1. Background
The levels of obesity and diabetes continue to increase around the world on an epidemic scale [
1]. Between 2011 and 2012, more than one-third of the American population was clinically obese. Obesity and diabetes have become huge public health problems in the United States and around the world [
2,
3]. On a global scale, obesity is a more serious health problem than hunger, and it is the main cause of death and disability worldwide. The public health burden of obesity is expected to increase in the next few years [
4]. The medical expenses of obese people are on average 42% higher than normal-weight patients, whilst the medical expenses of diabetic patients are about twice those of non-diabetic patients [
1,
5,
6].
The World Health Organization (WHO) defines obesity as “abnormal or excessive fat accu mulation with health risks”. Hippocrates wrote that obesity is not only a disease, but also a precursor to other diseases [
7]. Body mass index (BMI) has been widely used since it was proposed in 1972 [
7]. BMI is divided into four categories, Class I (Underweight) < 18.5, Class II (Healthy): 18.5 to 24.9, Class III (Overweight): 25 to 29.9, and Class IV (Obese): 30+. Overweight and obesity have a major impact on the physical, psychological, and social health of individuals and have a negative impact on society in the form of increased medical expenditure [
7,
8]. Traditional diet, cereals, and low-fat, mixed dishes have been replaced by high-fat meat diets, while the intake of cereals and vegetables has been reduced, leading to increased intake of animal foods, processed foods, sugar-sweetened beverages, and processed foods high in fat, energy, sugar, and salt [
9,
10]. This shift has led to a major change in the macronutrient composition of the Chinese diet. The shift from a high-carbohydrate diet to a high-fat diet is accompanied by negative changes in health. Overweight and obesity have become a prominent problem for adults. The prevalence increased from 16.4% and 3.6% in 1982 to 30.1% and 11.9% in 2012 [
11,
12,
13]. The contribution of fat-energy intake exceeded the recommended value of the Chinese Dietary Guidelines (CDG) and increased from 18.4% in 1982 to 32.9% in 2012 [
14,
15,
16].
According to the China Cardiovascular Disease Report, 11 million patients suffer from coronary heart disease. The incidence of cardiovascular diseases in China is on the rise, accounting for more than 40% of disease deaths [
17]. In 2016, the mortality rate of AMI in urban areas in China was 58.69/100,000, and the mortality rate of AMI in rural areas was 74.72/100,000 [
18].
Obesity and diabetes are multifactorial, complex diseases that are also preventable in most cases [
19,
20] and significantly increase the risk of coronary atherosclerosis [
21]. Atherosclerotic cardiovascular disease (ACD) is the leading cause of global mortality [
5]. Over time, high body mass index (BMI), blood pressure, blood glucose, and cholesterol have different effects on the mortality rate in patient groups of different incomes [
22,
23]. In the past 20 years, high-income countries have been able to reduce the impact of these risk factors, whilst the mortality rate in low- and middle-income countries due to high BMI and hyperglycemia has increased [
23]. The increase in mortality can be attributed to population growth and aging, as well as important changes in the dietary system [
10]. It is not known whether differences in the dietary intake of trace elements contribute to the development of coronary atherosclerosis [
24,
25].
Research studies have shown that trace elements in the body are closely related to many disease including coronary heart diseases [
26,
27]. In a previous study from our lab, we found that zinc ions are related to menopause, and supplementation of zinc ions has a positive effect on the treatment of coronary heart disease [
26]. Kalita H et al. confirmed that changes in the content of trace elements can improve insulin resistance caused by type 2 diabetes [
28]. The study demonstrated that magnesium and manganese are cofactors of a variety of related enzymes, and low levels of these elements increase the risk of metabolic syndrome. Furthermore, Li et al. found that serum selenium levels are significantly related to the all-cause mortality of men and women but are more significant in women with coronary heart disease [
29].
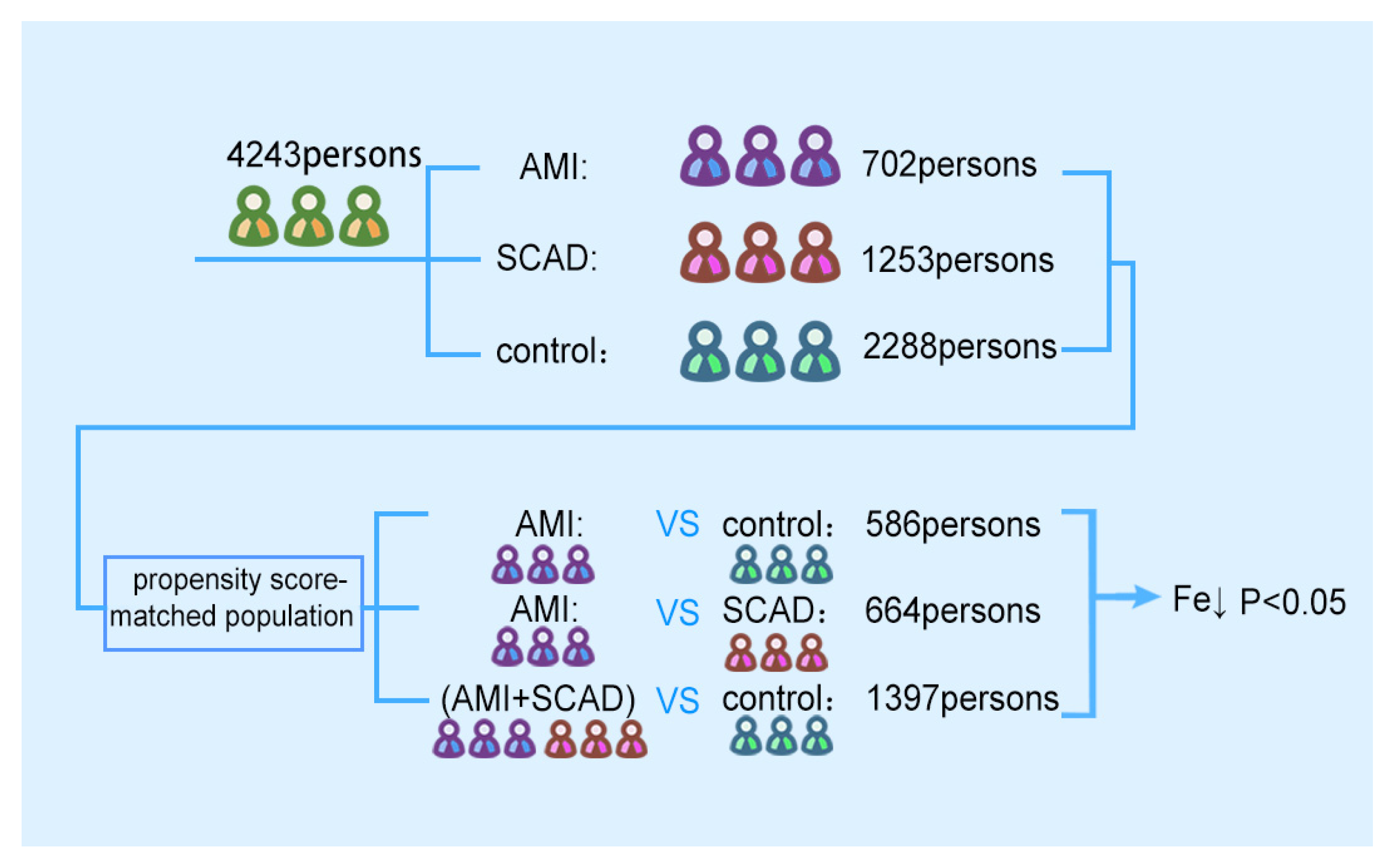
The purpose of this research was to explore the relationship between serum trace elements and coronary atherosclerosis. Based on big data, fasting peripheral blood samples of 4243 patients were collected to detect the levels of sodium, potassium, magnesium, calcium, and iron. The difference in serum iron concentration between different groups was analyzed to determine whether the serum iron level is a risk factor for coronary heart disease.
4. Discussion
Iron is an element that is essential for several physiological processes and plays important roles in cell metabolism through iron-containing proteins and enzymes. These proteins and enzymes maintain mitochondrial function, DNA synthesis and repair, and cell growth and death [
32,
33,
34]. Iron is the main component of hemoglobin that is essential for the production of red blood cells and oxygen transport. However, iron may also be toxic at high concentrations as it can generate reactive oxygen species (ROS) and oxidize biomolecules through toxic hydroxyl radicals generated by the Fenton reaction [
35]. Iron is also a key factor in determining the toxicity of bacteria [
36].
Iron homeostasis is strictly controlled by the interactions of various iron processing tissues and cells (including macrophages, red blood cells, hepatocytes, and duodenal epithelial cells) and is regulated by the hepcidin–ferroportin axis. Most of the required iron is recovered from aging red blood cells by the reticulum–endothelial system. The extra demand for iron is fine-tuned by adjusting the amount of iron absorbed by the intestinal cells.
Dysregulated iron intake or output can cause illness. Iron is involved in the pathogenesis of atherosclerotic coronary heart disease (CAD) which was first proposed by Sullivan [
35,
37]. Free iron can accelerate the oxidation of low density lipoproteins (LDLs). Low density lipoproteins are then absorbed by low-density lipoprotein receptors on macrophages, which leads to the recruitment of foam cells. Foam cell infiltration and necrotic core expansion are key events in the formation of coronary atherosclerosis [
38]. Macrophages play a central role in the progression of coronary atherosclerosis, and different subtypes of macrophages have been detected in atherosclerotic plaques [
39]. Lipid uptake is the main stimulating factor for the differentiation of M1 macrophages in plaques which can induce the production of inflammatory cytokines and the formation of foam cells [
40]. M1 macrophages can also induce SMC proliferation and migration from the media to the intima through paracrine actions and are considered to promote coronary atherosclerosis.
MMP-1, MMP-3, and MMP-9 released by M1 cells can hydrolyze collagen fibers in the fibrous cap which may lead to plaque instability [
41]. Conversely, M2 macrophages are stimulated by Th2-type cytokines (such as IL-10, IL-4, and IL-13) to produce anti-inflammatory cytokines. M2 macrophages are thought to balance the inflammatory response to promote the resolution of inflammation and tissue repair. The M1/M2 paradigm provides a simplified framework to understand the function of macrophages in an injury environment. M1 macrophages are rich in ferritin and are prone to iron accumulation, whilst M2 macrophages can metabolize and export iron. The turnover rate of iron in M1 and M2 macrophages is different and can result in the development of coronary atherosclerosis.
In organisms, iron is usually combined with biomolecules such as heme resulting in an iron-deficient environment in the body. Bacteria must adapt to this environment through a series of iron acquisition mechanisms [
36]. Siderophores are involved in these processes and have a high affinity for iron ions and can capture iron from protein-iron complexes [
42]. Another way of ingesting iron relies on direct contact between the pathogen and the iron source [
43,
44]. AS is an inflammatory disease and has a higher risk of bacterial infection compared to patients without AS. Patients with AS present increased opportunities for bacteria to use iron which is supported by the observations of a decreasing trend in iron in this study.
γ-glutamyl transpeptidase (γ-GT) is present in serum and the plasma membrane of almost all cells. γ-GT initiates the hydrolysis of extracellular glutathione (GSH). GSH is a tripeptide in which cysteine is located between α-glycine and γ-glutamic acid residues. It is known that cysteine and other thiol compounds promote LDL oxidation by reducing Fe(III) to redox-active Fe(II). This process requires the consumption of trace iron in AS [
45]. Therefore, it is feasible that patients with AS have lower concentrations of iron ions.
Magnesium (Mg) is a cofactor for various enzymes related to diabetes. Low levels of magnesium and manganese increase the risk of metabolic syndrome, which affects glucose metabolism. The lack of magnesium ions can lead to increased levels of total glycerides and total cholesterol, leading to coronary atherosclerosis [
46,
47]. Zinc (Zn) acts as a membrane stabilizer in vascular endothelial cells and participates in numerous signal transduction pathways in the cell, preventing the cell structure from being damaged by unstable factors such as oxidized lipids [
48,
49]. At the same time, there is a zinc regulation pathway in the blood vessel wall, which is combined with the production and effect of NO and is destroyed when zinc deficiency occurs, thereby affecting the onset and treatment of vascular diseases [
50]. Serum calcium (Ca) levels are strictly regulated by parathyroid hormone and vitamin D. The imbalance of calcium ion in the patient’s body leads to secondary hyperparathyroidism, which in turn leads to the occurrence of coronary atherosclerosis [
51].
IMT is a noninvasive marker of the severity of carotid atherosclerosis. There is no final conclusion on whether iron ion concentration changes arterial intima-media thickness [
52], and some studies have suggested that iron ion concentration has no relationship with IMT [
53]. ABI index can reflect the degree of lower extremity arterial stenosis and is an index to identify peripheral atherosclerosis. An increasing number of studies have linked ABI with coronary atherosclerosis, but its clinical value still needs to be judged. A 2019 study found that ABI can detect carotid atherosclerosis in healthy residents. It can prevent and help patients who have been identified as healthy [
54].
There may be two main ways in which iron ions affect the occurrence of atherosclerosis. First, iron and hydrogen peroxide can oxidize a variety of matrices and cause biological damage. This reaction is called Fenton reaction. It is a complex reaction that can produce hydroxyl radicals and a higher oxidation state of iron [
55]. The combined iron can prevent the catalytic reaction of iron producing free radicals [
56]. Reactive oxygen species play a role in the development of cardiovascular disease by oxidizing low density lipoproteins (LDLs), which damage vascular endothelial cells. This process is not only the pathological mechanism of atherosclerosis but also the reason for the increase in arterial intima-media thickness. The second is inflammatory reaction. A randomized crossover study from different countries showed significant changes in postprandial oxidative stress, which may trigger inflammation and lead to endothelial dysfunction and atherosclerosis [
57]. Iron ions are different in diets of different cultures. At the same time, the inflammatory process may also be improved by some nutrients (such as vitamin D, Omega 3 fatty acids [
58,
59] and tryptophan [
60,
61]) or exacerbated by metabolites (such as cysteine) [
62]. Therefore, iron ions can promote the formation of free radicals or increase inflammatory response and establish a relationship with IMT and ABI.
At first, serum amyloid
p substance was recognized as a kind of amyloid deposition protein, and it is believed that it plays an important role in the occurrence and development of systemic amyloidosis. Studies have gradually found that SAP participates in various activities such as innate immunity, inflammatory response, and amyloidosis in the body [
63,
64,
65]. In recent years, the role of serum amyloid
p in the cardiovascular system has gradually been recognized [
66].
Isoprostaglandin is a stable metabolite of arachidonic acid. In normal human plasma and urine, its concentration is at least an order of magnitude higher than that of prostaglandins [
67]. The concentration of isoprostaglandins, especially 8-iso-PGF
2α, which is the most widely studied, increases under many pathophysiological conditions, including diabetes [
68], Alzheimer’s disease [
69], and allergic reactions [
70]. Whether they can be used as disease markers, however, is largely unknown. The potency of 8-iso-PGE
2 on 8-iso-PGF
2α and 8-iso-PGE
1 on 8-isoPGF
1α indicates that the E-ring compound is more potent than the F-ring compound. Secondly, 8-iso-PGE
2 is better than 8-iso-PGE
1, and 8-iso-PGF
2α is better than 8-isoPGF
1α, indicating that double-unsaturated compounds are stronger than mono-unsaturated compounds. Third, 8-iso-PGF
2α is much more potent than 8-iso-PGF
2β, which shows the importance of α configuration [
71]. This suggests that 8-iso-PGF
1α seems to be a partial agonist. Isoprostaglandins participate in the dynamic changes of atherosclerosis, which include vasodilation and contraction and platelet activation and inhibition [
72,
73]. This dynamic balance is the key to the stability of the cardiovascular system. This also suggests that follow-up studies can focus on the relationship between iron ions and isoprostaglandins.
There are many risk factors associated with coronary atherosclerosis including high blood pressure, diabetes, and smoking. Most of these risk factors can be modified through a healthy lifestyle or drugs [
74]. For example, eating foods rich in fiber can significantly reduce the risk of coronary atherosclerosis [
75]. Currently, coronary atherosclerosis is diagnosed based on multi-factor joint analysis [
76] that can be enhanced by assessing the roles of specific trace elements. The optimized intake of trace elements in the diet can reduce the occurrence and improve the prognosis of coronary atherosclerosis.
There are some limitations in this article. This study is a retrospective analysis. ABI and IMT are classic markers of peripheral vascular atherosclerosis and carotid atherosclerosis respectively. Increasing this part of indicators can extend coronary atherosclerosis to systemic atherosclerosis. In addition, it may be more interesting to increase relevant indicators of oxidative stress and study the relationship between trace elements and oxidative stress.