Critical Review for the Production of Antidiabetic Peptides by a Bibliometric Approach
Abstract
1. Introduction
2. Methodology
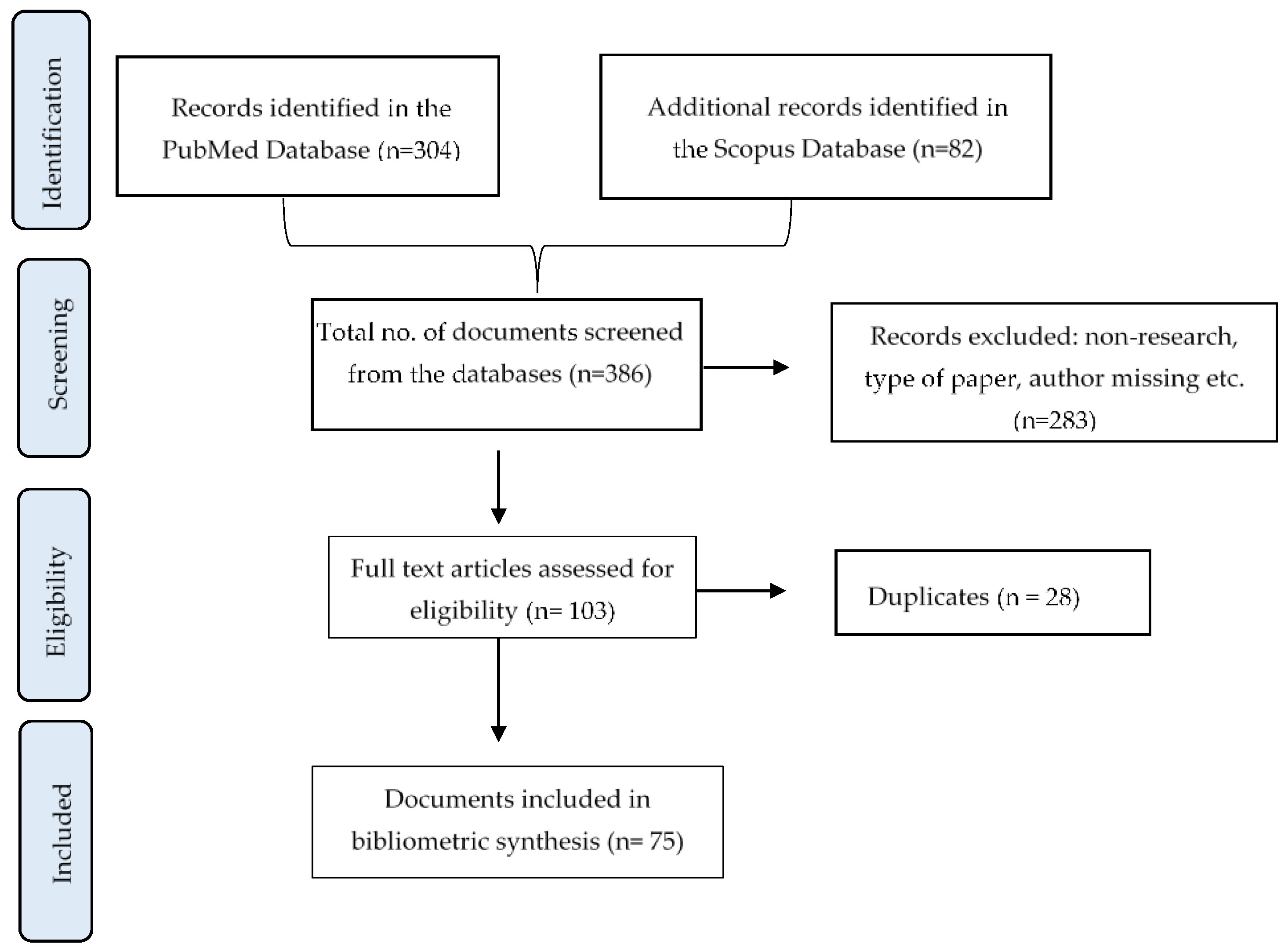
2.1. Search Strategy of the Bibliometric Analysis
2.2. Review Process and Selection Criteria
2.3. Data Extraction
3. Results and Discussion
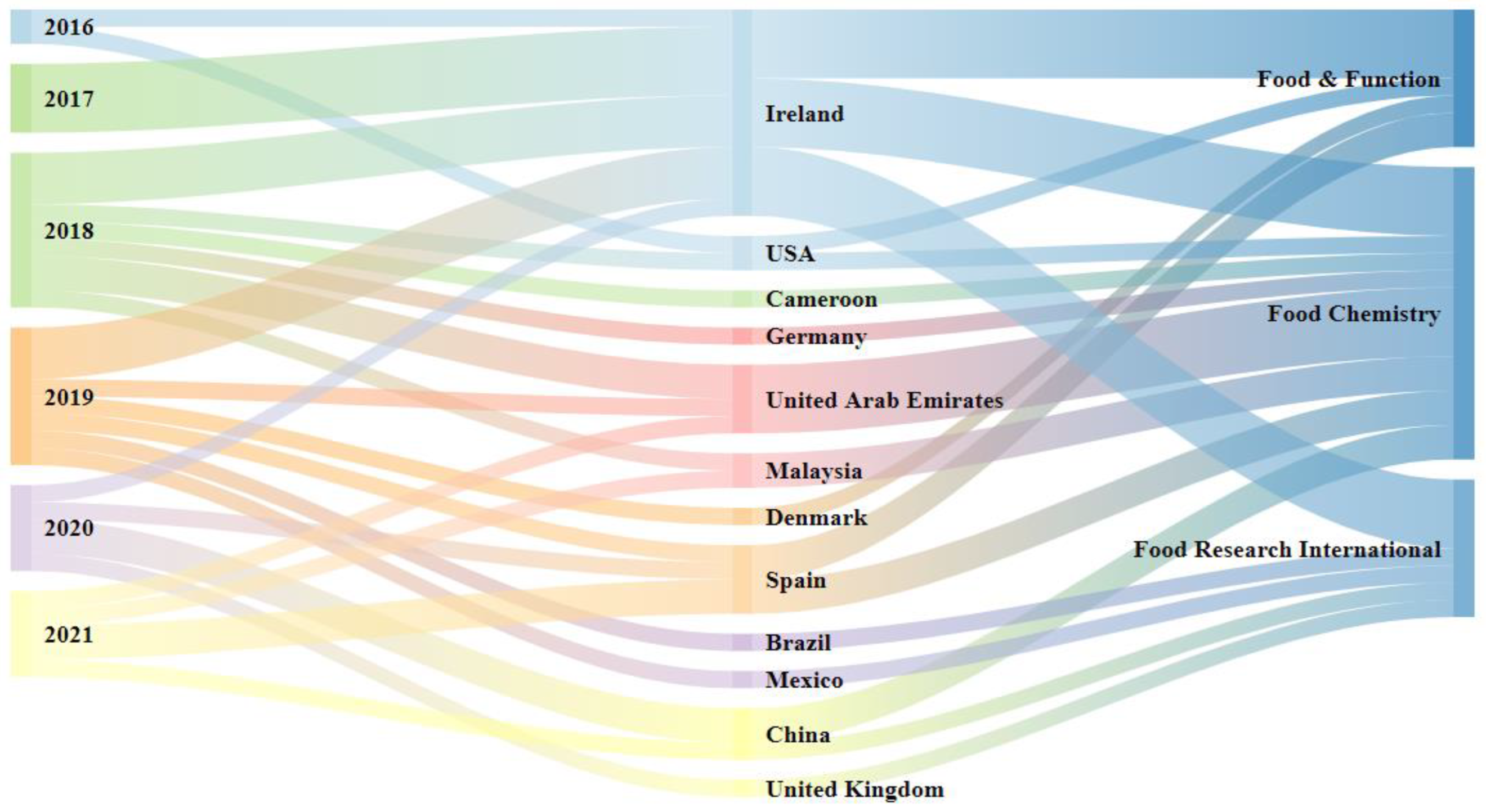
3.1. Number of Publications, Authors’ Countries/Territories, Publication Period, and Leading Journals
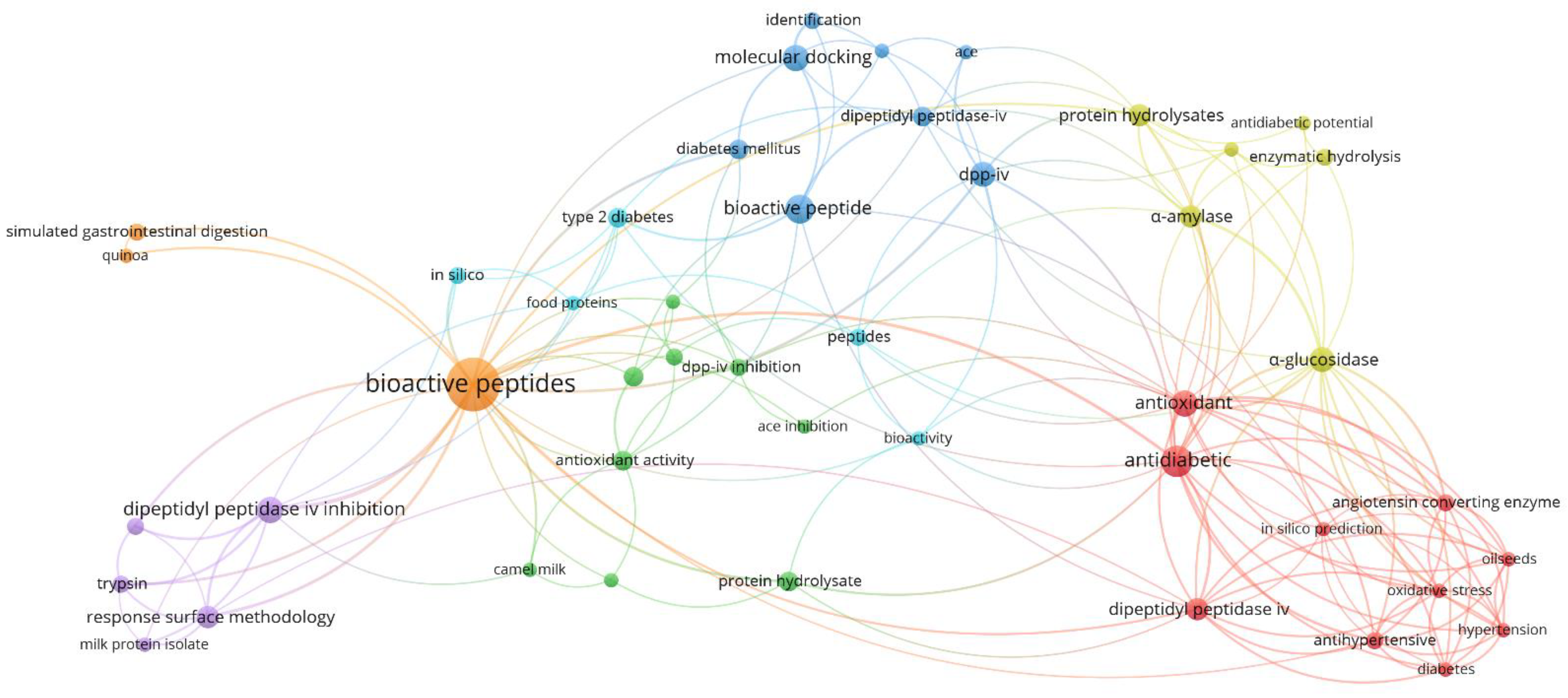
3.2. Author’s Keywords
3.3. The Most Used Proteases
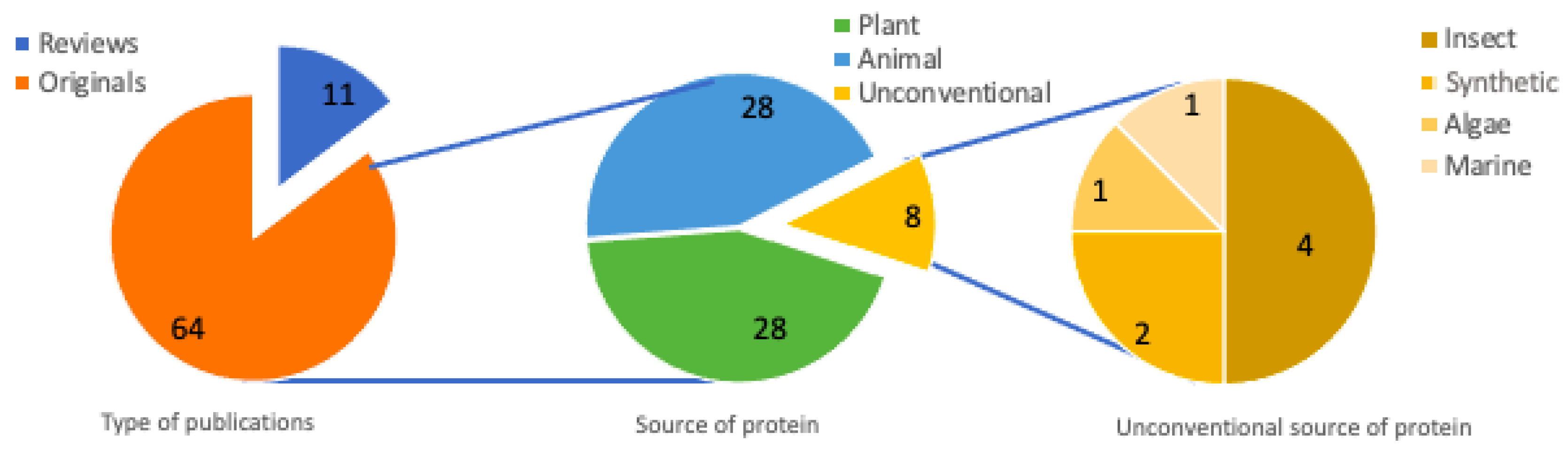
3.4. Main Protein Substrate Sources
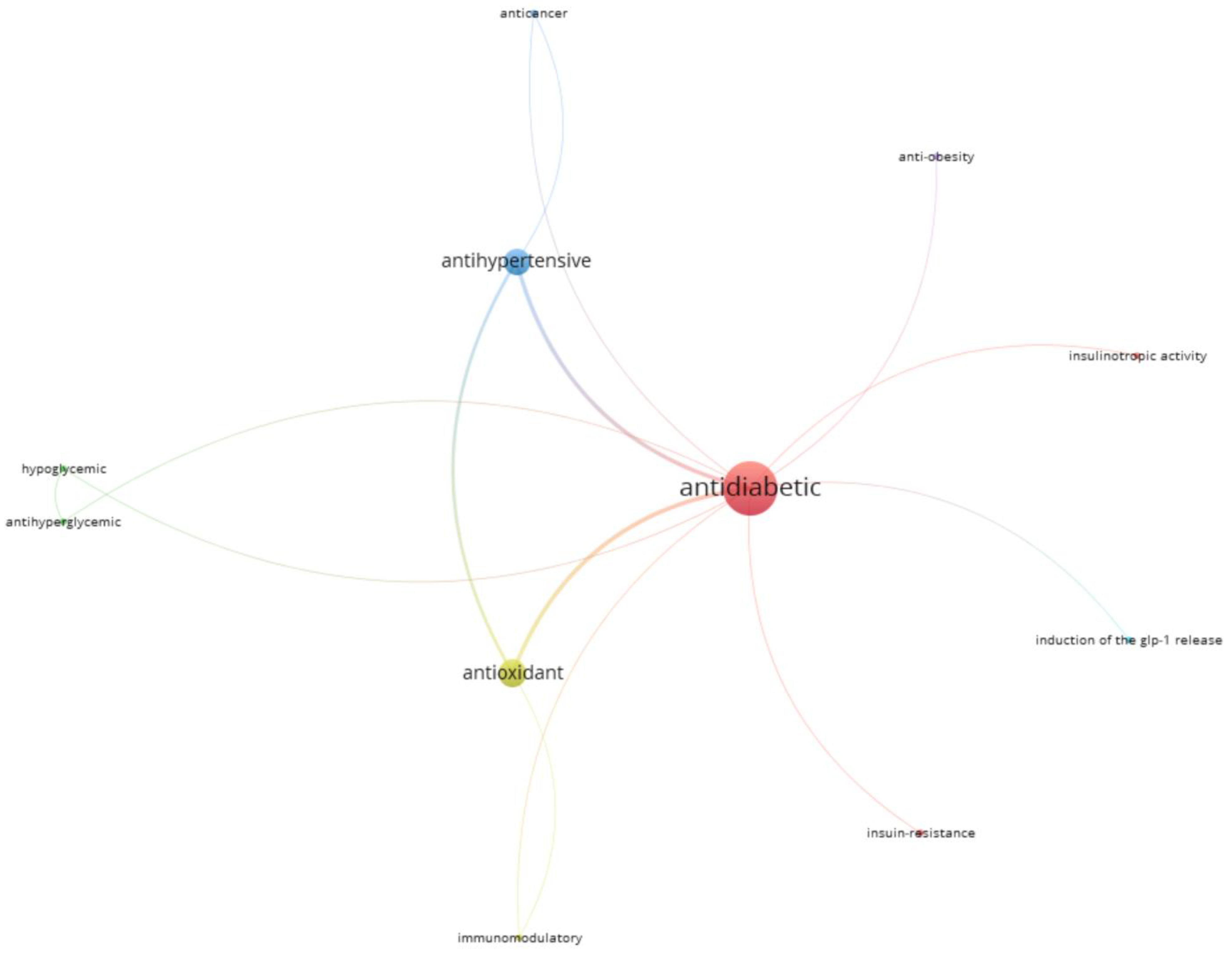
3.5. Bioactive Peptides with Multifunctional Activities
3.6. Type of Inhibition, Type of Analysis, and Type of Document
4. Challenges and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Acquah, C.; Dzuvor, C.K.O.; Tosh, S.; Agyei, D. Anti-diabetic effects of bioactive peptides: Recent advances and clinical implications. Crit. Rev. Food Sci. Nutr. 2020, 62, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2017, 43, e12451. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, J.; Yang, R.; Zhao, W. Bioactive peptides with antidiabetic properties: A review. Int. J. Food Sci. Technol. 2019, 54, 1909–1919. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Goswami, A.; Kalia, K.; Kate, A.S. Plant-Derived Bioactive Peptides: A Treatment to Cure Diabetes. Int. J. Pept. Res. Ther. 2020, 26, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xie, W.; Tan, Q.; Wu, L.; Zhu, S.; Zhu, H.; Qiu, J. Advance on anti-diabetic effects of protein hydrolysates and peptides derived from cereals and pseudocereals. E3S Web Conf. 2020, 189, 02030. [Google Scholar] [CrossRef]
- Linnenluecke, M.K.; Marrone, M.; Singh, A. Conducting systematic literature reviews and bibliometric analyses. Aust. J. Manag. 2020, 45, 175–194. [Google Scholar] [CrossRef]
- Randhawa, K.; Wilden, R.; Hohberger, J. A Bibliometric Review of Open Innovation: Setting a Research Agenda. J. Prod. Innov. Manag. 2016, 33, 750–772. [Google Scholar] [CrossRef]
- IDF. Diabetes Atlas. 2019. Available online: https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf/ (accessed on 23 June 2021).
- Jin, R.; Teng, X.; Shang, J.; Wang, D.; Liu, N. Identification of novel DPP–IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Res. Int. 2020, 133, 109161. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Production and identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from discarded Sardine pilchardus protein. Food Chem. 2020, 328, 127096. [Google Scholar] [CrossRef]
- Megrous, S.; Al-Dalali, S.; Zhao, X.; Chen, C.; Cao, Y.; Bourouis, I.; Mekkaoui, A.; Yang, Z.; Yang, Z. Evaluation of Antidiabetic Activities of Casein Hydrolysates by a Bacillus Metalloendopeptidase. Int. J. Pept. Res. Ther. 2020, 26, 2519–2527. [Google Scholar] [CrossRef]
- Han, R.; Álvarez, A.J.H.; Maycock, J.; Murray, B.S.; Boesch, C. Comparison of alcalase- and pepsin-treated oilseed protein hydrolysates—Experimental validation of predicted antioxidant, antihypertensive and antidiabetic properties. Curr. Res. Food Sci. 2021, 4, 141–149. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Mune, M.A.M.; Minka, S.R.; Henle, T. Investigation on antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activity of Bambara bean protein hydrolysates. Food Chem. 2018, 250, 162–169. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.; FitzGerald, R.J. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Res. Int. 2018, 106, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Azizi, M.H.; Gavlighi, H.A. Frationation of hydrolysate from corn germ protein by ultrafiltration: In vitro antidiabetic and antioxidant activity. Food Sci. Nutr. 2020, 8, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Antidiabetic Food-Derived Peptides for Functional Feeding: Production, Functionality and In Vivo Evidences. Foods 2020, 9, 983. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from vegetable protein sources. Food Chem. 2021, 354, 129473. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Bioactive Peptides from Germinated Soybean with Anti-Diabetic Potential by Inhibition of Dipeptidyl Peptidase-IV, α-Amylase, and α-Glucosidase Enzymes. Int. J. Mol. Sci. 2018, 19, 2883. [Google Scholar] [CrossRef]
- Gomez, H.L.R.; Peralta, J.P.; Tejano, L.A.; Chang, Y.-W. In Silico and In Vitro Assessment of Portuguese Oyster (Crassostrea angulata) Proteins as Precursor of Bioactive Peptides. Int. J. Mol. Sci. 2019, 20, 5191. [Google Scholar] [CrossRef]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide identification from a Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Le Maux, S.; Esteveny, C.; Fitzgerald, R.J. Response surface methodology applied to the generation of casein hydrolysates with antioxidant and dipeptidyl peptidase IV inhibitory properties. J. Sci. Food Agric. 2016, 97, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Ohara, A.; Cason, V.G.; Nishide, T.G.; de Matos, F.M.; de Castro, R.J.S. Improving the antioxidant and antidiabetic properties of common bean proteins by enzymatic hydrolysis using a blend of proteases. Biocatal. Biotransform. 2020, 39, 100–108. [Google Scholar] [CrossRef]
- Mudgil, P.; Kilari, B.P.; Kamal, H.; Olalere, O.A.; FitzGerald, R.J.; Gan, C.H.; Maqsood, S. Multifunctional bioactive peptides derived from quinoa protein hydrolysates: Inhibition of α-glucosidase, dipeptidyl peptidase-IV and angiotensin I converting enzymes. J. Cereal Sci. 2020, 96, 103130. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2016, 218, 396–405. [Google Scholar] [CrossRef]
- Akan, E. An evaluation of the in vitro antioxidant and antidiabetic potentials of camel and donkey milk peptides released from casein and whey proteins. J. Food Sci. Technol. 2020, 58, 3743–3751. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.; Ji, H. Purification, identification and molecular mechanism of two dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from Antarctic krill (Euphausia superba) protein hydrolysate. J. Chromatogr. B 2017, 1064, 56–61. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.; Ji, H. Two Novel Bioactive Peptides from Antarctic Krill with Dual Angiotensin Converting Enzyme and Dipeptidyl Peptidase IV Inhibitory Activities. J. Food Sci. 2017, 82, 1742–1749. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejía, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
- Valencia-Mejía, E.; Batista, K.A.; Fernández, J.J.A.; Fernandes, K.F. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.). Food Res. Int. 2019, 121, 238–246. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Zhu, S.-S.; He, M.-J.; Luo, F.; Fu, C.-Z.; Zou, T.-B. Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect. Mar. Drugs 2017, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Lamoureux, C.; FitzGerald, R.J. Generation of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides during the enzymatic hydrolysis of tropical banded cricket (Gryllodes sigillatus) proteins. Food Funct. 2018, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Luna-Vital, D.A.; De Mejía, E.G. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension and oxidative stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Jobe, B.; Kamal, H.; Alameri, M.; Al Ahbabi, N.; Maqsood, S. Dipeptidyl peptidase-IV, α-amylase, and angiotensin I converting enzyme inhibitory properties of novel camel skin gelatin hydrolysates. LWT 2019, 101, 251–258. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Serem, J.C.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R.M. New Antidiabetic Targets of α-Glucosidase Inhibitory Peptides, SVPA, SEPA, STYV and STY: Inhibitory Effects on Dipeptidyl Peptidase-IV and Lipid Accumulation in 3T3-L1 Differentiated Adipocytes with Scavenging Activities Against Methylglyoxal and Reactive Oxygen Species. Int. J. Pept. Res. Ther. 2020, 26, 1949–1963. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Identification of novel dipeptidyl peptidase IV (DPP-IV) inhibitory peptides in camel milk protein hydrolysates. Food Chem. 2018, 244, 340–348. [Google Scholar] [CrossRef]
- Park, B.Y.; Yoon, K.Y. Biological activity of enzymatic hydrolysates and the membrane ultrafiltration fractions from perilla seed meal protein. Czech J. Food Sci. 2019, 37, 180–185. [Google Scholar] [CrossRef]
- Uraipong, C.; Zhao, J. In vitro digestion of rice bran proteins produces peptides with potent inhibitory effects on α-glucosidase and angiotensin I converting enzyme. J. Sci. Food Agric. 2017, 98, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Kamal, H.; Kilari, B.P.; Salim, M.A.S.M.; Gan, C.-Y.; Maqsood, S. Simulated gastrointestinal digestion of camel and bovine casein hydrolysates: Identification and characterization of novel anti-diabetic bioactive peptides. Food Chem. 2021, 353, 129374. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ma, Y.; Sun, P.; Thakur, K.; Wang, S.; Zhang, J.; Wei, Z. Purification and characterization of α-glucosidase inhibitory peptides from defatted camellia seed cake. Int. J. Food Sci. Technol. 2020, 56, 138–147. [Google Scholar] [CrossRef]
- Gao, J.; Gong, H.; Mao, X. Dipeptidyl Peptidase-IV Inhibitory Activity and Related Molecular Mechanism of Bovine α-Lactalbumin-Derived Peptides. Molecules 2020, 25, 3009. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.-L.; Hussain, N.; Ujiroghene, O.J.; Pang, X.-Y.; Zhang, S.-W.; Lu, J.; Liu, L.; Lv, J.-P. Generation and characterization of dipeptidyl peptidase-IV inhibitory peptides from trypsin-hydrolyzed α-lactalbumin-rich whey proteins. Food Chem. 2020, 318, 126333. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Cadamuro, C.; Le Gouic, A.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of a camel whey protein enriched hydrolysate preparation. Food Chem. 2019, 279, 70–79. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Miralles, B.; Hernández-Ledesma, B. Release of multifunctional peptides from kiwicha (Amaranthus caudatus) protein under in vitro gastrointestinal digestion. J. Sci. Food Agric. 2019, 99, 1225–1232. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef]
- Xu, F.; Yao, Y.; Xu, X.; Wang, M.; Pan, M.; Ji, S.; Wu, J.; Jiang, D.; Ju, X.; Wang, L. Identification and Quantification of DPP-IV-Inhibitory Peptides from Hydrolyzed-Rapeseed-Protein-Derived Napin with Analysis of the Interactions between Key Residues and Protein Domains. J. Agric. Food Chem. 2019, 67, 3679–3690. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Q.; Lin, L.; Zeng, X.-A.; Sun, B.; Zhao, M. In Vitro Metabolic Stability of a Casein-Derived Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Peptide VPYPQ and Its Controlled Release from Casein by Enzymatic Hydrolysis. J. Agric. Food Chem. 2019, 67, 10604–10613. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R. Rational in silico design of novel α-glucosidase inhibitory peptides and in vitro evaluation of promising candidates. Biomed. Pharmacother. 2018, 107, 234–242. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, L.; Zhang, Y.; Sheng, N.-J.; Wang, Z.-K.; Wu, T.-Z.; Wang, X.-Z.; Wu, H. Rapid Identification of Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Peptides from Ruditapes philippinarum Hydrolysate. Molecules 2017, 22, 1714. [Google Scholar] [CrossRef] [PubMed]
- Taga, Y.; Hayashida, O.; Kusubata, M.; Ogawa-Goto, K.; Hattori, S. Production of a novel wheat gluten hydrolysate containing dipeptidyl peptidase-IV inhibitory tripeptides using ginger protease. Food Nutr. Sci. 2017, 81, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Peptides Derived from Soy and Lupin Protein as Dipeptidyl-Peptidase IV Inhibitors: In Vitro Biochemical Screening and in Silico Molecular Modeling Study. J. Agric. Food Chem. 2016, 64, 9601–9606. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Le Maux, S.; Hamayon, J.; FitzGerald, R.J. Strategies for the release of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides in an enzymatic hydrolyzate of α-lactalbumin. Food Funct. 2016, 7, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, K.; Fang, L.; Liu, C.; Min, W.; Liu, J. Evaluation of the antidiabetic activity of hydrolyzed peptides derived from Juglans mandshurica Maxim. fruits in insulin-resistant HepG2 cells and type 2 diabetic mice. J. Food Biochem. 2018, 42, e12518. [Google Scholar] [CrossRef]
- Casanova-Martí, A.; Bravo, F.I.; Serrano, J.; Ardévol, A.; Pinent, M.; Muguerza, B. Antihyperglycemic effect of a chicken feet hydrolysate via the incretin system: DPP-IV-inhibitory activity and GLP-1 release stimulation. Food Funct. 2019, 10, 4062–4070. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, L.; Song, W.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Separation, identification and molecular binding mechanism of dipeptidyl peptidase IV inhibitory peptides derived from walnut (Juglans regia L.) protein. Food Chem. 2021, 347, 129062. [Google Scholar] [CrossRef]
- Kehinde, B.A.; Sharma, P. Recently isolated antidiabetic hydrolysates and peptides from multiple food sources: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 322–340. [Google Scholar] [CrossRef]
- El Alameey, I.R.; Ahmed, H.H.; Abushady, M.M. Dipeptidyl Peptidase IV: A Target for Improving Metabolic Syndrome Components in Obese Children and Adolescents. Biomed. Pharmacol. J. 2019, 12, 1701–1713. [Google Scholar] [CrossRef]
- Trzaskalski, N.A.; Fadzeyeva, E.; Mulvihill, E.E. Dipeptidyl Peptidase-4 at the Interface between Inflammation and Metabolism. Clin. Med. Insights Endocrinol. Diabetes 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Uraipong, C.; Zhao, J. Rice bran protein hydrolysates exhibit strong in vitro α-amylase, β-glucosidase and ACE-inhibition activities. J. Sci. Food Agric. 2016, 96, 1101–1110. [Google Scholar] [CrossRef]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cheng, J.; Wu, H. Discovery of Food-Derived Dipeptidyl Peptidase IV Inhibitory Peptides: A Review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, S.; Mahoonak, A.; Mora, L.; Ghorbani, M.; Houshmand, G.; Toldrá, F. Pepsin Hydrolysis of Orange by-Products for the Production of Bioactive Peptides with Gastrointestinal Resistant Properties. Foods 2021, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Harnedy-Rothwell, P.A.; McLaughlin, C.M.; O’Keeffe, M.B.; Le Gouic, A.V.; Allsopp, P.J.; McSorley, E.M.; Sharkey, S.; Whooley, J.; McGovern, B.; O’Harte, F.P.; et al. Identification and characterization of peptides from a boarfish (Capros aper) protein hydrolysate displaying in vitro dipeptidyl peptidase-IV (DPP-IV) inhibitory and insulinotropic activity. Food Res. Int. 2020, 131, 108989. [Google Scholar] [CrossRef]
- Li, Y.; Aiello, G.; Bollati, C.; Bartolomei, M.; Arnoldi, A.; Lammi, C. Phycobiliproteins from Arthrospira platensis (Spirulina): A New Source of Peptides with Dipeptidyl Peptidase-IV Inhibitory Activity. Nutrients 2020, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Thermoase-hydrolysed pigeon pea protein and its membrane fractions possess in vitro bioactive properties (antioxidative, antihypertensive, and antidiabetic). J. Food Biochem. 2020, 45, e13429. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Pérez-Gálvez, R.; Guadix, A.; Guadix, E.M. Effect of ultrasound pretreatment and sequential hydrolysis on the production of Tenebrio molitor antidiabetic peptides. Food Bioprod. Process. 2020, 123, 217–224. [Google Scholar] [CrossRef]
- Yap, P.G.; Gan, C.Y. In vivo challenges of anti-diabetic peptide therapeutics: Gastrointestinal stability, toxicity and allergenicity. Trends Food Sci. Technol. 2020, 105, 161–175. [Google Scholar] [CrossRef]
- Zamudio, F.V.; Campos, M.R.S. Amaranth, quinoa and chia bioactive peptides: A comprehensive review on three ancient grains and their potential role in management and prevention of Type 2 diabetes. Crit. Rev. Food Sci. Nutr. 2020, 62, 2707–2721. [Google Scholar] [CrossRef]
- Connolly, A.; Cermeño, M.; Crowley, D.; O’Callaghan, Y.; O’Brien, N.M.; FitzGerald, R.J. Characterisation of the in vitro bioactive properties of alkaline and enzyme extracted brewers’ spent grain protein hydrolysates. Food Res. Int. 2019, 121, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R.M. Tuber Storage Proteins as Potential Precursors of Bioactive Peptides: An In Silico Analysis. Int. J. Pept. Res. Ther. 2019, 25, 437–446. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J.; Bąk, O.; Borowski, P. Meat Proteins as Dipeptidyl Peptidase IV Inhibitors and Glucose Uptake Stimulating Peptides for the Management of a Type 2 Diabetes Mellitus In Silico Study. Nutrients 2019, 11, 2537. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.D.C.L.; Berg, R.S.; Rønning, S.B.; Afseth, N.K.; Knutsen, S.H.; Staerk, D.; Wubshet, S.G. Peptides from chicken processing by-product inhibit DPP-IV and promote cellular glucose uptake: Potential ingredients for T2D management. Food Funct. 2019, 10, 1619–1628. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of camel milk protein hydrolysates generated with trypsin. J. Funct. Foods 2017, 34, 49–58. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Lalmahomed, M.; Paolella, S.; FitzGerald, R.J. Milk protein isolate (MPI) as a source of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Chem. 2017, 231, 202–211. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Hennemann, M.; Paolella, S.; FitzGerald, R.J. Generation of wheat gluten hydrolysates with dipeptidyl peptidase IV (DPP-IV) inhibitory properties. Food Funct. 2017, 8, 2249–2257. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mazzocchi, C.; Paolella, S.; FitzGerald, R.J. Release of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from milk protein isolate (MPI) during enzymatic hydrolysis. Food Res. Int. 2017, 94, 79–89. [Google Scholar] [CrossRef]
- Siow, H.-L.; Gan, C.-Y. Optimization Study in Extracting Anti-Oxidative and A-Amylase Inhibitor Peptides from Cumin Seeds (Cuminum cyminum). J. Food Biochem. 2016, 41, e12280. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Guadix, A.; Guadix, E.M. Identification of novel dipeptidyl peptidase IV and α-glucosidase inhibitory peptides from Tenebrio molitor. Food Funct. 2021, 12, 873–880. [Google Scholar] [CrossRef]
- Song, J.; Wang, Q.; Du, M.; Ji, X.; Mao, X. Identification of dipeptidyl peptidase-IV inhibitory peptides from mare whey protein hydrolysates. J. Dairy Sci. 2017, 100, 6885–6894. [Google Scholar] [CrossRef] [PubMed]
| Authors (Year) | Source of Protein | Peptide Sequence | Outcomes of Interest |
|---|---|---|---|
| Mudgil, et al., [42] | Bovine milk (Holstein Friesian) and dromedary camel milk (Camelus dromedarius, local breed) | FLWPEYGAL; LPTGWLM, MFE and GPAHCLL and HLPGRG; QNVLPLH and PLMLP | Both potent inhibitory effects against enzymatic markers involved in diabetes, e.g., α-amylase, α-glucosidase and DPP-IV |
| Rivero-Pino, et al. [19] | Soy, Lupine, and Quinoa | EPAAV, NPLL, and APFTVV | Soy the most activity but chickpea, lentil, and pea also showed potent DPP-IV inhibitory activity. |
| Feng, et al. [43] | Camellia seed cake (Camellia oleífera) | SPGYYDGR, GLTSLDRYK, and GHSLESIK | Alcalase and Asp 542 was recognized as the key target amino acid of a-glucosidase. |
| Gao, et al. [44] | Bovine α-lactalbumin | ELKDLKGY and ILDKVGINY | These two peptides could bind with DPP-IV. |
| Ibrahim, et al. [38] | Synthetic peptides | STYV; STY; SEPA; SVPA | α-glucosidase inhibitory activity: STYV > STY > SEPA > SVPA; DPP-IV: SVPA; In vitro studies: SEPA. |
| Jia, et al. [45] | Whey protein | LDQWLCEK, VGINYWLAHK, LDQWLCEKL, KILDKVGINYWLAHK, ILDKVGINYWLAHK | The peptide LDQWLCEKL exhibited the highest inhibitory activity. |
| Jin, et al. [10] | Atlantic salmon (Salmo salar) skin | LDKVFR | Hydrolysate with MW < 3 kDa was an excellent source of DPP-IV inhibitory peptides. |
| Nongonierma, et al. [46] | Camel milk (Camelius dromedaries) | VPV, VPF, LPVPQ, YPI, and VL | The stability of VPV to gastric and intestinal digestive enzymes suggests that it may have potential as an antidiabetic agent for humans. |
| Vilcacundo, et al. [47] | Kiwicha (Amaranthus caudatus) | FLISCLL, SVFDEELS, and DFIILE | ACE, DPP-IV, and colon cancer cell viability were obtained. These digests also showed moderate α-amylase inhibitory activity. |
| Wang, et al. [48] | Soy protein | LLPLPVLK; SWLRL and WLRL | Development of novel antidiabetic peptide nutraceuticals with α-glucosidase, DPP-IV, and ACE inhibitory potential. |
| Xu, et al. [49] | Rapeseed (Brassica napus) napin | PAGPF, KTMPGP, IPQVS, and ELHQEEPL | |
| Zheng, et al. [50] | Casein-derived synthetic peptide | VPYPQ | VPYPQ was a promising casein-derived DPP-IV inhibitor. |
| Ibrahim, et al. [51] | Synthetic peptides | SVPA and SEPA | Two novel and active α-glucosidase inhibitory peptides were identified; they could resist GIT digestion and have the potential to retard postprandial hyperglycemia in diabetic patients. |
| Mune, et al. [15] | Bambara bean | IP, LN, VE, and VY | After simulated digestion, thermolysin showed significantly higher ACE and DPP-IV inhibitory properties compared to the Alcalase. |
| Nongonierma, et al. [39] | Camel whey protein (Camelus dromedarius) | FLQY, FQLGASPY, ILDKEGIDY, ILELA, LLQLEAIR, LPVP, LQALHQGQIV, MPVQA, and SPVVPF | LPVP and MPVQA, with DPP-IV inhibition, were identified for the first time in camel milk protein hydrolysates. |
| Ji, et al. [29] | Antarctic krill (Euphausia superba) | AP and IPA | Can be considered as a promising source of DPP-IV inhibitory peptides for use as natural food ingredients against type 2 diabetes. |
| Ji, et al. [30] | Antarctic krill(Euphausia superba) | LVGPLP and PAL | These peptides exhibited dual inhibition of ACE and DPP-IV. |
| Liu, et al. [52] | Ruditapes philippinarum hydrolysate | LAPSTM | R. philippinarum-derived peptides may have potential as functional food ingredients for prevention of diabetes. |
| Mojica, et al. [36] | Common bean (Phaseolus vulgaris L.) | KKSSG, KTYGL, GGGLHK, and CPGNK | The first report. Significant antioxidant, antidiabetic, and antihypertensive properties were found after gastrointestinal simulated digestion, and inhibition of DPP-IV and α-glucosidase. |
| Taga, et al. [53] | Wheat gluten | GPG, QPQ, QPF, LPQ, and SPQ | The novel gluten hydrolysate prepared using ginger protease can be used as functional food for patients with type 2 diabetes. |
| Uraipong and Zhao [41] | Rice bran (cultivar Reiziq) | GE, GG, GP, EK, and GH | In vitro simulated human gastrointestinal digestion led to substantial hydrolysis of these proteins, and the resultant peptides possessed significant -glucosidase and ACE inhibitory activities. |
| Vilcacundo, et al. [26] | Quinoa (Chenopodium quinoa Willd.) | IQAEGGLT, DKDYPK, and GEHGSDGNV | The peptides generated showed ability to inhibit enzymes involved in incretin degradation and digestion of dietary carbohydrates. |
| Lammi, et al. [54] | Soy and Lupin Protein | Soy 1 (IAVPTGVA) and Lup 1 (LTFPGSAED) | Soy 1 (IAVPTGVA), Soy 2 (YVVNPDNDEN), Soy 3 (YVVNPDNNEN), Lup 1 (LTFPGSAED), Lup 2 (LILPKHSDAD), and Lup 3 (GQEQSHQDEGVIVR), were screened for their capacity to inhibit the activity of DPP-IV, using an in vitro bioassay against human recombinant DPP-IV. |
| Nongonierma, et al. [55] | Bovine α-lactalbumin | GY, GL, GI, NY, and WL | This preliminary study demonstrated the benefit of using a targeted approach combined with an experimental design for generation of dietary protein hydrolysates with DPP-IV inhibitory properties. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, T.C.; de Souza, T.S.P.; Fai, A.E.C.; Koblitz, M.G.B. Critical Review for the Production of Antidiabetic Peptides by a Bibliometric Approach. Nutrients 2022, 14, 4275. https://doi.org/10.3390/nu14204275
Farias TC, de Souza TSP, Fai AEC, Koblitz MGB. Critical Review for the Production of Antidiabetic Peptides by a Bibliometric Approach. Nutrients. 2022; 14(20):4275. https://doi.org/10.3390/nu14204275
Chicago/Turabian StyleFarias, Ticiane Carvalho, Thaiza Serrano Pinheiro de Souza, Ana Elizabeth Cavalcante Fai, and Maria Gabriela Bello Koblitz. 2022. "Critical Review for the Production of Antidiabetic Peptides by a Bibliometric Approach" Nutrients 14, no. 20: 4275. https://doi.org/10.3390/nu14204275
APA StyleFarias, T. C., de Souza, T. S. P., Fai, A. E. C., & Koblitz, M. G. B. (2022). Critical Review for the Production of Antidiabetic Peptides by a Bibliometric Approach. Nutrients, 14(20), 4275. https://doi.org/10.3390/nu14204275







