The Nutraceutical Genistein-Lycopene Combination Improves Bone Damage Induced by Glucocorticoids by Stimulating the Osteoblast Formation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental In Vivo Model of Glucocorticoid-Induced Osteoporosis
2.2. Primary Osteoblast Culture and Extraction
2.3. In Vitro Model of Glucocorticoid-Induced Osteoporosis
2.4. Alizarin Red S Staining
2.5. RT-qPCR
2.6. Histology
2.7. Microcomputed Tomography (µCT) Measurements
2.8. Statistical Evaluation
3. Results
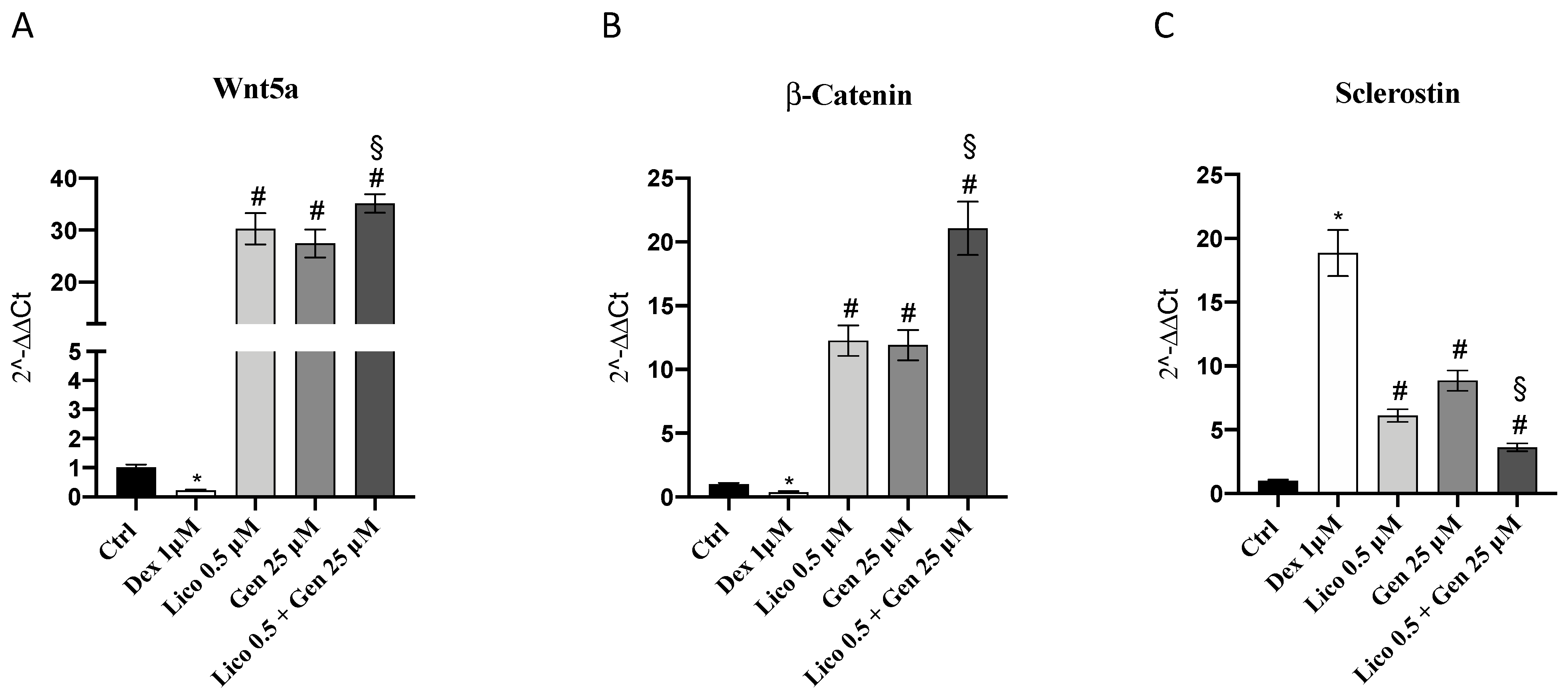
3.1. Genistein and Lycopene Treatment Stimulates Osteoblast Activity
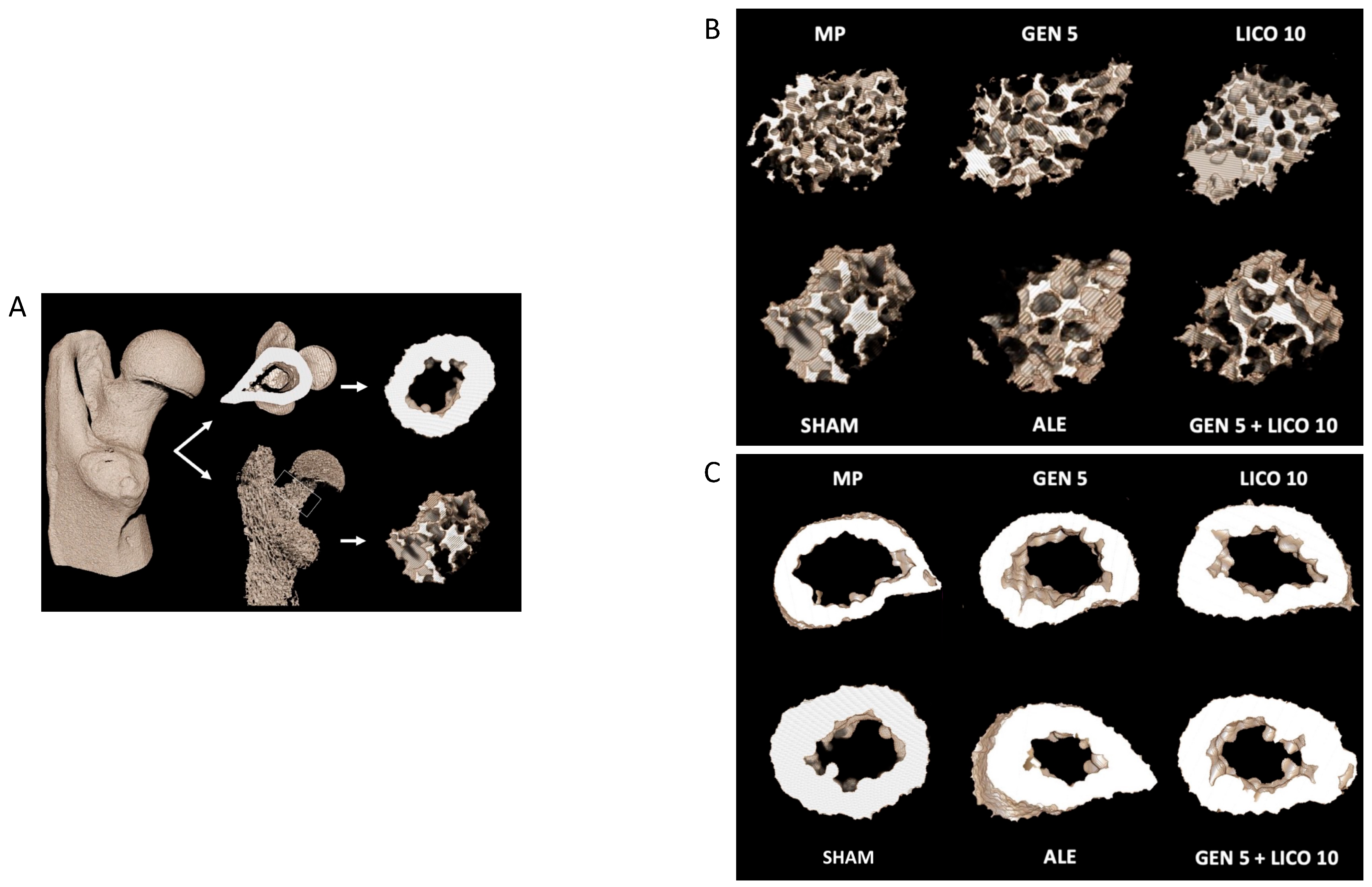
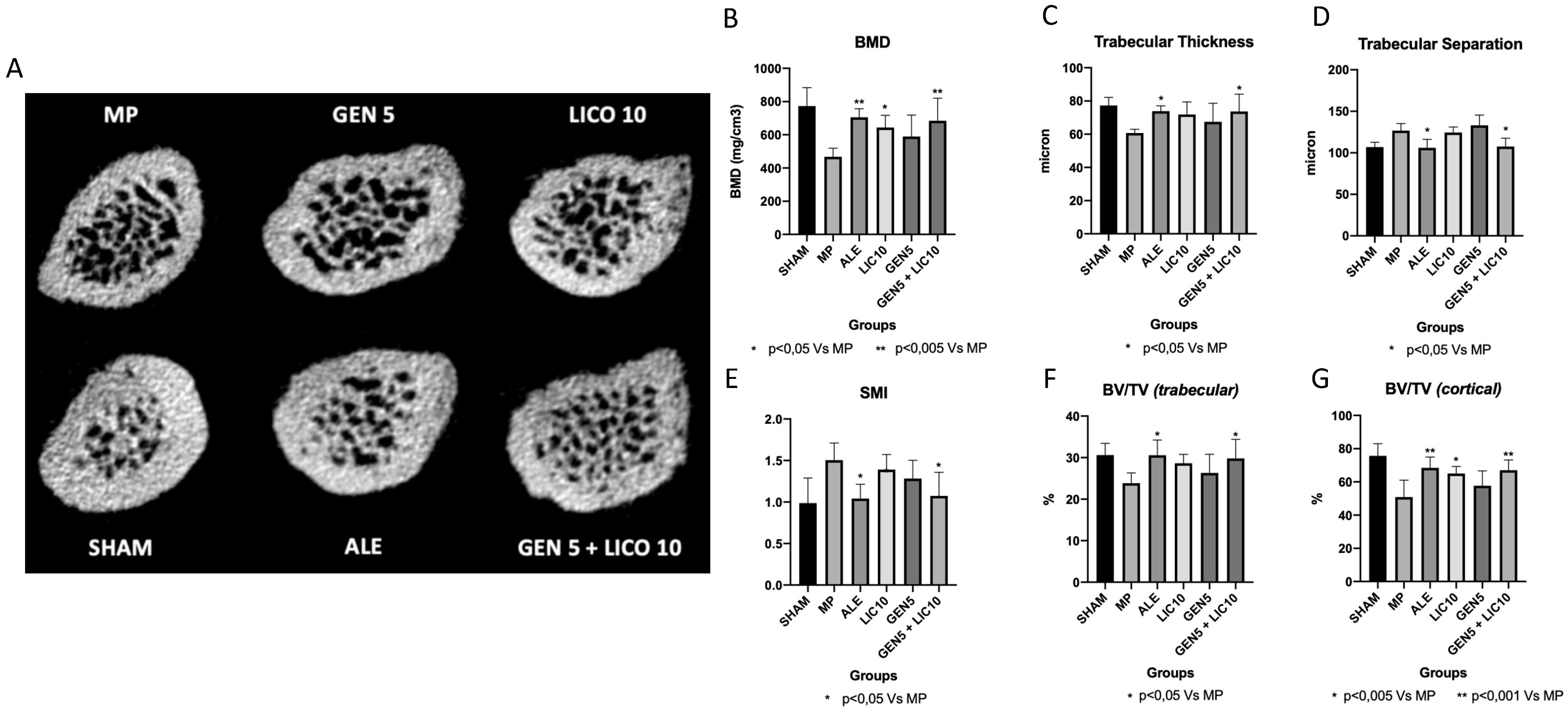
3.2. The Association of Genistein and Lycopene Improves Bone Microarchitecture and Volumetric BMD
3.3. The Combined Treatment of Genistein and Lycopene Improves Bone Structure
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erben, R.G. Hypothesis: Coupling between Resorption and Formation in Cancellous bone Remodeling is a Mechanically Controlled Event. Front. Endocrinol. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2012, 4, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Erfanian, A.; Rasti, B.; Manap, Y. Comparing the calcium bioavailability from two types of nano-sized enriched milk using in-vivo assay. Food Chem. 2017, 214, 606–613. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Rauner, M. Minireview: Live and let die: Molecular effects of glucocorticoids on bone cells. Mol. Endocrinol. 2009, 23, 1525–1531. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef]
- Hayhoe, R.P.G.; Lentjes, M.A.H.; Mulligan, A.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Br. J. Nutr. 2017, 117, 1439–1453. [Google Scholar] [CrossRef]
- Harahap, I.A.; Suliburska, J. Probiotics and Isoflavones as a Promising Therapeutic for Calcium Status and Bone Health: A Narrative Review. Foods 2021, 10, 2685. [Google Scholar] [CrossRef]
- Arcoraci, V.; Atteritano, M.; Squadrito, F.; D’Anna, R.; Marini, H.; Santoro, D.; Minutoli, L.; Messina, S.; Altavilla, D.; Bitto, A. Antiosteoporotic Activity of Genistein Aglycone in Postmenopausal Women: Evidence from a Post-Hoc Analysis of a Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 179. [Google Scholar] [CrossRef]
- Walallawita, U.S.; Wolber, F.M.; Ziv-Gal, A.; Kruger, M.C.; Heyes, J.A. Potential Role of Lycopene in the Prevention of Postmenopausal Bone Loss: Evidence from Molecular to Clinical Studies. Int. J. Mol. Sci. 2020, 21, 7119. [Google Scholar] [CrossRef]
- Mackinnon, E.; Venket Rao, A.; Rao, L. Dietary restriction of lycopene for a period of one month resulted in significantly increased biomarkers of oxidative stress and bone resorption in postmenopausal women. J. Nutr. Health Aging 2011, 15, 133–138. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Lv, H.; Yang, T.; He, A.; Wang, M.; Jia, H.; Ma, M.; Li, S. miR-27b attenuates dexamethasone-inhibited proliferation and osteoblastic differentiation in MC3T3-E1 cells by targeting PPARγ2. Exp. Ther. Med. 2022, 23, 127. [Google Scholar] [CrossRef]
- Picciolo, G.; Pallio, G.; Altavilla, D.; Vaccaro, M.; Oteri, G.; Irrera, N.; Squadrito, F. β-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors. Biomedicines 2020, 8, 164. [Google Scholar] [CrossRef]
- Benvenga, S.; Marini, H.R.; Micali, A.; Freni, J.; Pallio, G.; Irrera, N.; Squadrito, F.; Altavilla, D.; Antonelli, A.; Ferrari, S.M.; et al. Protective Effects of Myo-Inositol and Selenium on Cadmium-Induced Thyroid Toxicity in Mice. Nutrients 2020, 12, 1222. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Bachagol, D.; Joseph, G.S.; Ellur, G.; Patel, K.; Aruna, P.; Mittal, M.; China, S.P.; Singh, R.P.; Sharan, K. Stimulation of liver IGF-1 expression promotes peak bone mass achievement in growing rats: A study with pomegranate seed oil. J. Nutr. Biochem. 2018, 52, 18–26. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Tsai, M.T.; Wang, S.P.; Chen, Y.J.; Wu, J.; Hsu, J.T. Cortical Bone Morphological and Trabecular Bone Microarchitectural Changes in the Mandible and Femoral Neck of Ovariectomized Rats. PLoS ONE 2016, 11, e0154367. [Google Scholar] [CrossRef]
- Humphrey, E.L.; Williams, J.H.; Davie, M.W.; Marshall, M.J. Effects of dissociated glucocorticoid on OPG and RANKL in osteoblastic cells. Bone 2006, 38, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Benkusky, N.A.; Pike, J.W. The RUNX2 cistrome in osteoblasts: Characterization, down-regulation following differentiation, and relationship to gene expression. J. Biol. Chem. 2014, 289, 16016–16031. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carballo, E.; Ulsamer, A.; Susperregui, A.R.; Manzanares-Céspedes, C.; Sánchez-García, E.; Bartrons, R.; Rosa, J.L.; Ventura, F. Conserved regulatory motifs in osteogenic gene promoters integrate cooperative effects of canonical Wnt and BMP pathways. J. Bone Miner. Res. 2010, 26, 718–729. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Wattanapenpaiboon, N.; Lukito, W.; Wahlqvist, M.L.; Strauss, B.J. Dietary carotenoid intake as a predictor of bone mineral density. Asia Pac. J. Clin. Nutr. 2003, 12, 467–473. [Google Scholar]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological agents and natural compounds: Available treatments for osteoporosis. J. Physiol. Pharmacol. 2020, 71, 307–320. [Google Scholar]
- Papapoulos, S.E. Targeting sclerostin as potential treatment of osteoporosis. Ann. Rheum. Dis. 2011, 70 (Suppl. 1), i119–i122. [Google Scholar] [CrossRef]
- Bitto, A.; Burnett, B.P.; Polito, F.; Levy, R.M.; Marini, H.; Di Stefano, V.; Irrera, N.; Armbruster, M.A.; Minutoli, L.; Altavilla, D.; et al. Genistein aglycone reverses glucocorticoid-induced osteoporosis and increases bone breaking strength in rats: A comparative study with alendronate. Br. J. Pharmacol. 2009, 156, 1287–1295. [Google Scholar] [CrossRef]
- Semeghini, M.S.; Scalize, P.H.; Coelho, M.C.; Fernandes, R.R.; Pitol, D.L.; Tavares, M.S.; de Sousa, L.G.; Coppi, A.A.; Siessere, S.; Bombonato-Prado, K.F. Lycopene prevents bone loss in ovariectomized rats and increases the number of osteocytes and osteoblasts. J. Anat. 2022, 241, 729–740. [Google Scholar] [CrossRef]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S.; et al. Lycopene and bone: An in vitro investigation and a pilot prospective clinical study. J. Transl. Med. 2020, 18, 43. [Google Scholar] [CrossRef]
- Jain, S.K.; Roy, S.P.; Nagi, O.N. Alendronate induced femur fracture complicated with secondary hyperparathyroidism. Mymensingh Med. J. 2011, 20, 501–506. [Google Scholar]
- Li, Y.Q.; Xing, X.H.; Wang, H.; Weng, X.L.; Yu, S.B.; Dong, G.Y. Dose-dependent effects of genistein on bone homeostasis in rats’ mandibular subchondral bone. Acta Pharmacol. Sin. 2012, 33, 66–74. [Google Scholar] [CrossRef]
- Li, X.; Xue, W.; Cao, Y.; Long, Y.; Xie, M. Effect of lycopene on titanium implant osseointegration in ovariectomized rats. J. Orthop. Surg. Res. 2018, 13, 237. [Google Scholar] [CrossRef]


| Primer | Forward | Reverse |
|---|---|---|
| b-ALP | GCTGATCATTCCCACGTTTT | CTGGGCCTGGTAGTTGTTGT |
| RUNX-2 | CCCAGCCACCTTTACCTACA | TATGGAGTGCTGCTGGTCTG |
| Wnt5a | CAAATAGGCAGCCGAGAGAC | CTCTAGCGTCCACGAACTCC |
| β-Catenin | GTGCAATTCCTGAGCTGACA | CTTAAAGATGGCCAGCAAGC |
| Sclerostin | GCCGGACCTATACAGGACAA | CACGTAGCCCAACATCACAC |
| Nrf2 | CTCGCTGGAAAAAGAAGTGG | CCGTCCAGGAGTTCAGAGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannino, F.; D’Angelo, T.; Pallio, G.; Ieni, A.; Pirrotta, I.; Giorgi, D.A.; Scarfone, A.; Mazziotti, S.; Booz, C.; Bitto, A.; et al. The Nutraceutical Genistein-Lycopene Combination Improves Bone Damage Induced by Glucocorticoids by Stimulating the Osteoblast Formation Process. Nutrients 2022, 14, 4296. https://doi.org/10.3390/nu14204296
Mannino F, D’Angelo T, Pallio G, Ieni A, Pirrotta I, Giorgi DA, Scarfone A, Mazziotti S, Booz C, Bitto A, et al. The Nutraceutical Genistein-Lycopene Combination Improves Bone Damage Induced by Glucocorticoids by Stimulating the Osteoblast Formation Process. Nutrients. 2022; 14(20):4296. https://doi.org/10.3390/nu14204296
Chicago/Turabian StyleMannino, Federica, Tommaso D’Angelo, Giovanni Pallio, Antonio Ieni, Igor Pirrotta, Domenico Antonio Giorgi, Alessandro Scarfone, Silvio Mazziotti, Christian Booz, Alessandra Bitto, and et al. 2022. "The Nutraceutical Genistein-Lycopene Combination Improves Bone Damage Induced by Glucocorticoids by Stimulating the Osteoblast Formation Process" Nutrients 14, no. 20: 4296. https://doi.org/10.3390/nu14204296
APA StyleMannino, F., D’Angelo, T., Pallio, G., Ieni, A., Pirrotta, I., Giorgi, D. A., Scarfone, A., Mazziotti, S., Booz, C., Bitto, A., Squadrito, F., & Irrera, N. (2022). The Nutraceutical Genistein-Lycopene Combination Improves Bone Damage Induced by Glucocorticoids by Stimulating the Osteoblast Formation Process. Nutrients, 14(20), 4296. https://doi.org/10.3390/nu14204296







