Optimal Dietary Intake Composition of Choline and Betaine Is Associated with Minimized Visceral Obesity-Related Hepatic Steatosis in a Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Ultrasound Diagnosis of Hepatic Steatosis (HS)
2.3. Anthropometric Measurements on Body Composition, Adiposity Distribution and Obesity
2.4. Assessment of Habitual Dietary Intakes of Choline, Betaine and Folate by Quantitative Food Frequency Questionnaire (qFFQ)
2.5. Blood Metabolic Markers Measurements
2.6. Statistical Analysis
3. Results
3.1. Basic Data, Body Adiposity and Blood Metabolic Markers of the Study Participants
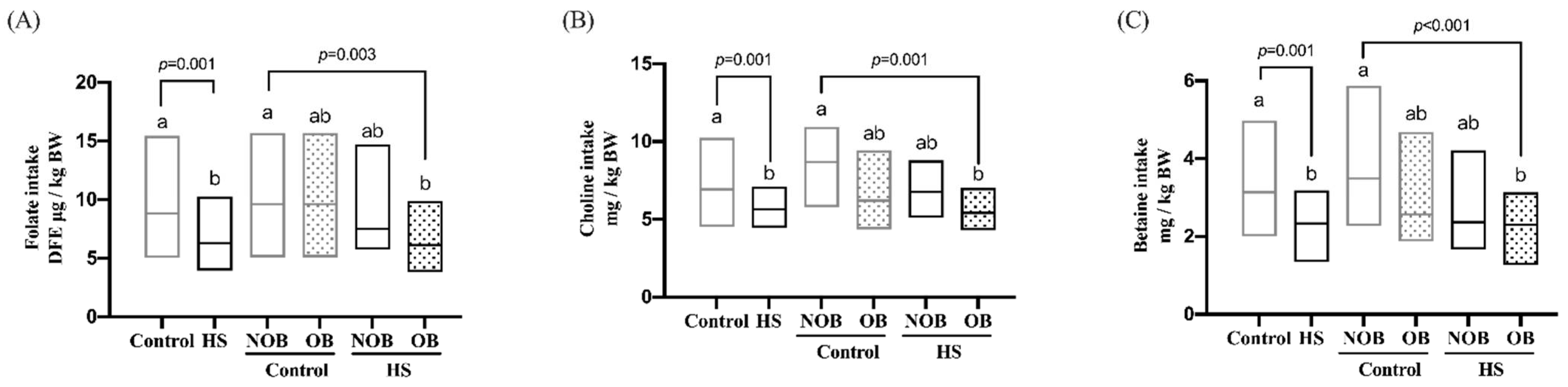
3.2. Individual Methyl-Donor Nutrients (MDNs) Intake of the HS and Obesity (OB)-Stratified Participants
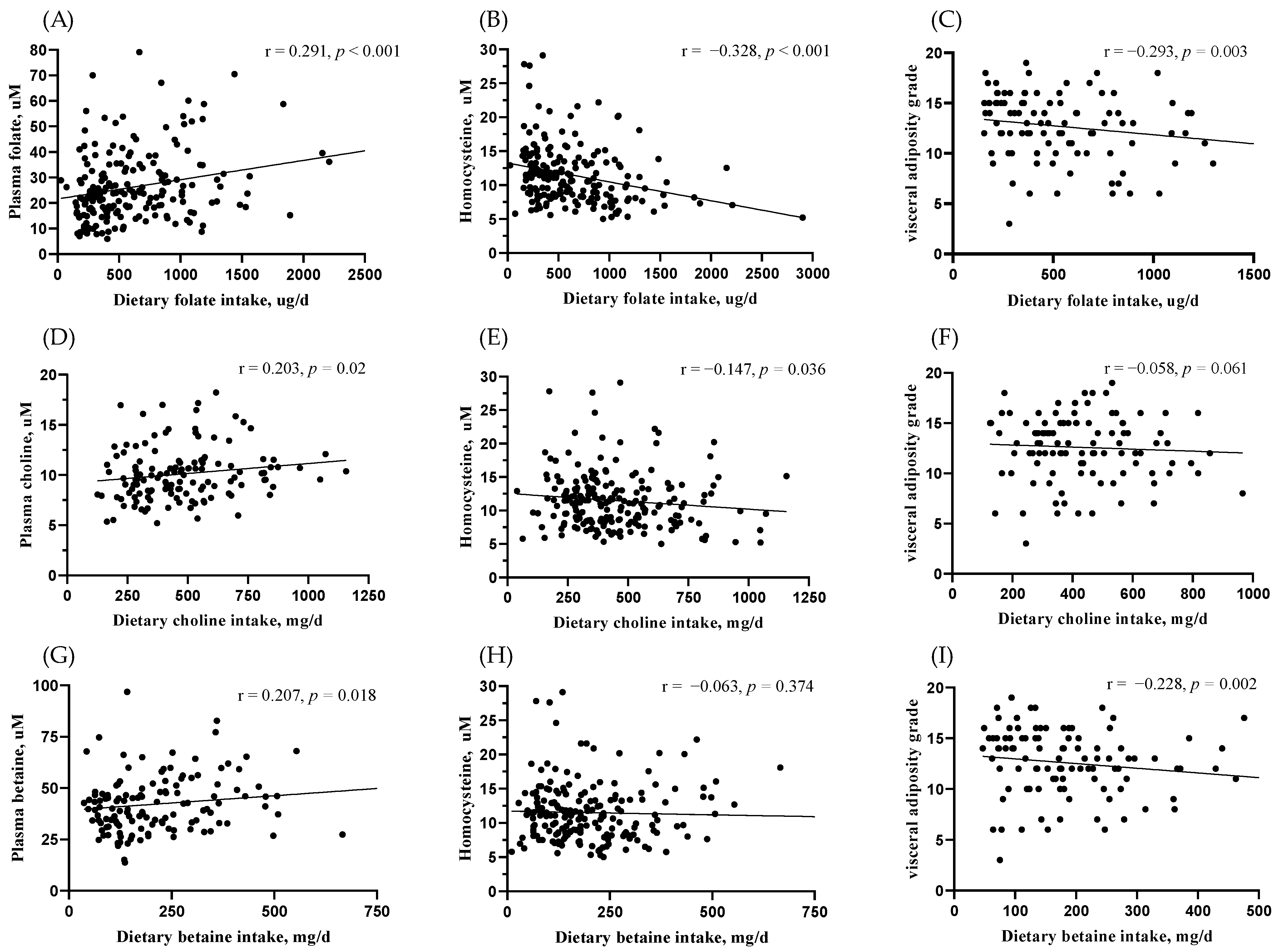
3.3. Correlation of Blood Marker-Validated MDNs Intake with Body Fat Distribution
3.4. Potential Dietary and Blood Determinants of HS in the Total and Visceral Obesity (VOB)-Stratified Participants
3.5. Quartile Intake of Individual MDN Associated with HS and VOB
3.6. Threshold Intakes of Individual MDN Associated with VOB-Related HS
3.7. Combined MDNs Intake Composition Associated with VOB-Related HS
4. Discussion
5. Limitations and Strengths of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabres, M.; Abbate, M.; Montemayor, S.; Mascaro, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, M.A.; Tur, J.A.; et al. Oxidative stress and pro-Inflammatory status in patients with non-alcoholic fatty liver disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef]
- Abbate, M.; Mascaro, C.M.; Montemayor, S.; Casares, M.; Gomez, C.; Ugarriza, L.; Tejada, S.; Abete, I.; Zulet, M.A.; Sureda, A.; et al. Non-alcoholic fatty liver disease is associated with kidney glomerular hyperfiltration in adults with metabolic syndrome. J. Clin. Med. 2021, 10, 1717. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Katzmarzyk, P.T.; Nichaman, M.Z.; Church, T.S.; Blair, S.N.; Ross, R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity 2006, 14, 336–341. [Google Scholar] [CrossRef]
- Noureddin, M.; Zelber-Sagi, S.; Wilkens, L.R.; Porcel, J.; Boushey, C.J.; Le Marchand, L.; Rosen, H.R.; Setiawan, V.W. Diet associations with nonalcoholic fatty liver disease in an ethnically diverse population: The multiethnic cohort. Hepatology 2020, 71, 1940–1952. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-carbon metabolism in health and disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Eudy, B.J.; Deminice, R. One-carbon metabolism in fatty liver disease and fibrosis: One-carbon to rule them all. J. Nutr. 2020, 150, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.; Kelly, K.B.; Al Rajabi, A.; Jacobs, R.L. Novel insights on interactions between folate and lipid metabolism. Biofactors 2014, 40, 277–283. [Google Scholar] [CrossRef]
- Zeisel, S.H. Metabolic crosstalk between choline/1-carbon metabolism and energy homeostasis. Clin. Chem Lab. Med. 2013, 51, 467–475. [Google Scholar] [CrossRef]
- Rizki, G.; Arnaboldi, L.; Gabrielli, B.; Yan, J.; Lee, G.S.; Ng, R.K.; Turner, S.M.; Badger, T.M.; Pitas, R.E.; Maher, J.J. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J. Lipid Res. 2006, 47, 2280–2290. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, P.J.; Nyirenda, M.J.; Walker, B.R. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes 2006, 55, 2015–2020. [Google Scholar] [CrossRef]
- Tan, H.L.; Mohamed, R.; Mohamed, Z.; Zain, S.M. Phosphatidylethanolamine N-methyltransferase gene rs7946 polymorphism plays a role in risk of nonalcoholic fatty liver disease: Evidence from meta-analysis. Pharm. Genom. 2016, 26, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.W.; Mehedint, M.G.; Garrow, T.A.; Zeisel, S.H. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J. Biol. Chem. 2011, 286, 36258–36267. [Google Scholar] [CrossRef]
- Sid, V.; Siow, Y.L.; Karmin, O. Role of folate in nonalcoholic fatty liver disease. Can. J. Physiol. Pharmacol. 2017, 95, 1141–1148. [Google Scholar] [CrossRef]
- Du, J.; Shen, L.; Tan, Z.; Zhang, P.; Zhao, X.; Xu, Y.; Gan, M.; Yang, Q.; Ma, J.; Jiang, A.; et al. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients 2018, 10, 131. [Google Scholar] [CrossRef]
- Sivanesan, S.; Taylor, A.; Zhang, J.; Bakovic, M. Betaine and choline improve lipid homeostasis in obesity by participation in mitochondrial oxidative demethylation. Front. Nutr. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Dahlhoff, C.; Worsch, S.; Sailer, M.; Hummel, B.A.; Fiamoncini, J.; Uebel, K.; Obeid, R.; Scherling, C.; Geisel, J.; Bader, B.L.; et al. Methyl-donor supplementation in obese mice prevents the progression of NAFLD, activates AMPK and decreases acyl-carnitine levels. Mol. Metab. 2014, 3, 565–580. [Google Scholar] [CrossRef]
- Yu, D.; Shu, X.O.; Xiang, Y.B.; Li, H.; Yang, G.; Gao, Y.T.; Zheng, W.; Zhang, X. Higher dietary choline intake is associated with lower risk of nonalcoholic fatty liver in normal-weight Chinese women. J. Nutr. 2014, 144, 2034–2040. [Google Scholar] [CrossRef]
- Guerrerio, A.L.; Colvin, R.M.; Schwartz, A.K.; Molleston, J.P.; Murray, K.F.; Diehl, A.; Mohan, P.; Schwimmer, J.B.; Lavine, J.E.; Torbenson, M.S.; et al. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2012, 95, 892–900. [Google Scholar] [CrossRef]
- Xia, M.F.; Bian, H.; Zhu, X.P.; Yan, H.M.; Chang, X.X.; Zhang, L.S.; Lin, H.D.; Hu, X.Q.; Gao, X. Serum folic acid levels are associated with the presence and severity of liver steatosis in Chinese adults. Clin. Nutr. 2018, 37, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Liu, Y.; Liu, Y.H.; Wang, X.; Guan, K.; Zhu, H.L. Higher serum concentrations of betaine rather than choline is associated with better profiles of DXA-derived body fat and fat distribution in Chinese adults. Int. J. Obes. 2015, 39, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, K.; Saarinen, M.T. Effect of dietary betaine on metabolic syndrome risk factors in Asian. J. Diabetes Metab. 2016, 7. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M., Jr.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial effects of betaine: A comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Lee, C.H.; Tzeng, M.S.; Huang, R.F.S. Dietary profile of folate intake in long-term post-stroke patients. Nutr. Res. 2005, 25, 465. [Google Scholar] [CrossRef]
- Cheng, C.P.; Chen, C.H.; Kuo, C.S.; Kuo, H.T.; Huang, K.T.; Shen, Y.L.; Chang, C.H.; Huang, R.F.S. Dietary choline and folate relationships with serum hepatic inflammatory injury markers in Taiwanese adults. Asia Pac. J. Clin. Nutr. 2017, 26, 642–649. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. Nutrition and Health Survey in Taiwan; Ministry of Health and Welfare: Taipei City, Taiwan, 2019.
- Chu, D.M.; Wahlqvist, M.L.; Chang, H.Y.; Yeh, N.H.; Lee, M.S. Choline and betaine food sources and intakes in Taiwanese. Asia Pac. J. Clin. Nutr. 2012, 21, 547–557. [Google Scholar] [PubMed]
- Patterson, K.Y.; Bhagwat, S.A.; Williams, J.A.; Howe, J.C.; Holden, J.M. USDA Database for the Choline Content of Common Foods: Release Two; U.S. Department of Agriculture: Beltsville, MD, USA, 2008.
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.; Mar, M.H.; Ranasinghe, A.; Swenberg, J.A.; Zeisel, S.H. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal. Chem. 2002, 74, 4734–4740. [Google Scholar] [CrossRef]
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008, 134, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Randell, E.; Pedram, P.; Yi, Y.; Gulliver, W.; Sun, G. Higher dietary choline and betaine intakes are associated with better body composition in the adult population of Newfoundland, Canada. PLoS ONE 2016, 11, e0155403. [Google Scholar] [CrossRef]
- Konstantinova, S.V.; Tell, G.S.; Vollset, S.E.; Ulvik, A.; Drevon, C.A.; Ueland, P.M. Dietary patterns, food groups, and nutrients as predictors of plasma choline and betaine in middle-aged and elderly men and women. Am. J. Clin. Nutr. 2008, 88, 1663–1669. [Google Scholar] [CrossRef]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Mollard, R.C.; Sénéchal, M.; MacIntosh, A.C.; Hay, J.; Wicklow, B.A.; Wittmeier, K.D.; Sellers, E.A.; Dean, H.J.; Ryner, L.; Berard, L.; et al. Dietary determinants of hepatic steatosis and visceral adiposity in overweight and obese youth at risk of type 2 diabetes. Am. J. Clin. Nutr. 2014, 99, 804–812. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, S.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Bao, X.; Gu, Y.; Sun, S.; Wang, X.; et al. Insoluble dietary fiber intake is associated with lower prevalence of newly-diagnosed non-alcoholic fatty liver disease in Chinese men: A large population-based cross-sectional study. Nutr. Metab. 2020, 17, 4. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, A.; Mao, L.; Quan, Y.; Cui, J.; Sun, Y. Association between dietary fiber intake and non-alcoholic fatty liver disease in adults. Front. Nutr. 2020, 7, 593735. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.M.; da Costa, K.A.; Kwock, L.; Stewart, P.W.; Lu, T.S.; Stabler, S.P.; Allen, R.H.; Zeisel, S.H. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 2007, 85, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.T.; O’Reilly, G.A.; Goran, M.I.; Weigensberg, M.J.; Spruijt-Metz, D.; Davis, J.N. Vegetable consumption is linked to decreased visceral and liver fat and improved insulin resistance in overweight Latino youth. J. Acad. Nutr. Diet. 2014, 114, 1776–1783. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Imoto, S.; Ihara, K.; Nakaji, S. Association between nutrients and visceral fat in healthy japanese adults: A 2-year longitudinal study: Micronutrients associated with visceral fat accumulation. Nutrients 2019, 11, 2698. [Google Scholar] [CrossRef]
- Bailey, B.W.; Sullivan, D.K.; Kirk, E.P.; Donnelly, J.E. Dietary predictors of visceral adiposity in overweight young adults. Br. J. Nutr. 2010, 103, 1702–1705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pereira, G.A.; Bressan, J.; Oliveira, F.L.P.; Sant’Ana, H.M.P.; Pimenta, A.M.; Lopes, L.L.; Hermsdorff, H.H.M. Dietary folate intake is negatively associated with excess body weight in Brazilian graduates and postgraduates (CUME Project). Nutrients 2019, 11, 518. [Google Scholar] [CrossRef]
- Li, Z.; Gueant-Rodriguez, R.M.; Quilliot, D.; Sirveaux, M.A.; Meyre, D.; Gueant, J.L.; Brunaud, L. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin. Nutr. 2018, 37, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.E.; Bassoli, B.K.; de Souza, C.A.; Deminice, R.; Jordão Júnior, A.A.; Paiva, S.A.; Dagli, M.L.; Ong, T.P.; Moreno, F.S. Folic acid supplementation during early hepatocarcinogenesis: Cellular and molecular effects. Int. J. Cancer 2011, 129, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Miller, J.W.; da Costa, K.A.; Nadeau, M.; Smith, D.; Selhub, J.; Zeisel, S.H.; Mason, J.B. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J. Nutr. 1994, 124, 2197–2203. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Zhao, Y.; Koonen, D.P.; Sletten, T.; Su, B.; Lingrell, S.; Cao, G.; Peake, D.A.; Kuo, M.S.; Proctor, S.D.; et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J. Biol. Chem. 2010, 285, 22403–22413. [Google Scholar] [CrossRef]
- Veenema, K.; Solis, C.; Li, R.; Wang, W.; Maletz, C.V.; Abratte, C.M.; Caudill, M.A. Adequate intake levels of choline are sufficient for preventing elevations in serum markers of liver dysfunction in Mexican American men but are not optimal for minimizing plasma total homocysteine increases after a methionine load. Am. J. Clin. Nutr. 2008, 88, 685–692. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline, an essential nutrient for humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Horita, D.A.; Hwang, S.; Stegall, J.M.; Friday, W.B.; Kirchner, D.R.; Zeisel, S.H. Two methods for assessment of choline status in a randomized crossover study with varying dietary choline intake in people: Isotope dilution MS of plasma and in vivo single-voxel magnetic resonance spectroscopy of liver. Am. J. Clin. Nutr. 2021, 113, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Best, C.H.; Ridout, J.H.; Lucas, C.C. Alleviation of dietary cirrhosis by betaine and other lipotropic agents. Nutr. Rev. 1969, 27, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S. Choline, other methyl-donors and epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, T.; Pini, M.; Zhou, Z.; Fantuzzi, G.; Song, Z. Betaine improved adipose tissue function in mice fed a high-fat diet: A mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G634–G642. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, L.C.; Sivanesan, S.; Zhang, J.; Wuyts, B.; Taylor, A.; Verbrugghe, A.; Bakovic, M. Choline supplementation restores substrate balance and alleviates complications of Pcyt2 deficiency. J. Nutr. Biochem. 2015, 26, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Grapentine, S.; Singh, R.K.; Basu, P.; Sivanesan, S.; Mattos, G.; Oresajo, O.; Cheema, J.; Demeke, W.; Dolinsky, V.W.; Bakovic, M. Pcyt2 Deficiency causes age-dependant development of non-alcoholic steatohepatitis and insulin resistance that could be attenuated with phospho-ethanolamine. Res. Sq. 2021, preprint. [Google Scholar] [CrossRef]
- Vance, D.E. Physiological roles of phosphatidylethanoamine N-methyltransferase. Biochim. Biophys. Acta 2013, 1831, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.B.; Kennelly, J.P.; Ordonez, M.; Nelson, R.; Leonard, K.; Stabler, S.; Gomez-Muñoz, A.; Field, C.J.; Jacobs, R.L. Excess folic acid increases lipid storage, weight gain, and adipose tissue inflammation in high fat diet-fed rats. Nutrients 2016, 8, 594. [Google Scholar] [CrossRef]
- Christensen, K.E.; Mikael, L.G.; Leung, K.Y.; Lévesque, N.; Deng, L.; Wu, Q.; Malysheva, O.V.; Best, A.; Caudill, M.A.; Greene, N.D.; et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015, 101, 646–658. [Google Scholar] [CrossRef]
| Variables | Control | HS | p Value |
|---|---|---|---|
| Sex | |||
| Men, n (%) | 47 (45) | 46 (44) | 0.84 |
| Age, y | 63 (55, 68) | 58 (46, 65) | 0.002 |
| BMI, kg/m2 | 22 (20, 24) | 27 (24, 29) | <0.001 |
| <18.5, n (%) | 9 (9) | 1 (1) | <0.001 |
| 18.5–24, n (%) | 63 (61) | 17 (16) | |
| 24–27, n (%) | 20 (19) | 34 (32) | |
| ≥27, n (%) | 12 (11) | 53 (51) | |
| Body composition | |||
| Skeletal muscle mass 3, % | 39 (35, 43) | 37 (34, 39) | 0.004 |
| Body fat 4, % | 28 (21, 33) | 32 (28, 36) | <0.001 |
| Waist/hip circumference | 0.8 (0.8, 0.9) | 0.9 (0.9, 1.0) | <0.001 |
| Visceral adiposity grade | 6 (5, 9) | 13 (10, 15) | <0.001 |
| Smoking status | |||
| Current and former smoker, n (%) | 17 (16) | 23 (22) | 0.36 |
| Alcohol use | |||
| Current and former, n (%) | 16 (15) | 19 (18) | 0.49 |
| Blood biochemical marker | |||
| Triglycerides, mg/dL | 72 (53, 95) | 135 (97, 187) | <0.001 |
| Total cholesterol, mg/dL | 197 (170, 224) | 191 (173, 215) | 0.44 |
| Plasma glucose, mg/dL | 98 (94, 104) | 104 (98, 116) | <0.001 |
| Insulin, μIU/ml | 7.9 (6.4, 10) | 13 (10, 19) | <0.001 |
| HOMA-IR | 1.9 (1.6, 2.7) | 3.7 (2.5, 5.7) | <0.001 |
| AST, U/L | 23 (19, 26) | 20 (17, 28) | 0.12 |
| ALT, U/L. | 19 (16, 25) | 24 (16, 38) | 0.04 |
| Independent Variables | Dependent Variables of Hepatic Steatosis | |||||
|---|---|---|---|---|---|---|
| Total Subjects | VOB | Non-VOB | ||||
| β | p Value | β | p Value | β | p Value | |
| Dietary intakes | ||||||
| Folate, DFE μg/day | 0.20 | 0.02 * | 0.24 | 0.07 | 0.09 | 0.5 |
| Choline, mg/day | −0.22 | 0.05 | -0.41 | 0.01 * | 0.05 | 0.8 |
| Betaine, mg/day | -0.04 | 0.6 | -0.01 | 0.9 | −0.13 | 0.4 |
| Lipid, g/day | 0.14 | 0.1 | 0.18 | 0.1 | −0.20 | 0.5 |
| Carbohydrate, g/day | 0.11 | 0.3 | 0.12 | 0.4 | −0.25 | 0.7 |
| Energy, Kcal/day | −0.17 | 0.3 | -0.04 | 0.8 | 0.27 | 0.8 |
| Fiber, g/day | −0.07 | 0.4 | -0.13 | 0.5 | 0.01 | 0.9 |
| Blood metabolic markers | ||||||
| Triglycerides, mg/dL | 0.28 | <0.001 *** | 0.32 | <0.001 *** | 0.15 | 0.09 |
| HOMA-IR index | 0.28 | <0.001 *** | 0.29 | 0.004 ** | 0.54 | <0.001 *** |
| Homocysteine, uM | 0.06 | 0.2 | 0.01 | 0.8 | -0.04 | 0.6 |
| Quartile MDNs Intake | HS | VOB |
|---|---|---|
| Choline intake mg/kg body weight | Model B odds ratio (95% CI) | Model B odds ratio (95% CI) |
| Q1: 3.7 (3.2, 4.1) | 1 (ref.) | 1 (ref.) |
| Q2: 5.2 (4.7, 5.7) | 1.3 (0.49–3.95) | 0.54 (0.16–1.8) |
| Q3: 7.0 (6.5, 7.5) | 1.1 (0.40–3.29) | 0.85 (0.25–2.8) |
| Q4: 10 (9.6, 13) | 0.32 * (0.11–0.97) | 0.28 * (0.08–0.95) |
| Folate intake DFE μg/kg body weight | Model A odds ratio (95% CI) | Model A odds ratio (95% CI) |
| Q1: 3.3 (2.6, 3.9) | 1 (ref.) | 1 (ref.) |
| Q2: 5.7 (5.0, 6.5) | 0.96 (0.36–2.62) | 0.83 (0.23–3.0) |
| Q3: 9.6 (8.1, 11) | 0.82 (0.30–2.20) | 0.83 (0.23–3.0) |
| Q4: 17 (14, 21) | 0.36 * (0.13–0.98) | 0.25 * (0.07–0.89) |
| Betaine intake mg/kg body weight | Model A odds ratio (95% CI) | Model A odds ratio (95% CI) |
| Q1: 1.1 (0.91, 1.3) | 1 (ref.) | 1 (ref.) |
| Q2: 2.1 (1.9, 2.3) | 0.79 (0.30–2.0) | 0.89 (0.26–3.0) |
| Q3: 3.1 (2.9, 3.6) | 0.86 (0.32–2.2) | 0.76 (0.22–2.6) |
| Q4: 5.6 (4.7, 6.9) | 0.31 * (0.12–0.85) | 0.26 * (0.08–0.89) |
| Visceral Obesity | Choline Intake 3 mg/kg Body Weight | Betaine Intake 4 mg/kg BW | Folate Intake 5 DFE ug/kg BW | |||
|---|---|---|---|---|---|---|
| Low <6.9 | High ≥6.9 | Low <3.1 | High ≥3.1 | Low <8.8 | High ≥8.8 | |
| Visceral adiposity grade | ||||||
| <10, n | 47 | 51 | 46 | 52 | 46 | 50 |
| ≥10, n | 76 | 33 | 80 | 29 | 73 | 36 |
| Model A: OR (95%CI) | ||||||
| <0 (ref.) | 1 (ref.) | 0.85 (0.2–3.1) | 1 (ref.) | 1.2 (0.3–4.3) | 1 (ref.) | 1.08 (0.3–3.8) |
| ≥10 | 22 * (8.1–63) | 10 * (2.9–37) | 26 * (9.4–75) | 12 * (3.3–45) | 26 * (8.9–76) | 13 * (3.9–48) |
| Model B: OR (95%CI) | ||||||
| <10 (ref.) | 1 (ref.) | 1.33 (0.2–7.2) | 1 (ref.) | 1.0 (0.21–5.5) | 1 (ref.) | 2.6 (0.5–13) |
| ≥10 | 22 * (6.5–80) | 9.6 * (1.9–46) | 14 * (4.4–50) | 14 * (2.8–70) | 19 * (5.2–74) | 30 * (5.7–156) |
| MDN Intake Composition | Fatty Liver | Mode 1 OR (95%CI) | Model 2 OR (95%CI) | Model 3 OR (95%CI) | Model 4 OR (95%CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | ||||||||
| Dietary Folate 3 (DFE ug/d) X Betaine 4 (mg/d) intake | |||||||||
| Folate | Betaine | ||||||||
| Low | Low | 62 | 76 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | ||
| Low | High | 5 | 4 | 0.46 (0.1–2.06) | 0.93 (0.14–6.08) | 0.55 (0.05–5.41) | 0.59 (0.07–5.16) | ||
| High | Low | 12 | 13 | 0.69 (0.28–1.72) | 1.32 (0.39–4.49) | 1.88 (0.47–7.55) | 2.10 (0.50–8.82) | ||
| High | High | 24 | 11 | 0.33 * (0.14–0.75) | 0.42 (0.15–1.21) | 0.42 (0.14–1.29) | 0.46 (0.14–1.54) | ||
| Dietary Folate 3 (DFE ug/d) X Choline 5 (mg/d) intake | |||||||||
| Folate | Choline | ||||||||
| Low | Low | 46 | 53 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | ||
| Low | High | 21 | 27 | 0.86 (0.39–1.85) | 0.67 (0.24–1.87) | 0.47 (0.14–1.56) | 0.4 (0.14–1.51) | ||
| High | Low | 5 | 4 | 0.58 (0.14–2.41) | 2.58 (0.45–14.9) | 4.18 (0.59–29.2) | 4.14 (0.51–33.3) | ||
| High | High | 31 | 20 | 0.42 * (0.19–0.89) | 0.44 (0.16–1.17) | 0.41 (0.14–1.21) | 0.53 (0.17–1.64) | ||
| Dietary Betaine 4 (mg/d) X Choline 5 (mg/d) intake | |||||||||
| Betaine | Choline | ||||||||
| Low | Low | 48 | 56 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | ||
| Low | High | 27 | 34 | 0.89 (0.45–1.79) | 0.58 (0.23–1.46) | 0.37 (0.12–1.14) | 0.32 (0.10–1.0) | ||
| High | Low | 4 | 2 | 0.51 (0.08–2.95) | 0.88 (0.11–7.11) | 0.33 (0.02–4.73) | 0.24 (0.02–3.57) | ||
| High | High | 25 | 13 | 0.33 * (0.14–0.78) | 0.31 * (0.10–0.92) | 0.26 * (0.08–0.84) | 0.19 * (0.05–0.69) | ||
| Dietary Choline 5 (mg/d) X Betaine 4 (mg/d) X Folate 3 (DFE ug/d) intake | |||||||||
| Choline | Betaine | Folate | |||||||
| Low | Low | Low | 43 | 51 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| One or two of the three MDN intake was low | 38 | 43 | 0.76 (0.4–1.5) | 0.89 (0.4–2.1) | 0.71 (0.3–1.9) | 0.72 (0.3–1.9) | |||
| High | High | High | 23 | 11 | 0.33 * (0.1–0.8) | 0.37 (0.1–1.1) | 0.33 (0.1–1.1) | 0.33 (0.1–1.1) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-Y.; Wu, C.-H.; Chang, C.-Y.; Lee, F.-J.; Wang, B.-W.; Doong, J.-Y.; Lin, Y.-S.; Kuo, C.-S.; Huang, R.-F.S. Optimal Dietary Intake Composition of Choline and Betaine Is Associated with Minimized Visceral Obesity-Related Hepatic Steatosis in a Case-Control Study. Nutrients 2022, 14, 261. https://doi.org/10.3390/nu14020261
Chang T-Y, Wu C-H, Chang C-Y, Lee F-J, Wang B-W, Doong J-Y, Lin Y-S, Kuo C-S, Huang R-FS. Optimal Dietary Intake Composition of Choline and Betaine Is Associated with Minimized Visceral Obesity-Related Hepatic Steatosis in a Case-Control Study. Nutrients. 2022; 14(2):261. https://doi.org/10.3390/nu14020261
Chicago/Turabian StyleChang, Ting-Yu, Chien-Hsien Wu, Chi-Yang Chang, Fu-Jen Lee, Bei-Wen Wang, Jia-Yau Doong, Yu-Shun Lin, Chang-Sheng Kuo, and Rwei-Fen S. Huang. 2022. "Optimal Dietary Intake Composition of Choline and Betaine Is Associated with Minimized Visceral Obesity-Related Hepatic Steatosis in a Case-Control Study" Nutrients 14, no. 2: 261. https://doi.org/10.3390/nu14020261
APA StyleChang, T.-Y., Wu, C.-H., Chang, C.-Y., Lee, F.-J., Wang, B.-W., Doong, J.-Y., Lin, Y.-S., Kuo, C.-S., & Huang, R.-F. S. (2022). Optimal Dietary Intake Composition of Choline and Betaine Is Associated with Minimized Visceral Obesity-Related Hepatic Steatosis in a Case-Control Study. Nutrients, 14(2), 261. https://doi.org/10.3390/nu14020261







