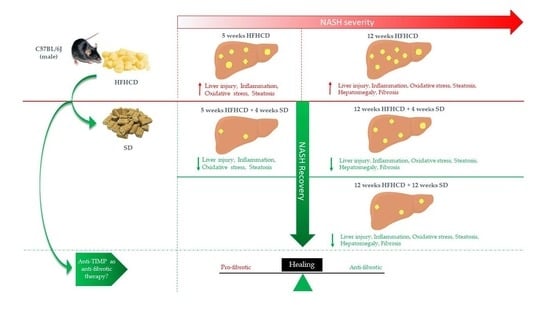
Switching to Regular Diet Partially Resolves Liver Fibrosis Induced by High-Fat, High-Cholesterol Diet in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
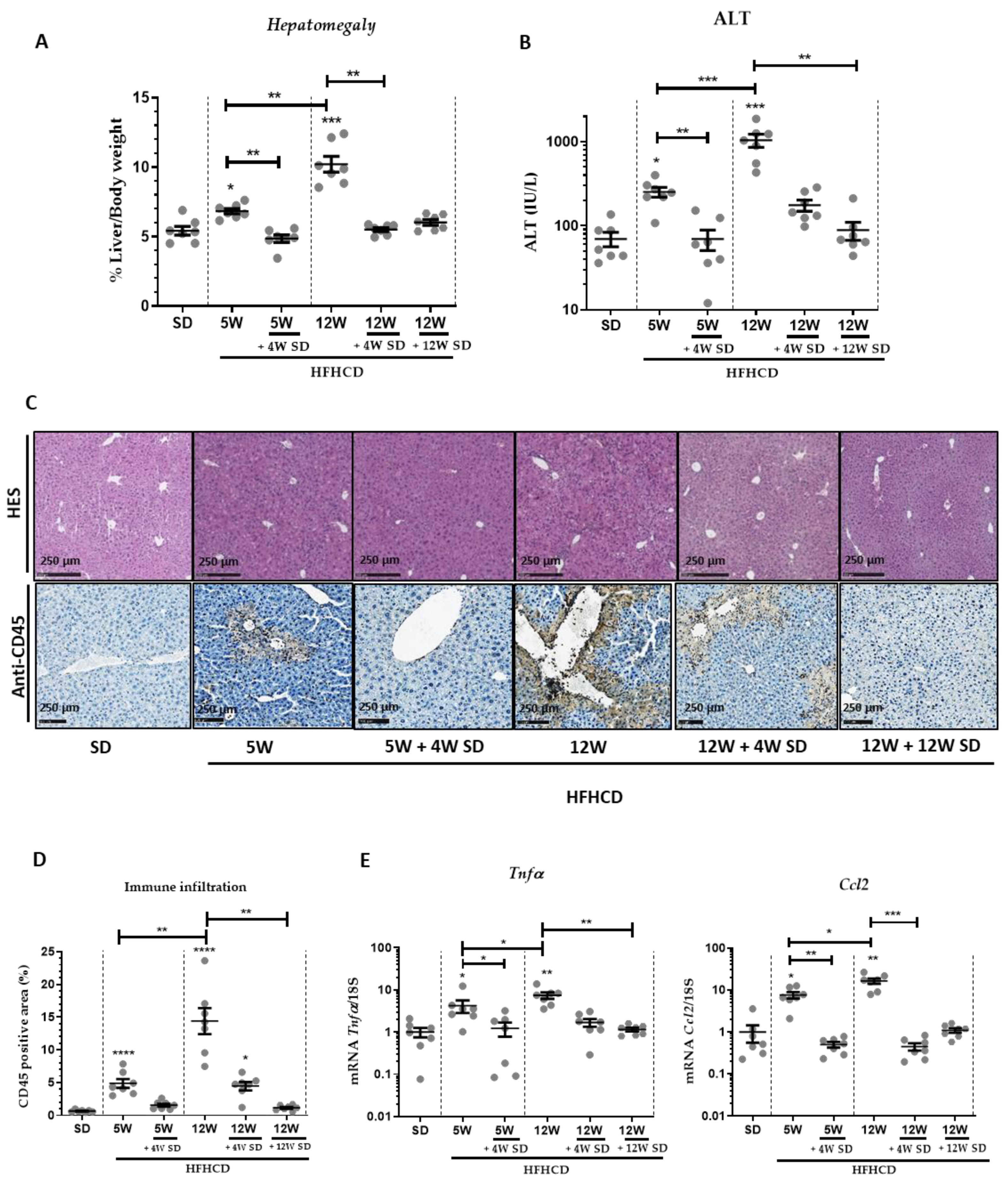
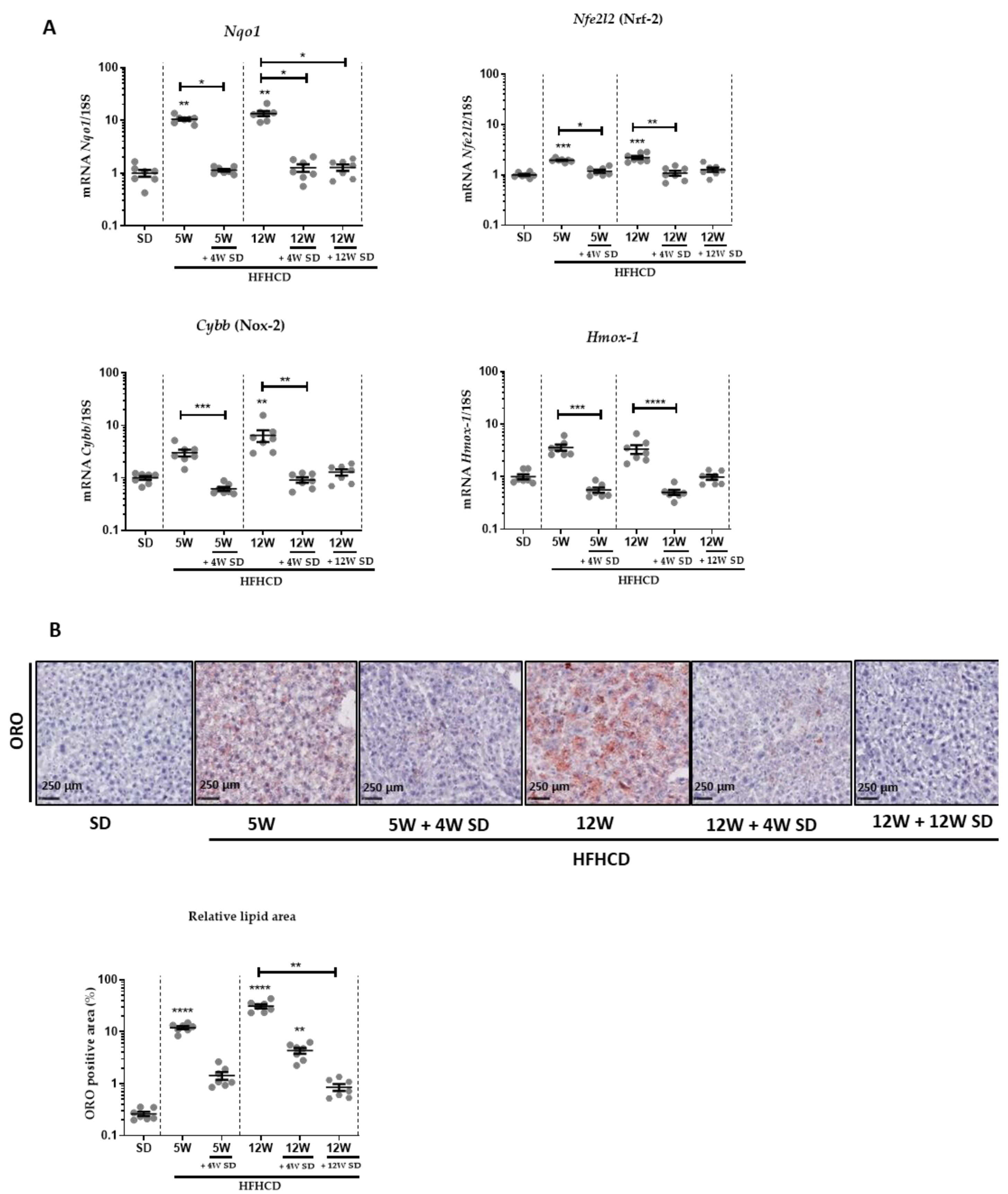
2.2. Histopathological and Biochemical Studies
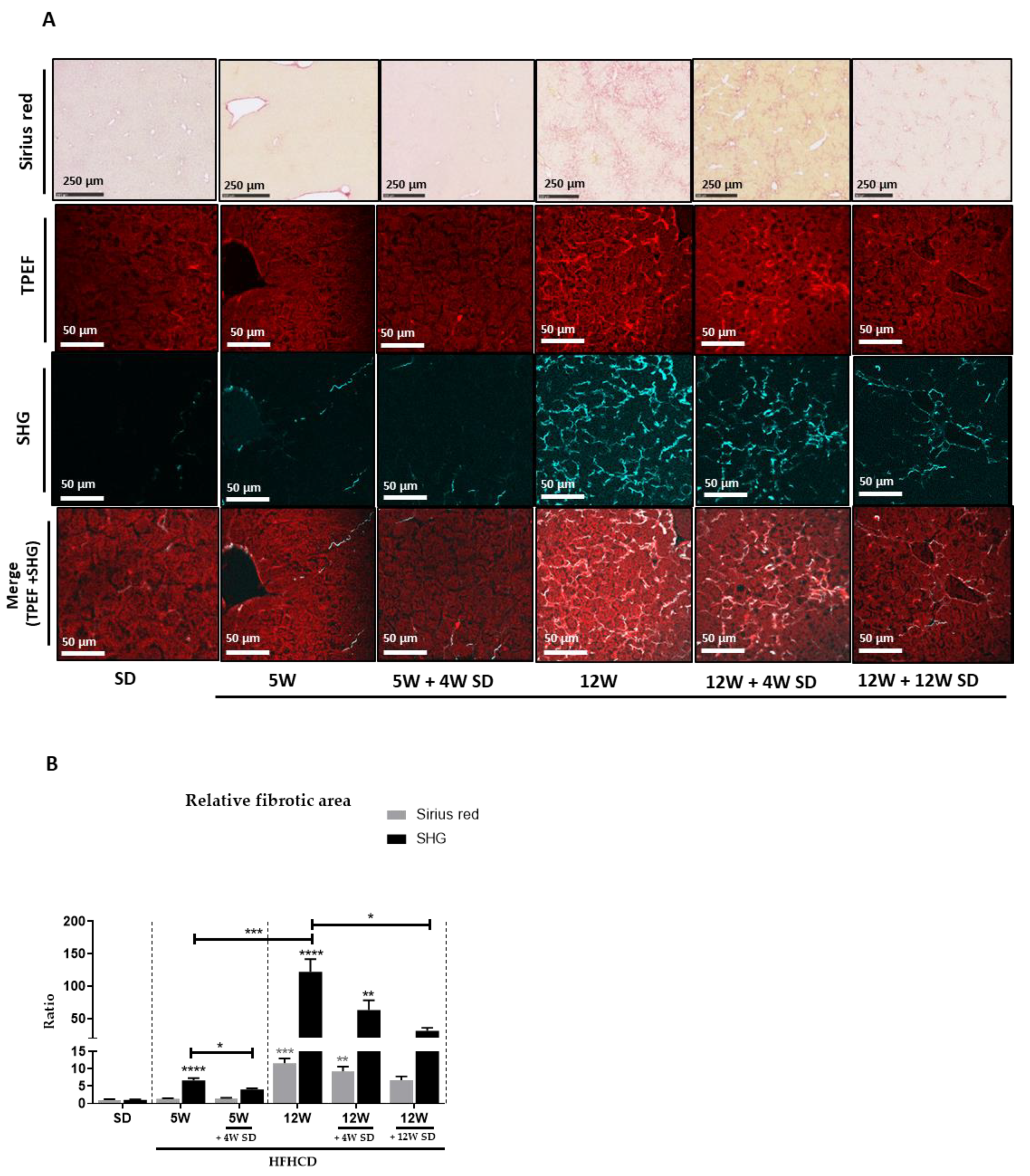
2.3. Imaging by Second Harmonic Generation (SHG)
2.4. RNA Isolation and RT-qPCR
2.5. Statistical Analysis
3. Results
3.1. HFHCD-Induced Liver Damage and Hepatomegaly Are Reversible after Switching to SD
3.2. HFHCD-Induced Fibrosis Is Partially Reversible
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Marchisello, S.; Di Pino, A.; Scicali, R.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview. Int. J. Mol. Sci. 2019, 20, 1948. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Kaplowitz, N.; Schwabe, R.F. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology 2014, 147, 765–783.e4. [Google Scholar] [CrossRef]
- Goldberg, D.; Ditah, I.C.; Saeian, K.; Lalehzari, M.; Aronsohn, A.; Gorospe, E.C.; Charlton, M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients with Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology 2017, 152, 1090–1099.e1. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Paradis, V. Liver extracellular matrix in health and disease. J. Pathol. 2003, 200, 504–515. [Google Scholar] [CrossRef]
- Zbodakova, O.; Chalupsky, K.; Tureckova, J.; Sedlacek, R. Metalloproteinases in liver fibrosis: Current insights. Met. Med. 2017, 4, 25–35. [Google Scholar] [CrossRef]
- Caligiuri, A.; Gentilini, A.; Pastore, M.; Gitto, S.; Marra, F. Cellular and Molecular Mechanisms Underlying Liver Fibrosis Regression. Cells 2021, 10, 2759. [Google Scholar] [CrossRef]
- Rockey, D.C. Liver Fibrosis Reversion After Suppression of Hepatitis B Virus. Clin. Liver Dis. 2016, 20, 667–679. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5, quiz e314–e365. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Argo, C.K. Reversing Advanced Hepatic Fibrosis in NASH: Clearly Possible, but Widely at Hand? Dig. Dis. Sci. 2015, 60, 810–812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iredale, J.P.; Benyon, R.C.; Pickering, J.; McCullen, M.; Northrop, M.; Pawley, S.; Hovell, C.; Arthur, M.J. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Investig. 1998, 102, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Lytle, K.A.; Jump, D.B. Is Western Diet-Induced Nonalcoholic Steatohepatitis in Ldlr-/- Mice Reversible? PLoS ONE 2016, 11, e0146942. [Google Scholar] [CrossRef]
- Mu, Y.P.; Ogawa, T.; Kawada, N. Reversibility of fibrosis, inflammation, and endoplasmic reticulum stress in the liver of rats fed a methionine-choline-deficient diet. Lab. Investig. J. Tech. Methods Pathol. 2010, 90, 245–256. [Google Scholar] [CrossRef]
- Takeuchi-Yorimoto, A.; Noto, T.; Yamada, A.; Miyamae, Y.; Oishi, Y.; Matsumoto, M. Persistent fibrosis in the liver of choline-deficient and iron-supplemented L-amino acid-defined diet-induced nonalcoholic steatohepatitis rat due to continuing oxidative stress after choline supplementation. Toxicol. Appl. Pharmacol. 2013, 268, 264–277. [Google Scholar] [CrossRef]
- Benyon, R.C.; Iredale, J.P. Is liver fibrosis reversible? Gut 2000, 46, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, P.; Dion, S.; Filliol, A.; Genet, V.; Lucas-Clerc, C.; Jean-Philippe, G.; Silvain, C.; Lecron, J.C.; Piquet-Pellorce, C.; Samson, M. Endogenous IL-33 has no effect on the progression of fibrosis during experimental steatohepatitis. Oncotarget 2017, 8, 48563–48574. [Google Scholar] [CrossRef]
- Simoes Eugénio, M.; Farooq, M.; Dion, S.; Devisme, C.; Raguenes-Nicol, C.; Piquet-Pellorce, C.; Samson, M.; Dimanche-Boitrel, M.T.; Le Seyec, J. Hepatocellular Carcinoma Emergence in Diabetic Mice with Non-Alcoholic Steatohepatitis Depends on Diet and Is Delayed in Liver Exhibiting an Active Immune Response. Cancers 2020, 12, 1491. [Google Scholar] [CrossRef]
- Han, Y.P. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J. Gastroenterol. Hepatol. 2006, 21 (Suppl. S3), S88–S91. [Google Scholar] [CrossRef]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Models Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Hemmann, S.; Graf, J.; Roderfeld, M.; Roeb, E. Expression of MMPs and TIMPs in liver fibrosis-a systematic review with special emphasis on anti-fibrotic strategies. J. Hepatol. 2007, 46, 955–975. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Friedman, S.L.; McCullough, A.J.; Dimick-Santos, L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: Findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology 2015, 61, 1392–1405. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Loomba, R.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; Ratziu, V.; et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018, 68, 361–371. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, J.; Spincemaille, P.; Vanhorebeek, I.; Van den Berghe, G.; Vander Elst, I.; Windmolders, P.; van Pelt, J.; van der Merwe, S.; Bedossa, P.; Nevens, F.; et al. Dietary intervention, but not losartan, completely reverses non-alcoholic steatohepatitis in obese and insulin resistant mice. Lipids Health Dis. 2017, 16, 46. [Google Scholar] [CrossRef]
- van den Hoek, A.M.; de Jong, J.; Worms, N.; van Nieuwkoop, A.; Voskuilen, M.; Menke, A.L.; Lek, S.; Caspers, M.P.M.; Verschuren, L.; Kleemann, R. Diet and exercise reduce pre-existing NASH and fibrosis and have additional beneficial effects on the vasculature, adipose tissue and skeletal muscle via organ-crosstalk. Metab. Clin. Exp. 2021, 124, 154873. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Tartaglione, E.V.; Kuver, R.; Haigh, W.G.; Farrell, G.C.; Subramanian, S.; Chait, A.; Yeh, M.M.; Quinn, L.S.; Ioannou, G.N. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology 2013, 57, 81–92. [Google Scholar] [CrossRef]
- Liang, J.Q.; Teoh, N.; Xu, L.; Pok, S.; Li, X.; Chu, E.S.H.; Chiu, J.; Dong, L.; Arfianti, E.; Haigh, W.G.; et al. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat. Commun. 2018, 9, 4490. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Kisseleva, T. Reversibility of liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2015, 39 (Suppl. S1), S60–S63. [Google Scholar] [CrossRef]
- Parsons, C.J.; Bradford, B.U.; Pan, C.Q.; Cheung, E.; Schauer, M.; Knorr, A.; Krebs, B.; Kraft, S.; Zahn, S.; Brocks, B.; et al. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology 2004, 40, 1106–1115. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, M.; Hameed, H.; Dimanche-Boitrel, M.-T.; Piquet-Pellorce, C.; Samson, M.; Le Seyec, J. Switching to Regular Diet Partially Resolves Liver Fibrosis Induced by High-Fat, High-Cholesterol Diet in Mice. Nutrients 2022, 14, 386. https://doi.org/10.3390/nu14020386
Farooq M, Hameed H, Dimanche-Boitrel M-T, Piquet-Pellorce C, Samson M, Le Seyec J. Switching to Regular Diet Partially Resolves Liver Fibrosis Induced by High-Fat, High-Cholesterol Diet in Mice. Nutrients. 2022; 14(2):386. https://doi.org/10.3390/nu14020386
Chicago/Turabian StyleFarooq, Muhammad, Huma Hameed, Marie-Thérèse Dimanche-Boitrel, Claire Piquet-Pellorce, Michel Samson, and Jacques Le Seyec. 2022. "Switching to Regular Diet Partially Resolves Liver Fibrosis Induced by High-Fat, High-Cholesterol Diet in Mice" Nutrients 14, no. 2: 386. https://doi.org/10.3390/nu14020386
APA StyleFarooq, M., Hameed, H., Dimanche-Boitrel, M.-T., Piquet-Pellorce, C., Samson, M., & Le Seyec, J. (2022). Switching to Regular Diet Partially Resolves Liver Fibrosis Induced by High-Fat, High-Cholesterol Diet in Mice. Nutrients, 14(2), 386. https://doi.org/10.3390/nu14020386