Abstract
Although massive progress in discovering allergic rhinitis (AR) aetiology has been made in recent years, its prevalence is still rising and it significantly impacts patients’ lives. That is why further and non-conventional research elucidating the role of new factors in AR pathogenesis is needed, facilitating discoveries of new treatment approaches. One of these factors is the gut microbiota, with its specific roles in health and disease. This review presents the process of gut microbiota development, especially in early life, focusing on its impact on the immune system. It emphasizes the link between the gut microbiota composition and immune changes involved in AR development. Specifically, it elucidates the significant link between bacteria colonizing the gut and the Th1/Th2 imbalance. Probiotics, prebiotics and bacterial lysates, which are medications that restore the composition of intestinal bacteria and indirectly affect the clinical course of AR, are also discussed.
1. Introduction
The human organism is not a sterile, closed system. Multiple microorganisms implant on its surfaces and interiors and develop into the local microbiota. The main places with bacterial presence are the gut, skin, and urogenital and respiratory tracts [1]. Figure 1 illustrates the crucial factors that influence gut microbiota development. In recent years, plenty of information has been compiled about microbiota’s significant impact on human health. Its ability to modulate immune system response remains one of the most popular issues of 21st century science.
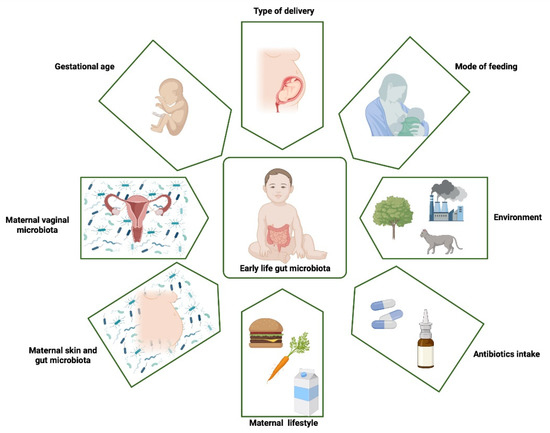
Figure 1.
The main factors that influence early-life gut microbiota. The first steps in gut colonization probably start already in utero and are influenced by maternal gut microbiota. More likely, a new-born is specifically colonized during birth, and the composition of bacteria localized in the intestine depends on the type of delivery. In the first two years of life, the gut microbiota composition is influenced by multiple factors, mainly mode of feeding, environment and antibiotics intake. Created with biorender.com, accessed on 24 September 2022.
A disturbance of the immune system is the cause of multiple diseases, including allergic ones [2]. Speculations on their development centre around innate and adaptive immune response abnormalities. Firstly, the hygiene hypothesis enlightens the importance of the proper innate response maturation. Secondly, the imbalance of Th1/Th2 responses seems to play a significant role in the pathogenesis of allergic diseases [3].
Allergic rhinitis (AR) is a widely prevalent condition of the upper respiratory tract. It affects mainly children with up to 40% of the population worldwide [4]. It is characterized by rhinorrhoea, sneezing, and a blocked and itchy nose [5]. Such symptoms affect patients’ quality of life and lead to multiple nuisances such as sleep disturbances, learning disabilities and changes in behaviour. Furthermore, AR promotes sinusitis, otitis and asthma exacerbations [6,7]. The treatment of AR is based mainly on nasal corticosteroids as well as nasal and oral H1-antihistamines; in addition, anticholinergic, antileukotriene and alpha-mimetic agents and chromones are used. However, the effectiveness of these medications is limited, and they are burdened with multiple side effects [8].
As mentioned before, the immune system is constantly stimulated by the plethora of ligands presented by bacteria colonizing the intestine, such as lipopolysaccharides, unmethylated CpG motifs, fatty acids and flagellin [9]. They are recognized by Toll-like receptors (TLRs) and stimulate the immune system. It shapes the differentiation of naïve T cells into Tregs, Th1, Th2 and Th17; modifies the levels of eosinophils, basophils and mast cells; and influences the production of IgE [10]. Considering that these processes are closely related to the composition of the gut microbiota, its significant role in the pathogenesis of allergic disorders remains valid [11].
This review aims to summarize the interplay between the gut microbiota and AR’s development and clinical course. Furthermore, immunomodulatory preparations that alter the composition of microbes present in the intestine and their effects in AR treatment are also discussed.
2. Gut Microbiota Development and the Risk of Allergic Rhinitis
2.1. Prenatal Period
The exact time of gut colonization has yet to become known. The ‘sterile womb paradigm’ has been the accepted dogma for years. It was believed that microbial implementation starts during or right after birth and depends on the delivery type [12]. However, at the end of the 20th century, the research on gut microbiota gathered pace, and some studies have proposed that not all healthy new-borns are born sterile. The microorganisms were found in the amniotic fluid [13,14], umbilical cord [15], placenta [14], and meconium [16,17]. In animal studies, bacteria in the foetal gut resembled those from the maternal intestine [16]. It can be suspected that multiple hormonal changes during the pregnancy provoke a transfer of bacteria from the intestinal epithelium to the placenta.
Nevertheless, maternal gut microbiota more likely produces compounds that affect the developing immune system of the foetus. Thorburn et al. showed that feeding pregnant mice a high-fibre diet yielded a distinctive gut microbiota, which increased the levels of the short-chain fatty acid (SCFA) acetate. It reduced the symptoms of allergic airways disease in cubs by enhancing Tregs number and function [18]. Furthermore, Venter et al. developed a maternal diet index during pregnancy that was associated with offspring allergy outcomes. Data suggest that high vegetables and yoghurt intake, which have been reported to increase the gut microbiome diversity, reduce the risk of AR, atopic dermatitis (AD) and asthma [19]. Notably, this microbiome diversity promotes higher levels of faecal butyrate, an SCFA that stimulates the development of Tregs and promotes macrophage differentiation [20,21]. Nevertheless, foods high in advanced glycosylated end-products reduce the diversity of bacteria present in the maternal intestine, which minimizes the production of metabolites favourable in foetal immune development [22]. Furthermore, not only diet during pregnancy has an impact on the gut microbiota composition. Numerous medications alter the microbial diversity and modulate the immature foetal immune system. In a mouse model, prenatal antibiotic exposure significantly altered new-borns’ gut microbiota composition. The treatment decreased the presence of Bacteroides spp. and Firmicutes spp. and significantly increased the relative abundance of Proteobacteria spp. in the intestine compared with the control group [23]. Remarkably, Bacteroides spp. secrete a polysaccharide that promotes the differentiation of Tregs and influences the Th1/Th2 balance [24].
2.2. Birth Mode
As mentioned in the previous paragraph, the mode of birth for years was considered to be the main factor influencing the gut microbiota composition. Indeed, during vaginal birth, an infant is exposed to microbes from the maternal intestine, urogenital tract and skin, while a Caesarean section provokes colonization mainly by bacteria associated with the skin, mouth and hospital environment [25]. Notably, not all microbes can colonize a new-born’s gut. Microbiota compositions differ among children born in different ways until the end of the first year of life. The intestine of infants born vaginally presents a greater concentration of Bacteroides spp., Bifidobacteria spp., and Lactobacillus spp. On the other hand, the microbiome of new-borns delivered by Caesarean section is composed mainly of Staphylococcus spp., Streptococcus spp., and Clostridium spp. [26]. Of note, bacteria species have their own abilities to modulate the immune system. For instance, Pang et al. showed that Bacteroides spp. diminishes the secretion of Th2 cytokines (IL-4, IL-5 and IL-13) and activates Tregs, which ameliorates allergic airway inflammation [27]. Furthermore, Lactobacillus spp. promotes the maintenance of Th1/Th2 balance, decreases levels of proinflammatory cytokines (IL-6 and TNF-alfa) and inhibits IgE secretion [28]. On the other hand, Staphylococcus aureus enterotoxins act as superantigens and promote Th2 type immune response, IgE secretion and eosinophilic inflammation [29]. These interplays seem to validate the belief that the risk of allergy is enhanced in children born by Caesarean section. Nevertheless, appropriate maturation of the gut microbiota in the first year of life could mitigate these abnormalities [30].
2.3. Early Childhood
Many factors impact the acquisition and evolution of gut microbiota in early childhood. Among them, breastfeeding status seems to be the most influential. Breast milk is the most recommended first source of nutrition. It supports growth and development and provides passive immunity to protect against pathogens [31]. Moreover, a specific composition of human milk significantly impacts bacteria species colonizing the foetal gut [32]. Breastfed infants have a specific high amount of Bifidobacterium spp., a genus known for its numerous health benefits. Furthermore, exclusively breastfed children have increased taxa levels used as probiotics such as Lactobacillus johnsonii, paracasei/casei and Bifidobacterium longum. However, formula-fed infants’ gut is markedly similar to that of older children and colonized by Clostridium difficile, Granulicatella adiacens, Citrobacter spp. and Enterobacter spp. in tremendous amount [33].
Bifidobacterium and Lactobacillus spp. in human milk activate IgA producing plasma cells in the neonatal gut [34]. Furthermore, they are noted to control local inflammation by mucosal host–microbiota crosstalk and are associated with a lower risk of allergy later in life [35].
Interestingly, a short exposure to antibiotics, especially in the first two years of life, can shift the gut microbiota to long-term dysbiosis. Loss of diversity or specific important taxa, shifts in metabolic capacity and reduced resistance against pathogens may indicate post-antibiotic dysbiosis [36]. Notably, the overgrowth of Enterobacteriaceae and reduced diversity of Firmicutes and Bacteroidetes are typical of the described phenomenon [37]. These disturbances increase the host’s vulnerability to the invasion of Clostridium difficile [38] and vancomycin-resistant Enterococcus [39]. The retrospective cohort study by Mitre et al. showed a significant link between antibiotics’ treatment in infancy and development of allergic disease. For instance, allergic rhinitis was increased by 75% in children exposed to antibiotics [40].
Studies suggest that the perturbations in the intestinal microbes’ composition may increase the risk of allergy [1,11,23,33,41]. However, the microbiota status in early life seems to be the most important in the immune system development. For instance, neonatal mice treated with streptomycin and vancomycin had a reduced diversity of gut microbiota and an increased risk of asthma development. However, similar effects were not observed in adult mice [41]. It is presumed that similar phenomena can be observed in humans.
3. Gut Microbiota Composition in Health and in Allergic Rhinitis
The intestinal microbiota contains more than 1500 bacteria species [42]. Some bacteria species are typical for all healthy individuals. Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria make up to 90% of the total microbial population [43]. Remarkably, altered gut diversity is more common in patients suffering from allergic diseases. Liu et al. enrolled 93 AR patients and 72 healthy controls (HCs) and analysed their gut microbiota composition. Research showed that AR patients had a significantly lower microbiota diversity with an increased abundance of Bacteroidetes and lower levels of Actinobacteria and Proteobacteria than HCs [44]. Similar findings were shown by Watts et al. and Zhu et al., who also pointed out the reduced abundance of Clostridiales in AR patients [45,46]. Interestingly, not all allergic diseases are characterized by the same bacteria species colonizing the gut. The microbiota differs substantially between patients with AD, chronic urticaria and AR, which indicates that intestinal bacteria colonies differ significantly between patients with allergic skin disease and allergic nasal disease. Furthermore, it validates the existence of the gut–skin and gut–nose axes [47].
4. Shaping the Immune System by Gut Microbiota
As mentioned before, the gut microbiota acts as a new organ that influences the development of the immune system. The term “gut–organ axis” points to the significant crosstalk between the bacteria species colonizing the intestine and processes taking place in the nose, lungs, brain and skin. The microbiota of a healthy infant born vaginally and fed with breast milk correctly shapes its immune system. However, every disturbance of gut composition can negatively influence immature immunity and disrupt innate and adaptive response. Figure 2 explains the link between altered gut microbiota composition and its impact on the immune system in AR patients.
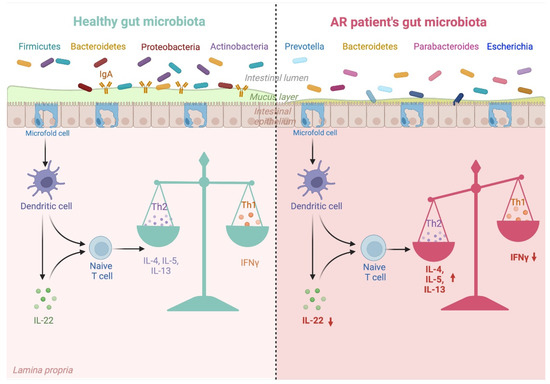
Figure 2.
Gut microbiota dysbiosis in AR patients and its impact on Th1/Th2 balance. Gut microbiota composition differs between healthy individuals and AR patients. Gut microbiota typical for AR promotes unfavourable changes in cytokines, which promote the Th1/Th2 imbalance involved in AR development. Created with biorender.com, accessed on 24 September 2022.
The intestinal epithelial cells (IECs; microfold cells) bridge the bacteria and the host’s immune system. They translate the commensal bacteria-derived signals (bacterial metabolites, bacterial components, and bacteria themselves) and send them to mucosal immune cells. Such crosstalk, where IECs play a crucial role in intestinal immunity, was seen in germ-free mice colonized with segmented filamentous bacteria. The microbes colonized the intestine and, via IECs, induced the production of serum amyloid A, which improved the Th17 differentiation and IL-22 production [48].
The immune cells mainly engaged in the crosstalk with bacteria colonizing the intestine are primarily seen in the lamina propria. Among them, the most common are dendritic cells (DCs), Tregs, NK cells and CD4+ T cells.
DCs have a crucial role in interacting with innate and adaptive immune responses. They migrate to secondary lymphoid tissues and stimulate CD4+ T cells to differentiate into subtypes based on the activation signal. The commensal bacteria-derived metabolites influence the DCs’ functions. For instance, SCFAs suppress IL-12 and increase IL-10 and IL-23 production [49,50]. Furthermore, they decrease the levels of CCL3, CCL4, CCL5, CXCL9, CXCL10 and CXCL11, indirectly regulating T cell functions [51]. Moreover, they induce B-cell IgA class switching and IgA production and regulate other adaptive response cell functions via DCs’ modulation [52].
Macrophages associated with the gut epithelium have a broad spectrum of functions. They can ingest pathogens, produce multiple cytokines that affect other immune cells and support the maintenance of Tregs. Liu et al. showed that SCFAs produced by microbes promote anti-inflammatory IL-10 secretion by macrophages [53]. Furthermore, they shift macrophages’ metabolism, reduce mTOR kinase activity and increase anti-microbial peptide production [54].
In this review, the interplay between the gut microbiota and T cells is limited to the microbes’ impact on the Th1/Th2 balance and its significant role in AR development. Notably, in early life, systemic immune responses are biased toward Th2 [55]. Therefore, the proper composition of bacteria in the intestine is an issue that provides mentioned balance maintenance. Qian et al. investigated the effect of various early life exposures on gut microbial colonization in mice. The research showed that diversity of the intestinal flora in early life influences the levels of IL-4 and IFN-γ, which may prevent airway inflammation in asthma via regulating the Th1/Th2 balance [56]. Furthermore, Jakobsson et al. pointed out that lower microbial diversity during the first two years of life delays the colonization of Bacteroidetes and results in reduced Th1-type response [12].
Type 2 innate lymphoid cells (ILC2) are innate immune cells that are deprived of surface markers, which makes them difficult to identify. They mirror the Th2 type cells and have a considerable role in allergy development [57]. Notably, the gut microbiota impacts the migration of ILC2s from the gut to the lung through the gut–lung axis. For instance, Proteobacteria significantly facilitate said migration and promote the production of IL-33 [58]. Furthermore, Chua et al. associated the development of respiratory allergies with the increased abundance of Ruminococcus gnavus. They showed that intestinal dysbiosis stimulated ILC2s and DCs to produce type 2 cytokines and promoted lung infiltration by eosinophils and mastocytes [59]. On the other hand, SCFAs derived from fermentation of dietary fibres by the gut microbiota inhibit the function of ILC2s and prevent lung inflammation [60]. Thus, studies suggest that the activity of ILC2s is modulated by the gut microbiota, but its underlying mechanisms are still insufficiently elucidated.
5. Preparations Affecting Gut Microbiota Composition and Their Effects in Allergic Rhinitis Treatment
With rising allergy prevalence, knowledge of microbiota situated in the gastrointestinal tract and its beneficial effects becomes increasingly important. On the one hand, probiotics, prebiotics and symbiotics affect the gut microbiota composition by counteracting the activity of undesirable bacteria and by modulating the host’s metabolism. On the other hand, bacterial lysates (BLs) indirectly influence the gut environment by stimulating DCs in the gut mucosa and by modulating the immune response. Finally, foecal microbiota transplantation (FMT), as a novel therapy, is likely to ensure stable gut microbiota maintenance.
5.1. Probiotics
The World Health Organization defines probiotics as “live strains of strictly selected microorganisms which, when administered in adequate amounts, confer a health benefit on the host” [61]. Noticeably, not all beneficial bacteria species can meet the requirements to become probiotics. The safety of the bacteria strain is defined by its origin, the absence of pathogenicity, and the antibiotic resistance profile. Moreover, probiotics should be easy to produce and should easily maintain their properties through the distribution process [62].
Probiotic products contain of one or more bacteria strains. The most used are Lactobacillus, Bifidobacterium, Lactococcus, Streptococcus and Enterococcus [63]. Their main advantage is the ability to ensure a proper balance between microorganisms that impact human’s health. They promote antagonism through the production of antimicrobial products, compete with pathogens, inhibit bacteria toxin production and modulate the host’s immune system [64].
Several human studies have evaluated the efficacy of probiotics in the prevention and treatment of allergic diseases, including AR. The first clinical trial that examined the impact of Lactobacillus GG on atopic disease was conducted by Kalliomäki et al. in 2001. Firstly, mothers with a family history of allergy were prenatally supplemented with Lactobacillus GG strains. Secondly, their infants received the same strain for the first six months of life. The research showed that the frequency of AD in the probiotic group was half that of the placebo group (23% vs. 46%), which took notice on the promising efficacy of probiotics in the prevention of allergic diseases [65]. Afterwards, Wang et al. investigated the effect of fermented milk containing Lactobacillus paracasei-33 (LP-33) on the quality of life (QOL) of patients with AR. The results suggested that LP-33-fortified fermented milk intake for 30 days can effectively and safely improve AR patients’ QOL and may be used as an alternative treatment [66]. Researchers encouraged by the promising outcomes of mentioned studies decided to examine whether the bacteria strains’ efficacy depends on their activity. Peng et al. compared the impact of heat-killed LP-33 with its live strains in treating AR induced by house dust mite in human subjects. After the 30-day treatment, the outcomes were comparable. The heat-killed LP-33 was not inferior to the live variant, and both interventions improved the QOL of patients with AR compared with the placebo group [67]. Recently, Yan et al. collected 30 randomized controlled trials (RCTs) with probiotics as an intervention in AR and prepared their meta-analysis. It showed that compared with the placebo group, the Rhinitis Quality of Life (RQLQ) global score, RQLQ nasal score and Rhinitis Total Symptom Score (RTSS) for nasal symptoms were significantly improved after probiotic supplementation. However, there was no significant difference between the placebo and the probiotic group in the blood eosinophil count, RQLQ eye score, RTSS global score, RTSS eye score, and total and antigen-specific serum IgE levels. Noticeably, the vast majority of included studies used probiotics composed of Lactobacillus and Bifidobacterium strains. Most studies did not report any adverse effects. However, some studies lacked quantifiable data, and their outcomes were incomplete; thus, there is an urgent need to conduct more high-quality RCTs [68].
The mechanism of probiotics’ action in reducing the risk and improving the clinical course of AR is not fully understood. However, Kukkonen et al. showed that probiotic supplementation might be linked with a high secretion of mucosal IgA, which participates in antigen elimination [69]. Furthermore, the consumption of probiotics may reduce the secretion of antigen-specific IgE and Th2 cytokines (IL-4, IL-13) [70].
It is worth pointing out that the International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis recommends considering probiotics as an adjuvant therapy for patients with AR due to their minimal harm and proven efficacy in improving symptoms [71].
5.2. Prebiotics
Prebiotics are specific dietary ingredients that affect the composition and activity of the gut microbiota. To become supplements, they must meet some inclusion criteria. Firstly, prebiotics must be resistant to gastric acidity, hydrolysis by enzymes and gastrointestinal absorption. Secondly, they should be fermented by intestinal microbiota and able to stimulate the growth of beneficial bacteria [72]. The most used prebiotics are lactitol, lactulose, inulin, lactosucrose, fructooligosaccharides, galactooligosaccharides and soy oligosaccharides [73]. They may be used as an alternative to probiotics or as a support for them.
Only one study examined the prebiotics’ impact on AR adults with high IgE levels. For 52 weeks, patients received lactosucrose, and their serum IgE levels were measured. After a year of treatment, serum IgE levels (especially to pollen allergens) significantly decreased, accompanied by the relief of allergic symptoms [74]. Furthermore, Derakhshan et al. investigated the effect of dried Ma-al-Shaeer (a traditional, rich-in-fibre, Iranian medicine with a formulation based on barley) versus fexofenadine on adult AR patients. Enrolled participants received orally mentioned preparations twice a day for 14 consecutive days. The clinical course of AR was improved in both groups, whereas nasal congestion, post-nasal drip and headache scores were significantly decreased in the Ma-al-Shaeer group [75].
Prebiotics are widely used as supplementation to milk formula for infants. Arslanoglu et al. evaluated the protective effect of prebiotic oligosaccharides against allergy. In this RCT, healthy infants with a risk of atopy were fed prebiotic-supplemented or placebo-supplemented formula during the first six months of life. The follow-up period lasted for five years. The cumulative incidences of allergy manifestation were significantly lower in the prebiotic supplemented group. The intervention was particularly beneficial in allergic rhinoconjunctivitis and allergic urticaria prevention [76].
In a mouse model of allergy, 2’-fucosyllactose and 6’-sialyllactose stimulated IL-10 production and stabilized mast cells [77]. The study by Gourbeyre et al. showed that sensitized mice supplemented with prebiotics or not had similar levels of IgE, IgG1, IL-4, IL-17 and allergy symptoms. However, the levels of IgG2a, specific IgA, IL-10, TGF-β and IFN-γ were significantly higher in the prebiotic treated group. This suggests that, in the mouse model, the exposure to prebiotics during perinatal and postweaning periods induces the highest expression of biomarkers related to tolerance without affecting biomarkers related to allergy [78].
To conclude, there are still insufficient data regarding prebiotics use in the prevention and treatment of AR. Nevertheless, their ability to modulate cytokine release seems to be a new, promising approach to the treatment of allergic diseases.
5.3. Bacterial Lysates
BLs are immunomodulatory preparations consisting of antigens derived from respiratory tract pathogens. The most common are Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pyogenes, Streptococcus viridans, Staphylococcus aureus, Klebsiella pneumoniae and Klebsiella ozaenae [79]. The preparation can be obtained using chemical or mechanical lysis. Different production methods can result in different immune effects. BLs can be administered orally, intranasally and sublingually [80]. This review discusses oral administration due to its impact on the gut environment.
As mentioned before, BLs’ mechanism of action is based upon natural exposure to pathogen antigens and the following immune response. They activate DCs through Toll-like receptors, promoting antiviral cytokines release, NK cell activation, and the restoration of the Th1/Th2 balance. The in-depth mechanism of BLs’ action is described in our previous publication [81].
Only five studies investigated BLs’ impact on the AR course. Two of them used orally administered OM-85. Koatz et al. conducted an open-label, sequential study concerning the use of OM-85 on respiratory tract infection rates, primary disease exacerbation rates and symptom severity in patients with AR, asthma or chronic obstructive pulmonary disease. Patients received the preparation in three cycles consisting of 10 consecutive days of intake followed by a 20-day break. They showed that OM-85 therapy reduced the number of respiratory tract infections and AR exacerbations, and the severity of allergic symptoms in comparison to the previous year when patients received only standard optimized care. Moreover, an increase in serum and salivary IgA levels has been demonstrated [82]. To further expand the research, Meng et al. evaluated its clinical effects in 60 patients with perennial AR. Enrolled participants were administered with OM-85 following the same regimen as in the previously mentioned study. After the treatment, the OM-85 group presented a significant decrease in total nasal symptom score, itching score, nasal rhinorrhoea score, sneezing score and medication score. Furthermore, an increase in nasal IFN-γ and decreases in nasal IL-4 and IL-13 levels, and the number of eosinophils in nasal swabs were observed [83].
BLs do not affect the intestinal bacteria directly; however, the cytokines stimulated by them may affect the gut environment. Van Averbeke et al. suggest that host immunity influences the composition of gut microbiota. In a murine model, they characterized the humoral, cellular and cytokine immunity and associated alterations in the gut microbiota of naïve wild-type mice and mice with deficiencies in Th2 responses or both Th1 and Th2 responses. The research showed that wild-type mice were enriched in bacteria, which are able to stimulate beneficial SCFAs production. Furthermore, the Th1-leaning compared to the naïve wild-type mice presented less microbial diversity. These data suggest that alterations in the Th1/Th2 balance or complete ablation of Th1/Th2 responses can significantly alter gut microbiota composition and function [84].
5.4. Fecal Microbiota Transplantation
FMT is the procedure in which the healthy donor’s stool is transformed into faecal suspension and is administered to the patient’s intestine to re-establish the balance of the gut microbiota [85]. Currently, there are no studies concerning its use in AR treatment; however, it may be a promising way to restore the intestine bacteria composition. Potentially, it may be more effective than probiotics due to its significantly more abundant infused microorganisms and the ability to permanently colonize the gut [86].
This presumption is being upheld by the study by Mashiah et al., who investigated the effects of FMT in adult AD patients. It was shown that the Scoring Atopic Dermatitis Score (SCORAD) significantly decreased after FMT. Moreover, weekly topical usage of corticosteroids was diminished during the study and follow-up period. Metagenomic analysis of the gut microbiota showed a significant bacterial strain transmission from donors to patients. No adverse effects of the treatment were observed [87]. Nevertheless, there are still insufficient data concerning its use in allergic diseases; thus, more large sample studies are needed.
6. Conclusions
In summary, it seems highly probable that gut microbiota plays a role in the pathogenesis of AR. With ever-growing evidence published in that field, the hope for the new prevention and therapy of AR is growing in parallel.
It is believed that immune system imbalance is linked to decreased gut microbiota composition. Prenatal, neonatal and early childhood bacteria colonization alters the gut environment and impacts the developing immune system. Thus, early-life disturbances in colonization may affect future host’s immunity and lead to allergy. However, probiotics, prebiotics and BLs seem promising therapeutic approaches to restore this imbalance.
In the future, it will probably be possible to identify patients with an exceptionally high allergy risk or even modulate intestinal microbiota effectively and permanently. Nevertheless, more rigorous and detailed trials are demanded to draw definitive conclusions.
Author Contributions
Conceptualization, A.K.; methodology, A.K., M.K. and P.C.; investigation, A.K., M.K. and P.C.; resources, A.K., M.K. and P.C.; data curation, A.K., M.K. and P.C.; writing—original draft preparation, A.K., M.K. and P.C.; writing—review and editing, A.K. and K.J.; visualization, A.K. and K.J.; supervision, K.J. and A.E.; project administration, A.K. and K.J.; funding acquisition, K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davenport, E.R.; Sanders, J.G.; Song, S.J.; Amato, K.R.; Clark, A.G.; Knight, R. The human microbiome in evolution. BMC Biol. 2017, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Schwartz, G.; Bernstein, J.A. Allergic Rhinitis: Mechanisms and Treatment. Immunol. Allergy. Clin. N. Am. 2016, 36, 261–278. [Google Scholar] [CrossRef]
- Haapakoski, R.; Karisola, P.; Fyhrquist, N.; Savinko, T.; Lehtimaki, S.; Wolff, H.; Lauerma, A.; Alenius, H. Toll-like receptor activation during cutaneous allergen sensitization blocks development of asthma through IFN-gamma-dependent mechanisms. J. Invest. Dermatol. 2013, 133, 964–972. [Google Scholar] [CrossRef]
- Cingi, C.; Bayar Muluk, N.; Scadding, G.K. Will every child have allergic rhinitis soon? Int. J. Pediatr. Otorhinolaryngol. 2019, 118, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Small, P.; Keith, P.K.; Kim, H. Allergic rhinitis. Allergy Asthma. Clin. Immunol. 2018, 14, 51. [Google Scholar] [CrossRef]
- Sih, T.; Mion, O. Allergic rhinitis in the child and associated comorbidities. Pediatr. Allergy Immunol. 2010, 21, e107–e113. [Google Scholar] [CrossRef] [PubMed]
- Mir, E.; Panjabi, C.; Shah, A. Impact of allergic rhinitis in school going children. Asia Pac. Allergy 2012, 2, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Schunemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80. [Google Scholar] [CrossRef]
- Platt, A.M.; Mowat, A.M. Mucosal macrophages and the regulation of immune responses in the intestine. Immunol. Lett. 2008, 119, 22–31. [Google Scholar] [CrossRef]
- Romagnani, S. Regulation of the T cell response. Clin. Exp. Allergy 2006, 36, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Cosola, C.; Rocchetti, M.T.; Gesualdo, L. Gut Microbiota, the Immune System, and Cytotoxic T Lymphocytes. Methods Mol. Biol. 2021, 2325, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Bjorksten, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Romero, R.; Amogan, H.P.; Kusanovic, J.P.; Bik, E.M.; Gotsch, F.; Kim, C.J.; Erez, O.; Edwin, S.; Relman, D.A. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: A molecular and culture-based investigation. PLoS ONE 2008, 3, e3056. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef]
- Jimenez, E.; Fernandez, L.; Marin, M.L.; Martin, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodriguez, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.; Marin, M.L.; Martin, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernandez, L.; Rodriguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Nomoto, K.; Kawashima, K.; Nagata, S.; Yamashiro, Y. Ontogenesis of the Gut Microbiota Composition in Healthy, Full-Term, Vaginally Born and Breast-Fed Infants over the First 3 Years of Life: A Quantitative Bird’s-Eye View. Front Microbiol. 2017, 8, 1388. [Google Scholar] [CrossRef]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Palumbo, M.P.; Glueck, D.H.; Sauder, K.A.; O’Mahony, L.; Fleischer, D.M.; Ben-Abdallah, M.; Ringham, B.M.; Dabelea, D. The maternal diet index in pregnancy is associated with offspring allergic diseases: The Healthy Start study. Allergy 2022, 77, 162–172. [Google Scholar] [CrossRef]
- Obata, Y.; Furusawa, Y.; Hase, K. Epigenetic modifications of the immune system in health and disease. Immunol. Cell Biol. 2015, 93, 226–232. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
- Smith, P.K.; Masilamani, M.; Li, X.M.; Sampson, H.A. The false alarm hypothesis: Food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Chou, H.C.; Yang, Y.S.H. Maternal Antibiotic Treatment Disrupts the Intestinal Microbiota and Intestinal Development in Neonatal Mice. Front Microbiol. 2021, 12, 684233. [Google Scholar] [CrossRef] [PubMed]
- Erturk-Hasdemir, D.; Oh, S.F.; Okan, N.A.; Stefanetti, G.; Gazzaniga, F.S.; Seeberger, P.H.; Plevy, S.E.; Kasper, D.L. Symbionts exploit complex signaling to educate the immune system. Proc. Natl. Acad. Sci. USA 2019, 116, 26157–26166. [Google Scholar] [CrossRef]
- Shaterian, N.; Abdi, F.; Ghavidel, N.; Alidost, F. Role of cesarean section in the development of neonatal gut microbiota: A systematic review. Open Med. 2021, 16, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.D.P.; Ayres, L.F.A.; Barreto, D.S.; Henriques, B.D.; Prado, M.; Passos, C.M.D. Acquisition of microbiota according to the type of birth: An integrative review. Rev. Lat. Am. Enfermagem. 2021, 29, e3446. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Jiang, Y.; Li, A.; Zhang, J.; Chen, M.; Hu, L.; Li, Z.; Wang, D. Bacteroides thetaiotaomicron Ameliorates Experimental Allergic Airway Inflammation via Activation of ICOS(+)Tregs and Inhibition of Th2 Response. Front Immunol. 2021, 12, 620943. [Google Scholar] [CrossRef] [PubMed]
- Steiner, N.C.; Lorentz, A. Probiotic Potential of Lactobacillus Species in Allergic Rhinitis. Int. Arch. Allergy. Immunol. 2021, 182, 807–818. [Google Scholar] [CrossRef]
- Flora, M.; Perrotta, F.; Nicolai, A.; Maffucci, R.; Pratillo, A.; Mollica, M.; Bianco, A.; Calabrese, C. Staphylococcus Aureus in chronic airway diseases: An overview. Respir. Med. 2019, 155, 66–71. [Google Scholar] [CrossRef]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmso, M.; Shah, S.; Christensen, E.D.; Chawes, B.L.; Bonnelykke, K.; Brix, S.; et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 2020, 12, eaax9929. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, eaax9929. [Google Scholar] [CrossRef]
- Mosca, F.; Gianni, M.L. Human milk: Composition and health benefits. Pediatr. Med. Chir. 2017, 39, 155. [Google Scholar] [CrossRef]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 852. [Google Scholar] [CrossRef]
- Fernandez, L.; Ruiz, L.; Jara, J.; Orgaz, B.; Rodriguez, J.M. Strategies for the Preservation, Restoration and Modulation of the Human Milk Microbiota. Implications for Human Milk Banks and Neonatal Intensive Care Units. Front Microbiol. 2018, 9, 2676. [Google Scholar] [CrossRef]
- Suzuki, S.; Shimojo, N.; Tajiri, Y.; Kumemura, M.; Kohno, Y. Differences in the composition of intestinal Bifidobacterium species and the development of allergic diseases in infants in rural Japan. Clin. Exp. Allergy 2007, 37, 506–511. [Google Scholar] [CrossRef]
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef]
- Jernberg, C.; Lofmark, S.; Edlund, C.; Jansson, J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef]
- Schubert, A.M.; Sinani, H.; Schloss, P.D. Antibiotic-Induced Alterations of the Murine Gut Microbiota and Subsequent Effects on Colonization Resistance against Clostridium difficile. mBio 2015, 6, e00974. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Mitre, E.; Susi, A.; Kropp, L.E.; Schwartz, D.J.; Gorman, G.H.; Nylund, C.M. Association between Use of Acid-Suppressive Medications and Antibiotics during Infancy and Allergic Diseases in Early Childhood. JAMA Pediatr. 2018, 172, e180315. [Google Scholar] [CrossRef]
- Russell, S.L.; Gold, M.J.; Hartmann, M.; Willing, B.P.; Thorson, L.; Wlodarska, M.; Gill, N.; Blanchet, M.R.; Mohn, W.W.; McNagny, K.M.; et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012, 13, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Tierney, B.T.; Yang, Z.; Luber, J.M.; Beaudin, M.; Wibowo, M.C.; Baek, C.; Mehlenbacher, E.; Patel, C.J.; Kostic, A.D. The Landscape of Genetic Content in the Gut and Oral Human Microbiome. Cell Host Microbe 2019, 26, 283–295 e288. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.K.; Kamm, M.A.; Teo, S.M.; Inouye, M.; Wagner, J.; Kirkwood, C.D. Recent advances in characterizing the gastrointestinal microbiome in Crohn’s disease: A systematic review. Inflamm. Bowel Dis. 2015, 21, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tao, J.; Li, J.; Cao, X.; Li, Y.; Gao, X.; Fu, Y. Dysbiosis of Fecal Microbiota in Allergic Rhinitis Patients. Am. J. Rhinol. Allergy 2020, 34, 650–660. [Google Scholar] [CrossRef]
- Watts, A.M.; West, N.P.; Zhang, P.; Smith, P.K.; Cripps, A.W.; Cox, A.J. The Gut Microbiome of Adults with Allergic Rhinitis Is Characterised by Reduced Diversity and an Altered Abundance of Key Microbial Taxa Compared to Controls. Int. Arch Allergy Immunol. 2021, 182, 94–105. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, F.; Wan, W.; Yu, B.; Tang, L.; Yang, Y.; Du, Y.; Chen, Z.; Xu, H. Gut microbial characteristics of adult patients with allergy rhinitis. Microb. Cell Fact. 2020, 19, 171. [Google Scholar] [CrossRef]
- Su, Y.J.; Luo, S.D.; Hsu, C.Y.; Kuo, H.C. Differences in gut microbiota between allergic rhinitis, atopic dermatitis, and skin urticaria: A pilot study. Medicine 2021, 100, e25091. [Google Scholar] [CrossRef]
- Sano, T.; Huang, W.; Hall, J.A.; Yang, Y.; Chen, A.; Gavzy, S.J.; Lee, J.Y.; Ziel, J.W.; Miraldi, E.R.; Domingos, A.I.; et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 2016, 164, 324. [Google Scholar] [CrossRef]
- Berndt, B.E.; Zhang, M.; Owyang, S.Y.; Cole, T.S.; Wang, T.W.; Luther, J.; Veniaminova, N.A.; Merchant, J.L.; Chen, C.C.; Huffnagle, G.B.; et al. Butyrate increases IL-23 production by stimulated dendritic cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1384–G1392. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Min, J.; Wang, J.; Wu, H.; Zeng, Y.; Chen, S.; Chu, Z. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 2012, 277, 66–73. [Google Scholar] [CrossRef]
- Nastasi, C.; Candela, M.; Bonefeld, C.M.; Geisler, C.; Hansen, M.; Krejsgaard, T.; Biagi, E.; Andersen, M.H.; Brigidi, P.; Odum, N.; et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015, 5, 16148. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal. Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-kappaB pathway in RAW264.7 cells. Inflammation 2012, 35, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Cahenzli, J.; Koller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.J.; Kang, S.M.; Xie, J.L.; Huang, L.; Wen, Q.; Fan, Y.Y.; Lu, L.J.; Jiang, L. Early-life gut microbial colonization shapes Th1/Th2 balance in asthma model in BALB/c mice. BMC Microbiol. 2017, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Lin, P.; Gao, P.; Wang, Z.; Guo, K.; Qin, S.; Zhou, C.; Wang, B.; Wu, E.; Khan, N.; et al. Gut Microbiota Regulate Gut-Lung Axis Inflammatory Responses by Mediating ILC2 Compartmental Migration. J. Immunol. 2021, 207, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.H.; Chou, H.C.; Tung, Y.L.; Chiang, B.L.; Liao, C.C.; Liu, H.H.; Ni, Y.H. Intestinal Dysbiosis Featuring Abundance of Ruminococcus gnavus Associates with Allergic Diseases in Infants. Gastroenterology 2018, 154, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.; Wang, B.; Shafiei Jahani, P.; Hurrell, B.P.; Banie, H.; Aleman Muench, G.R.; Maazi, H.; Helou, D.G.; Howard, E.; Galle-Treger, L.; et al. Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress ILC2-Dependent Airway Inflammation. Front Immunol. 2019, 10, 2051. [Google Scholar] [CrossRef] [PubMed]
- FAO. Guidelines for the Evaluation of Probiotics in Food. In Proceedings of the Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, ON, Canada, 30 April–1 May 2002. [Google Scholar]
- European Food Safety Authority (EFSA). Opinion of the Scientific Committee on a request from EFSA related to a generic approach to the safety assessment by EFSA of microorganisms used in food/feed and the production of food/feed additives. EFSA J. 2005, 226, 1–12. [Google Scholar]
- Simon, O. Microorganisms as feed additives—Probiotics. Adv. Pork Prod. 2005, 16, 161–167. [Google Scholar]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef]
- Wang, M.F.; Lin, H.C.; Wang, Y.Y.; Hsu, C.H. Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr. Allergy Immunol. 2004, 15, 152–158. [Google Scholar] [CrossRef]
- Peng, G.C.; Hsu, C.H. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr. Allergy Immunol. 2005, 16, 433–438. [Google Scholar] [CrossRef]
- Yan, S.; Ai, S.; Huang, L.; Qiu, C.; Zhang, F.; He, N.; Zhuang, X.; Zheng, J. Systematic review and meta-analysis of probiotics in the treatment of allergic rhinitis. Allergol. Immunopathol. 2022, 50, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, K.; Kuitunen, M.; Haahtela, T.; Korpela, R.; Poussa, T.; Savilahti, E. High intestinal IgA associates with reduced risk of IgE-associated allergic diseases. Pediatr. Allergy Immunol. 2010, 21, 67–73. [Google Scholar] [CrossRef]
- Kang, M.G.; Han, S.W.; Kang, H.R.; Hong, S.J.; Kim, D.H.; Choi, J.H. Probiotic NVP-1703 Alleviates Allergic Rhinitis by Inducing IL-10 Expression: A Four-week Clinical Trial. Nutrients 2020, 12, 1427. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.K.; Lin, S.Y.; Toskala, E.; Orlandi, R.R.; Akdis, C.A.; Alt, J.A.; Azar, A.; Baroody, F.M.; Bachert, C.; Canonica, G.W.; et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int. Forum Allergy Rhinol. 2018, 8, 108–352. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Olveira, G.; Gonzalez-Molero, I. An update on probiotics, prebiotics and symbiotics in clinical nutrition. Endocrinol. Nutr. 2016, 63, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Nagamine, T. The effect of prebiotic lactosucrose on serum IgE levels in allergic people: A pilot study in Japan. Intern. Med. J. 2018, 25, 389–390. [Google Scholar]
- Derakhshan, A.; Khodadoost, M.; Ghanei, M.; Gachkar, L.; Hajimahdipour, H.; Taghipour, A.; Yousefi, J.; Khoshkhui, M.; Azad, F.J. Effects of a Novel Barley-Based Formulation on Allergic Rhinitis: A Randomized Controlled Trial. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Boehm, G.; Wienz, F.; Stahl, B.; Bertino, E. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J. Biol. Regul. Homeost. Agents 2012, 26, 49–59. [Google Scholar]
- Castillo-Courtade, L.; Han, S.; Lee, S.; Mian, F.M.; Buck, R.; Forsythe, P. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy 2015, 70, 1091–1102. [Google Scholar] [CrossRef]
- Gourbeyre, P.; Desbuards, N.; Gremy, G.; Tranquet, O.; Champ, M.; Denery-Papini, S.; Bodinier, M. Perinatal and postweaning exposure to galactooligosaccharides/inulin prebiotics induced biomarkers linked to tolerance mechanism in a mouse model of strong allergic sensitization. J. Agric. Food Chem. 2013, 61, 6311–6320. [Google Scholar] [CrossRef]
- Bessler, W.G.; Vor dem Esche, U.; Masihi, N. The bacterial extract OM-85 BV protects mice against influenza and Salmonella infection. Int. Immunopharmacol. 2010, 10, 1086–1090. [Google Scholar] [CrossRef]
- Cazzola, M.; Anapurapu, S.; Page, C.P. Polyvalent mechanical bacterial lysate for the prevention of recurrent respiratory infections: A meta-analysis. Pulm. Pharmacol. Ther. 2012, 25, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kaczynska, A.; Klosinska, M.; Janeczek, K.; Zarobkiewicz, M.; Emeryk, A. Promising Immunomodulatory Effects of Bacterial Lysates in Allergic Diseases. Front Immunol. 2022, 13, 907149. [Google Scholar] [CrossRef] [PubMed]
- Koatz, A.M.; Coe, N.A.; Ciceran, A.; Alter, A.J. Clinical and Immunological Benefits of OM-85 Bacterial Lysate in Patients with Allergic Rhinitis, Asthma, and COPD and Recurrent Respiratory Infections. Lung 2016, 194, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, P.; Li, Y.; Chen, J.; Wang, L.; He, L.; Xie, J.; Gao, X. Broncho-vaxom alleviates persistent allergic rhinitis in patients by improving Th1/Th2 cytokine balance of nasal mucosa. Rhinology 2019, 57, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Van Averbeke, V.; Berkell, M.; Mysara, M.; Rodriguez-Ruiz, J.P.; Xavier, B.B.; De Winter, F.H.R.; Jongers, B.; Jairam, R.K.; Hotterbeekx, A.; Goossens, H.; et al. Host Immunity Influences the Composition of Murine Gut Microbiota. Front Immunol. 2022, 13, 828016. [Google Scholar] [CrossRef] [PubMed]
- Shouval, R.; Geva, M.; Nagler, A.; Youngster, I. Fecal Microbiota Transplantation for Treatment of Acute Graft-versus-Host Disease. Clin. Hematol. Int. 2019, 1, 28–35. [Google Scholar] [CrossRef]
- Grehan, M.J.; Borody, T.J.; Leis, S.M.; Campbell, J.; Mitchell, H.; Wettstein, A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J. Clin. Gastroenterol. 2010, 44, 551–561. [Google Scholar] [CrossRef]
- Mashiah, J.; Karady, T.; Fliss-Isakov, N.; Sprecher, E.; Slodownik, D.; Artzi, O.; Samuelov, L.; Ellenbogen, E.; Godneva, A.; Segal, E.; et al. Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun. Inflamm. Dis. 2022, 10, e570. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).