 | 1.41 | 2.29 | 1.81 | 1.42 | 2.30 |
| Atopic diseases, No. | 1 vs. 0 | 1.38 | 1.05 | 1.82 | 1.39 | 1.05 | 1.83 |
| >1 vs. 0 | 2.84 | 2.12 | 3.82 | 2.86 | 2.13 | 3.84 | |
| 95% CI | 95% CI | ||||||
|---|---|---|---|---|---|---|---|
| Outcome Events | OR ab | Lower | Upper | OR ac | Lower | Upper | |
| Asthma | Yes vs. No | 1.62 | 1.09 | 2.41 | 1.75 | 1.11 | 2.76 |
| Atopic dermatitis | Yes vs. No | 2.36 | 1.76 | 3.16 | 2.35 | 1.75 | 3.17 |
| Allergic rhinitis | Yes vs. No | 1.35 | 1.02 | 1.78 | 1.39 | 1.05 | 1.83 |
| Allergic rhinoconjunctivitis | Yes vs. No | 2.99 | 1.80 | 4.97 | 2.88 | 1.77 | 4.69 |
| Food allergy | Yes vs. No | 1.95 | 1.42 | 2.68 | 2.04 | 1.47 | 2.82 |
| Any allergy | Yes vs. No | 1.87 | 1.42 | 2.45 | 1.86 | 1.41 | 2.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Sato, M.; Saito-Abe, M.; Miyaji, Y.; Shimada, M.; Sato, C.; Nishizato, M.; Kumasaka, N.; Mezawa, H.; Yamamoto-Hanada, K.; et al. Allergic Disorders and Risk of Anemia in Japanese Children: Findings from the Japan Environment and Children’s Study. Nutrients 2022, 14, 4335. https://doi.org/10.3390/nu14204335
Yang L, Sato M, Saito-Abe M, Miyaji Y, Shimada M, Sato C, Nishizato M, Kumasaka N, Mezawa H, Yamamoto-Hanada K, et al. Allergic Disorders and Risk of Anemia in Japanese Children: Findings from the Japan Environment and Children’s Study. Nutrients. 2022; 14(20):4335. https://doi.org/10.3390/nu14204335
Chicago/Turabian StyleYang, Limin, Miori Sato, Mayako Saito-Abe, Yumiko Miyaji, Mami Shimada, Chikako Sato, Minaho Nishizato, Natsuhiko Kumasaka, Hidetoshi Mezawa, Kiwako Yamamoto-Hanada, and et al. 2022. "Allergic Disorders and Risk of Anemia in Japanese Children: Findings from the Japan Environment and Children’s Study" Nutrients 14, no. 20: 4335. https://doi.org/10.3390/nu14204335
APA StyleYang, L., Sato, M., Saito-Abe, M., Miyaji, Y., Shimada, M., Sato, C., Nishizato, M., Kumasaka, N., Mezawa, H., Yamamoto-Hanada, K., Ohya, Y., & on behalf of the Japan Environment and Children’s Study (JECS) Group. (2022). Allergic Disorders and Risk of Anemia in Japanese Children: Findings from the Japan Environment and Children’s Study. Nutrients, 14(20), 4335. https://doi.org/10.3390/nu14204335





