Caregiver Nutritional Health Outcomes of the Simple Suppers Study: Results from a 10 Week, Two-Group Quasi-Experimental Family Meals Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
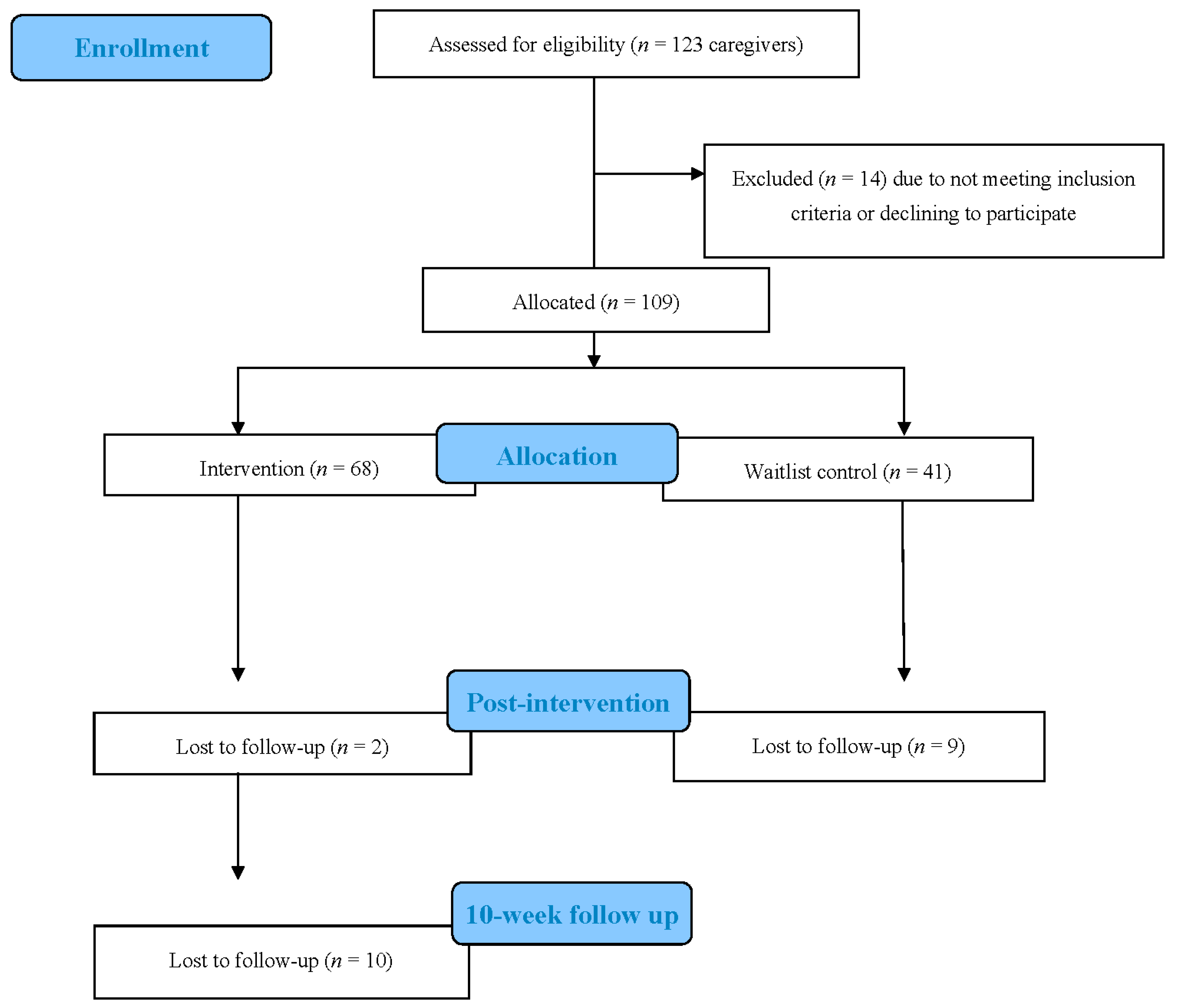
2.3. Participants
2.4. Data Collection
2.5. Intervention
2.6. Outcome Measures
2.7. Process Measures
2.8. Sample Size and Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogden, C.L.; Carroll, M.D.; Fryar, C.D.; Flegal, K.M. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief 2015, 219, 1–8. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26633046 (accessed on 1 December 2021).
- Chang, V.W.; Lauderdale, D.S. Income Disparities in Body Mass Index and Obesity in the United States, 1971-2002. Arch. Intern. Med. 2005, 165, 2122–2128. [Google Scholar] [CrossRef]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; Inge, T.H.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar]
- Nakeshbandi, M.; Maini, R.; Daniel, P.; Rosengarten, S.; Parmar, P.; Wilson, C.; Kim, J.M.; Oommen, A.; Mecklenburg, M.; Salvani, J.; et al. The impact of obesity on COVID-19 complications: A retrospective cohort study. Int. J. Obes. 2020, 44, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Shear, C.L.; Burke, G.L.; Srinivasan, S.R.; Webber, L.S.; Harsha, D.W.; Berenson, G.S. Persistence of juvenile-onset obesity over eight years: The Bogalusa Heart Study. Am. J. Public Health 1987, 77, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.L.; Fisher, J.O. Development of Eating Behaviors Among Children and Adolescents. Pediatrics 1998, 101, 539–549. [Google Scholar] [CrossRef]
- Savage, J.S.; Fisher, J.O.; Birch, L.L. Parental Influence on Eating Behavior: Conception to Adolescence. J. Law Med. Ethic 2007, 35, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Berge, J.M.; MacLehose, R.F.; Larson, N.; Laska, M.; Neumark-Sztainer, D. Family Food Preparation and Its Effects on Adolescent Dietary Quality and Eating Patterns. J. Adolesc. Health 2016, 59, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulkerson, J.A.; Kubik, M.Y.; Story, M.; Lytle, L.; Arcan, C. Are There Nutritional and Other Benefits Associated with Family Meals Among At-Risk Youth? J. Adolesc. Health 2009, 45, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, N.I.; Story, M.; Eisenberg, M.E.; Neumark-Sztainer, D. Food Preparation and Purchasing Roles among Adolescents: Associations with Sociodemographic Characteristics and Diet Quality. J. Am. Diet. Assoc. 2006, 106, 211–218. [Google Scholar] [CrossRef]
- Gillman, M.W.; Rifas-Shiman, S.L.; Frazier, A.L.; Rockett, H.R.; Camargo, C.A., Jr.; Field, A.E.; Colditz, G.A.; Berkey, C.S. Family dinner and diet quality among older children and adolescents. Arch. Fam. Med. 2000, 9, 235–240. [Google Scholar] [CrossRef]
- Neumark-Sztainer, D.; Hannan, P.J.; Story, M.; Croll, J.; Perry, C. Family meal patterns: Associations with sociodemographic characteristics and improved dietary intake among adolescents. J. Am. Diet. Assoc. 2003, 103, 317–322. [Google Scholar] [CrossRef]
- Gunther, C.; Rogers, C.; Holloman, C.; Hopkins, L.C.; Anderson, S.E.; Miller, C.K.; Copeland, K.A.; Dollahite, J.S.; Pratt, K.J.; Webster, A.; et al. Child diet and health outcomes of the simple suppers program: A 10-week, 2-group quasi-experimental family meals trial. BMC Public Health 2019, 19, 1657. [Google Scholar] [CrossRef]
- Fulkerson, J.A.; Friend, S.; Horning, M.; Flattum, C.; Draxten, M.; Neumark-Sztainer, D.; Gurvich, O.; Garwick, A.; Story, M.; Kubik, M.Y. Family Home Food Environment and Nutrition-Related Parent and Child Personal and Behavioral Outcomes of the Healthy Home Offerings via the Mealtime Environment (HOME) Plus Program: A Randomized Controlled Trial. J. Acad. Nutr. Diet. 2017, 118, 240–251. [Google Scholar] [CrossRef]
- Utter, J.; Denny, S.; Farrant, B.; Cribb, S. Feasibility of a Family Meal Intervention to Address Nutrition, Emotional Wellbeing, and Food Insecurity of Families with Adolescents. J. Nutr. Educ. Behav. 2019, 51, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Mullins, J.T.; Walters, J.; Najor, J.; Mikuška, J.; Courtney, A.; Norman-Burgdolf, H. Evaluation of a social marketing approach to healthy, home-cooked family meals for low-income mothers of young children. Transl. Behav. Med. 2020, 10, 1312–1321. [Google Scholar]
- Litterbach, E.-K.V.; Campbell, K.J.; Spence, A.C. Family meals with young children: An online study of family mealtime characteristics, among Australian families with children aged six months to six years. BMC Public Health 2017, 17, 111. [Google Scholar] [CrossRef] [Green Version]
- Rogers, C.; Anderson, S.E.; Dollahite, J.S.; Hill, T.F.; Holloman, C.; Miller, C.K.; Pratt, K.J.; Gunther, C. Methods and design of a 10-week multi-component family meals intervention: A two group quasi-experimental effectiveness trial. BMC Public Health 2017, 17, 50. [Google Scholar] [CrossRef] [Green Version]
- Bartholomew, L.K.; Parcel, G.S.; Kok, G. Intervention Mapping: A Process for Developing Theory and Evidence-Based Health Education Programs. Health Educ. Behav. 1998, 25, 545–563. [Google Scholar] [CrossRef]
- Fink, S.K.; Racine, E.F.; Mueffelmann, R.E.; Dean, M.N.; Herman-Smith, R. Family Meals and Diet Quality Among Children and Adolescents in North Carolina. J. Nutr. Educ. Behav. 2014, 46, 418–422. [Google Scholar] [CrossRef]
- Gable, S.; Chang, Y.; Krull, J.L. Television Watching and Frequency of Family Meals Are Predictive of Overweight Onset and Persistence in a National Sample of School-Aged Children. J. Am. Diet. Assoc. 2007, 107, 53–61. [Google Scholar] [CrossRef]
- Rollins, B.Y.; Belue, R.Z.; Francis, L.A. The Beneficial Effect of Family Meals on Obesity Differs by Race, Sex, and Household Education: The National Survey of Children’s Health, 2003–2004. J. Am. Diet. Assoc. 2010, 110, 1335–1339. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, S.J.; Hanning, R.M.; McGoldrick, K.; Brown, K.S. Healthy eating index-C is positively associated with family dinner frequency among students in grades 6–8 from Southern Ontario, Canada. Eur. J. Clin. Nutr. 2010, 64, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Bandura, A. Social Foundations of Thought and Action: A Social Cognitive Theory; Prentice Hall: Edgewood Cliffs, NJ, USA, 1986. [Google Scholar]
- Blumberg, J.; Bialostosky, K.; Hamilton, W.L.; Briefel, R.R. U.S. Household Food Security Survey Module: Six-Item Short Form. Am. J. Public Health 1999, 89, 1231–1234. [Google Scholar] [CrossRef] [Green Version]
- Bliss, R. Researchers produce innovation in dietary recall. Agric. Res. 2004, 52, 10–12. [Google Scholar]
- University of Minnesota Nutrition Coordinating Center. Nutrition Data System for Research. 2015. Available online: http://www.ncc.umn.edu (accessed on 1 December 2021).
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the Healthy Eating Index: HEI-2010. J. Acad. Nutr. Diet. 2013, 113, 569–580. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010, 7th ed.; U.S. Government Printing Office: Washington, DC, USA, 2010.
- Centers for Disease Control and Prevention. Anthropometry Procedures Manual; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2007; p. 102.
- Bohman, B.; Nyberg, G.; Sundblom, E.; Elinder, L.S. Validity and Reliability of a Parental Self-Efficacy Instrument in the Healthy School Start Prevention Trial of Childhood Obesity. Health Educ. Behav. 2013, 41, 392–396. [Google Scholar] [CrossRef]
- Cullen, K.W.; Baranowski, T.; Rittenberry, L.; Cosart, C.; Owens, E.; Hebert, D.; de Moor, C. Socioenvironmental influences on children’s fruit, juice and vegetable con-sumption as reported by parents: Reliability and validity of measures. Public Health Nutr. 2000, 3, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.M.; Wagner, A.K.; Kennel, J.; Miller, C.K.; Holloman, C.; Pennywitt, J.; Battista, M.; Murray, R.D.; Rogers, C.A.; Gunther, C.W. Determining the Feasibility and Acceptability of a Nutrition Education and Cooking Program for Preschoolers and Their Families Delivered Over the Dinner Hour in a Low-Income Day Care Setting. Infant Child Adolesc. Nutr. 2014, 6, 144–151. [Google Scholar] [CrossRef]
- Taveras, E.M.; Rifas-Shiman, S.L.; Berkey, C.S.; Rockett, H.R.; Field, A.E.; Frazier, A.L.; Colditz, G.A.; Gillman, M.W. Family Dinner and Adolescent Overweight. Obes. Res. 2005, 13, 900–906. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software: Release 15; StataCorp LLC: College Station, TX, USA, 2017. [Google Scholar]
- Fruh, S.M.; Mulekar, M.S.; Crook, E.; Hall, H.R.; Adams, J.; Lemley, T. The Family Meal Challenge: A Faith-Based Intervention to Empower Families. J. Christ. Nurs. 2018, 35, 191–197. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief. 2017, 270, 1–8. [Google Scholar]
- Sobal, J.; Hanson, K. Family meals and body weight in US adults. Public Health Nutr. 2011, 14, 1555–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.C.; Sobal, J. Family meals and body weight. Analysis of multiple family members in family units. Appetite 2011, 57, 517–524. [Google Scholar] [CrossRef]
- Tumin, R.; Anderson, S.E. Television, Home-Cooked Meals, and Family Meal Frequency: Associations with Adult Obesity. J. Acad. Nutr. Diet. 2017, 117, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Berge, J.M.; MacLehose, R.F.; Loth, K.A.; Eisenberg, M.E.; Fulkerson, J.A.; Neumark-Sztainer, D. Family meals. Associations with weight and eating behaviors among mothers and fathers. Appetite 2012, 58, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Utter, J.; Larson, N.; Berge, J.M.; Eisenberg, M.E.; Fulkerson, J.A.; Neumark-Sztainer, D. Family meals among parents: Associations with nutritional, social and emotional wellbeing. Prev. Med. 2018, 113, 7–12. [Google Scholar] [CrossRef]
- Bandura, A. Social cognitive theory. Ann. Child Dev. 1989, 6, 1–60. [Google Scholar]
- Heaney, C.A.; Israel, B.A. Social networks and social support. Health Behav. Health Educ. Theory Res. Pract. 2008, 4, 189–210. [Google Scholar]
| Characteristics | Total (n = 98) | Intervention (n = 66) | Waitlist Control (n = 32) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Sex (female) b, n (%) | n = 96 | n = 65 | n = 31 | 0.49 |
| Female | 95 (98.96) | 64 (98.46) | 31 (100.00) | |
| Male | 1 (1.04) | 1 (1.54) | 0 (0.00) | |
| Race c, n (%) | n = 96 | n = 65 | n = 31 | 0.07 |
| Black | 59 (61.46) | 39 (60.0) | 20 (64.52) | |
| White | 28 (29.17) | 17 (26.15) | 11 (35.48) | |
| Other d | 9 (9.38) | 9 (13.85) | 0 (0.00) | |
| Age (years) c, n (%) | n = 91 | n = 62 | n = 29 | 0.36 |
| 18–30 | 9 (9.89) | 7 (11.29) | 2 (6.90) | |
| 31–40 | 46 (50.55) | 27 (43.55) | 19 (65.52) | |
| 41+ | 36 (39.56) | 28 (45.16) | 8 (27.59) | |
| Household Income Status b,e, n (%) | n = 91 | n = 62 | n = 29 | 0.81 |
| Low Income | 36 (39.56) | 24 (38.71) | 12 (41.38) | |
| Non-Low Income | 55 (60.44) | 38 (61.29) | 17 (58.62) | |
| Home Food Security c,f, n (%) | n = 94 | n = 63 | n = 31 | 0.10 |
| High/Marginal Food Security | 59 (62.77) | 35 (55.56) | 24 (77.42) | |
| Low Food Security | 19 (20.21) | 16 (25.40) | 3 (9.68) | |
| Very Low Food Security | 16 (17.02) | 12 (19.05) | 4 (12.90) | |
| Anthropometrics and Biometrics g | ||||
| BMI (kg/m2) h, mean (SE) | n = 86 | n = 58 | n = 28 | 0.35 |
| 32.62 (1.13) | 31.88 (1.28) | 34.16 (2.25) | ||
| Weight Status c, n (%) | n = 87 | n = 58 | n = 28 | 0.77 |
| Underweight (BMI < 18.5) | 2 (2.33) | 2 (3.45) | 0 (0.00) | |
| Normal Weight (BMI 18.5–24.9) | 22 (25.58) | 13 (22.41) | 9 (32.14) | |
| Overweight (BMI 25–29.9) | 17 (19.77) | 12 (20.69) | 5 (17.86) | |
| Obese (BMI ≥30) | 45 (52.33) | 31 (53.44) | 14 (50.00) | |
| Waist Circumference (cm) h,i, mean (SE) | n = 88 | n = 59 | n = 29 | 0.62 |
| 102.84 (2.23) | 102.06 (2.61) | 104.43 (4.24) | ||
| Blood Pressure (mmHg) h, mean (SE) | n = 90 | n = 61 | n = 29 | |
| Systolic Blood Pressure | 128.33 (1.72) | 127.90 (2.08) | 129.22 (3.10) | 0.72 |
| Diastolic Blood Pressure | 81.94 (1.28) | 80.95 (1.41) | 84.02 (2.63) | 0.26 |
| Outcomes | Intervention | Waitlist Control | β | p-Value | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | Δ | T0 | T1 | Δ | ||||
| Dietary Intake a,b,c,d, mean (SE) (n = 40) | |||||||||
| HEI e Total Score f (100 maximum) | 58.68 (1.87) | 55.52 (3.05) | −3.16 (3.43) | 58.50 (2.71) | 61.57 (2.98) | 3.07 (3.94) | −6.53 | 0.17 | (−15.75, 2.69) |
| HEI Component Scores | |||||||||
| Adequacy | |||||||||
| Total fruits (0–5) | 2.00 (0.32) | 1.80 (0.42) | −0.20 (0.44) | 1.82 (0.48) | 2.08 (0.67) | 0.26 (0.93) | −0.45 | 0.53 | (−1.86, 0.96) |
| Whole fruits (0–5) | 2.5 (0.38) | 1.85 (0.43) | −0.70 (0.46) | 2.87 (0.67) | 2.21 (0.69) | −0.66 (0.99) | −0.38 | 0.60 | (−1.83, 1.05) |
| Total vegetables (0–5) | 3.00 (0.28) | 3.44 (0.31) | 0.44 (0.40) | 2.56 (0.51) | 3.10 (0.50) | 0.55 (0.55) | 0.04 | 0.93 | (−0.94, 1.03) |
| Greens and beans (0–5) | 2.94 (0.39) | 2.17 (0.43) | −0.77 (0.56) | 0.97 (0.56) | 2.52 (0.68) | 1.54 (0.89) | −0.39 | 0.62 | (−1.99, 1.19) |
| Whole grains (0–10) | 4.01 (0.75) | 2.24 (0.76) | −1.77 (1.00) | 5.39 (1.36) | 3.37 (1.15) | −2.01 (1.84) | −1.34 | 0.28 | (−3.80, 1.11) |
| Dairy (0–10) | 5.39 (0.56) | 5.26 (0.65) | −0.14 (0.83) | 4.82 (0.97) | 4.43 (1.04) | −0.39 (1.22) | 0.74 | 0.51 | (−1.43, 2.89) |
| Total protein foods (0–5) | 4.49 (0.19) | 6.76 (0.58) | 2.26 (0.63) | 4.68 (0.15) | 6.37 (0.94) | 1.70 (0.93) | 0.57 | 0.57 | (−1.41, 2.54) |
| Seafood and plant proteins (0–5) | 2.94 (0.43) | 4.72 (0.86) | 1.78 (0.96) | 2.5 (0.75) | 5.27 (1.40) | 2.77 (1.27) | −0.75 | 0.57 | (−3.36, 1.85) |
| Fatty acids (0–10) | 7.70 (0.40) | 6.81 (0.39) | −0.89 (0.48) | 7.76 (0.81) | 7.94 (0.56) | 0.18 (1.12) | −1.53 | 0.02 | (−2.83, −0.23) |
| Moderation | |||||||||
| Refined grains (0–10) | 7.86 (0.53) | 5.21 (0.74) | −2.65 (0.84) | 8.79 (0.45) | 6.83 (0.99) | −1.96 (1.13) | −1.39 | 0.25 | (−3.79, 1.00) |
| Sodium (0–10) | 7.38 (0.52) | 7.30 (0.60) | −0.08 (0.66) | 6.94 (1.21) | 5.50 (1.37) | −1.44 (1.21) | 1.85 | 0.08 | (−0.23, 3.95) |
| Empty calories (0–20) | 8.40 (0.60) | 7.66 (0.77) | −0.74 (1.01) | 9.41 (0.53) | 9.43 (0.44) | 0.02 (0.73) | −2.54 | 0.03 | (−4.87, −0.22) |
| Total Energy (kcal/day) | 2031.79 (108.38) | 1885.56 (138.77) | −446.23 (167.54) | 1820.91 (184.16) | 2014.68 (224.83) | 193.78 (197.18) | −485.85 | 0.04 | (−935.94, −15.76) |
| Anthropometrics and Biometrics c,d | |||||||||
| BMI, mean (SE) (kg/m2) (n = 94) | 31.66 (1.30) | 30.85 (1.29) | −0.81 (0.24) | 34.39 (2.10) | 34.74 (2.15) | 0.35 (0.31) | −1.40 | <0.001 | (−2.17, −0.64) |
| Waist Circumference, mean (SE) (cm) (n = 78) g | 99.69 (2.83) | 99.47 (2.95) | −0.22 (0.88) | 104.85 (4.06) | 104.11 (4.25) | −0.73 (0.86) | 0.08 | 0.95 | (−2.28, 2.44) |
| Systolic Blood Pressure, mean (SE) (mmHg) (n = 94) | 127.69 (2.07) | 125.14 (2.26) | −2.55 (2.24) | 129.41 (3.05) | 130.84 (3.41) | 1.43 (2.83) | −6.28 | 0.04 | (−12.34, −0.21) |
| Diastolic Blood Pressure, mean (SE) (mmHg) (n = 94) | 80.88 (1.42) | 80.15 (1.91) | −0.73 (1.83) | 83.90 (2.58) | 83.05 (2.84) | −0.85 (2.39) | −1.36 | 0.64 | (−7.15, 4.42) |
| Personal Determinantsc, mean (SE) (n = 97) | |||||||||
| Self-Efficacy h | 94.70 (1.82) | 103.61 (2.06) | 8.91 (2.15) | 96.45 (3.20) | 97.26 (2.73) | 0.81 (2.89) | 6.27 | 0.03 | (0.70, 11.83) |
| Menu Planning i | 24.39 (0.44) | 26.36 (0.60) | 1.97 (0.58) | 24.77 (0.63) | 24.60 (0.86) | −0.17 (0.76) | 1.73 | 0.06 | (−0.09, 3.55) |
| Dietary Intake a,b,c, Mean (SE) | |||||||
|---|---|---|---|---|---|---|---|
| Outcomes | T0 (n = 54) | Δ T0 to T1 (n = 30) | p-Value i | Δ T1 to T2 (n = 20) | p-Value i | Δ T0 to T2 (n = 22) | p-Value i |
| HEI d Total Score e (100 maximum) | 56.11 (1.61) | −3.81 (3.23) | 0.25 | 2.74 (4.41) | 0.55 | −0.20 (3.11) | 0.95 |
| HEI Component Scores | |||||||
| Adequacy | 1.66 (0.24) | −0.46 (0.45) | 0.32 | 0.66 (0.62) | 0.30 | 0.41 (0.47) | 0.39 |
| Total fruits (0–5) | 2.16 (0.29) | −0.89 (0.45) | 0.06 | 0.28 (0.67) | 0.68 | 0.57 (0.90) | 0.54 |
| Whole fruits (0–5) | 3.15 (0.22) | 0.38 (0.38) | 0.32 | 0.55 (0.54) | 0.33 | 0.70 (0.45) | 0.87 |
| Total vegetables (0–5) | 2.69 (0.31) | −0.88 (0.54) | 0.11 | 1.10 (0.80) | 0.18 | −0.10 (0.66) | 0.89 |
| Greens and beans (0–5) | 4.06 (0.58) | −1.87 (0.94) | 0.06 | 1.35 (1.34) | 0.33 | −0.26 (1.34) | 0.85 |
| Whole grains (0–10) | 5.26 (0.43) | 0.02 (0.80) | 0.98 | −0.65 (0.84) | 0.45 | −1.11 (1.13) | 0.34 |
| Dairy (0–10) | 4.32 (0.18) | 2.22 (0.59) | <0.001 | −1.67 (0.70) | 0.03 | 0.56 (0.61) | 0.37 |
| Total protein foods (0–5) | 2.22 (0.32) | 1.66 (0.93) | 0.08 | −2.02 (0.88) | 0.03 | 0.09 (0.60) | 0.88 |
| Seafood and plant proteins (0–5) | 7.26 (0.31) | −0.77 (0.46) | 0.11 | 0.98 (0.61) | 0.12 | 0.34 (0.76) | 0.66 |
| Fatty acids (0–10) | 1.66 (0.24) | −0.46 (0.45) | 0.32 | 0.66 (0.62) | 0.30 | 0.41 (0.47) | 0.39 |
| Moderation | |||||||
| Refined grains (0–10) | 7.14 (0.47) | −2.85 (0.80) | <0.01 | 0.59 (1.46) | 0.69 | −2.08 (1.32) | 0.13 |
| Sodium (0–10) | 7.14 (0.42) | 0.04 (0.63) | 0.95 | −0.39 (0.74) | 0.60 | 0.10 (0.82) | 0.90 |
| Empty calories (0–20) | 8.57 (0.41) | −0.69 (0.95) | 0.47 | 2.40 (0.92) | 0.02 | 1.21 (1.05) | 0.26 |
| Total Energy (kcal/day) | 1878.31 (97.93) | −445.65 (156.29) | <0.01 | −83.13 (235.33) | 0.73 | −289.36 (142.30) | 0.05 |
| Anthropometrics and Biometrics, mean (SE) | |||||||
| Outcomes | T0 | Δ T0 to T1 | p-value h | Δ T1 to T2 | p-value h | Δ T0 to T2 | p-value h |
| BMI (kg/m2) (n = 63) | 31.66 (1.30) | −0.81 (0.24) | <0.01 | −0.04 (0.40) | 0.82 | −0.85 (0.45) | 0.07 |
| Waist Circumference f (cm) (n = 48) | 99.69 (2.83) | −0.22 (0.88) | 0.80 | −0.43 (1.55) | 0.79 | −0.65 (1.45) | 0.66 |
| Systolic Blood Pressure (mmHg) (n = 63) | 127.70 (2.07) | −2.55 (2.24) | 0.26 | 2.18 (3.10) | 0.49 | −0.37 (3.20) | 0.91 |
| Diastolic Blood Pressure (mmHg) (n = 63) | 80.88 (1.42) | −0.73 (1.83) | 0.69 | −1.85 (3.00) | 0.55 | −2.57 (3.02) | 0.40 |
| Personal Determinants, mean (SE) (n = 66) | |||||||
| Outcomes | T0 | Δ T0 to T1 | p-value h | Δ T1 to T2 | p-value h | Δ T0 to T2 | p-value h |
| Self-Efficacy g | 94.70 (1.82) | 8.91 (2.15) | <0.001 | 0.31 (1.93) | 0.87 | 9.22 (2.26) | <0.001 |
| Menu Planning h | 24.39 (0.44) | 1.97 (0.58) | 0.001 | 0.33 (0.47) | 0.49 | 2.31 (0.58) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopkins, L.C.; Holloman, C.; Webster, A.; Labyk, A.N.; Penicka, C.; May, L.; Sharn, A.; Gupta, S.; Schier, H.; Kennel, J.; et al. Caregiver Nutritional Health Outcomes of the Simple Suppers Study: Results from a 10 Week, Two-Group Quasi-Experimental Family Meals Intervention. Nutrients 2022, 14, 250. https://doi.org/10.3390/nu14020250
Hopkins LC, Holloman C, Webster A, Labyk AN, Penicka C, May L, Sharn A, Gupta S, Schier H, Kennel J, et al. Caregiver Nutritional Health Outcomes of the Simple Suppers Study: Results from a 10 Week, Two-Group Quasi-Experimental Family Meals Intervention. Nutrients. 2022; 14(2):250. https://doi.org/10.3390/nu14020250
Chicago/Turabian StyleHopkins, Laura C., Christopher Holloman, Alison Webster, Allison N. Labyk, Christine Penicka, Leah May, Amy Sharn, Shivani Gupta, Heather Schier, Julie Kennel, and et al. 2022. "Caregiver Nutritional Health Outcomes of the Simple Suppers Study: Results from a 10 Week, Two-Group Quasi-Experimental Family Meals Intervention" Nutrients 14, no. 2: 250. https://doi.org/10.3390/nu14020250
APA StyleHopkins, L. C., Holloman, C., Webster, A., Labyk, A. N., Penicka, C., May, L., Sharn, A., Gupta, S., Schier, H., Kennel, J., & Gunther, C. (2022). Caregiver Nutritional Health Outcomes of the Simple Suppers Study: Results from a 10 Week, Two-Group Quasi-Experimental Family Meals Intervention. Nutrients, 14(2), 250. https://doi.org/10.3390/nu14020250





