An Earlier First Meal Timing Associates with Weight Loss Effectiveness in A 12-Week Weight Loss Support Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Physical Measurement
2.4. Physical Activity
2.5. Circadian Timing of Daily Behaviors and Dietary Intake
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oishi, K.; Shirai, H.; Ishida, N. CLOCK is involved in the circadian transactivation of peroxisome- proliferator-activated receptor α (PPARα) in mice. Biochem. J. 2005, 386, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [Green Version]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Martha, H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froy, O. Metabolism and circadian rhythms—Implications for obesity. Endocr. Rev. 2010, 31, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Thomas, E.A.; Zaman, A.; Cornier, M.A.; Catenacci, V.A.; Tussey, E.J.; Grau, L.; Arbet, J.; Broussard, J.L.; Rynders, C.A. Later meal and sleep timing predicts higher percent body fat. Nutrients 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Timing of breakfast, lunch, and dinner. Effects on obesity and metabolic risk. Nutrients 2019, 11, 2624. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Feng, W.; Wang, F.; Li, P.; Li, Z.; Li, M.; Tse, G.; Vlaanderen, J.; Vermeulen, R.; Tse, L.A. Meta-analysis on shift work and risks of specific obesity types. Obes. Rev. 2018, 19, 28–40. [Google Scholar] [CrossRef]
- Gluck, E.M.; Venti, C.A.; Salbe, A.D.; Krakoff, J. Nighttime eating: Commonly observed and related to weight gain in an inpatient food intake study. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, J.; Eguchi, E.; Nagaoka, K.; Ito, T.; Ogino, K. Association of night eating habits with metabolic syndrome and its components: A longitudinal study. BMC Public Health 2018, 18, 1366. [Google Scholar] [CrossRef]
- Dashti, H.S.; Gómez-Abellán, P.; Qian, J.; Esteban, A.; Morales, E.; Scheer, F.A.J.L.; Garaulet, M. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am. J. Clin. Nutr. 2021, 113, 154–161. [Google Scholar] [CrossRef]
- Allison, K.C.; Hopkins, C.M.; Ruggieri, M.; Spaeth, A.M.; Ahima, R.S.; Zhang, Z.; Taylor, D.M.; Goel, N. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr. Biol. 2021, 31, 650–657.e3. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, P.M.; Purificación Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Yu-Chi Lee, P.; Ordovás, P.J.M.; Scheer, P.F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Lozano, T.; Vidal, J.; de Hollanda, A.; Scheer, F.A.J.L.; Garaulet, M.; Izquierdo-Pulido, M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin. Nutr. 2016, 35, 1308–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allaf, M.; Elghazaly, H.; Mohamed, O.G.; Fareen, M.F.K.; Zaman, S.; Salmasi, A.M.; Tsilidis, K.; Dehghan, A. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2021, 1, CD013496. [Google Scholar] [CrossRef]
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Zhao, L.; Turner-Mcgrievy, G.M.; Ortaglia, A. Associations between fasting duration, timing of first and last meal, and cardiometabolic endpoints in the national health and nutrition examination survey. Nutrients 2021, 13, 2686. [Google Scholar] [CrossRef]
- Michiwaki, R.; Tajiri, E.; Hatamoto, Y.; Matsumoto, N.; Tanaka, S.; Yoshimura, E. Effects of using a smartphone application for step counting on weight loss and physical activity while supporting weight loss. J. Jpn. Soc. Study Obes. 2021, 27, 90–98. (In Japanese) [Google Scholar]
- Ohkawara, K.; Oshima, Y.; Hikihara, Y.; Ishikawa-Takata, K.; Tabata, I.; Tanaka, S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br. J. Nutr. 2011, 105, 1681–1691. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Camhi, S.M.; Troiano, R.P. A catalog of rules, variables, and definitions applied to accelerometer data in the national health and nutrition examination Survey, 2003–2006. Prev. Chronic Dis. 2012, 9, E113. [Google Scholar] [CrossRef] [Green Version]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Amagasa, S.; Inoue, S.; Murayama, H.; Fujiwara, T.; Kikuchi, H.; Fukushima, N.; MacHida, M.; Chastin, S.; Owen, N.; Shobugawa, Y. Changes in rural older adults’ sedentary and physically-active behaviors between a non-snowfall and a snowfall season: Compositional analysis from the NEIGE study. BMC Public Health 2020, 20, 1248. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Satoshi Sasaki, M.K.K. Validation of Self-Administered Dietary Assessment Questionnaires Developed for Japanese Subjects: Systematic Review. J. Community Nutr. 2003, 5, 83–92. [Google Scholar]
- Sasaki, S.; Yanagibori, R.; Amano, K. Self-administered diet history questionnaire developed for health education: A relative validation of the test-version by comparison with 3-day diet record in women. J. Epidemiol. 1998, 8, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Jacob, R.; Tremblay, A.; Panahi, S.; Provencher, V.; Drapeau, V. Is the timing of food intake a potential indicator of low weight loss responders? A secondary analysis of three weight loss studies. Clin. Obes. 2020, 10, e12360. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef]
- Kahleova, H.; Lloren, J.I.; Mashchak, A.; Hill, M.; Fraser, G.E. Meal frequency and timing are associatedwith changes in body mass index in Adventist Health Study 2. J. Nutr. 2017, 147, 1722–1728. [Google Scholar] [CrossRef] [Green Version]
- Raynor, H.A.; Li, F.; Cardoso, C. Daily pattern of energy distribution and weight loss. Physiol. Behav. 2018, 192, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Schembre, S.M.; Wen, C.K.; Davis, J.N.; Shen, E.; Nguyen-Rodriguez, S.T.; Belcher, B.R.; Hsu, Y.W.; Weigensberg, M.J.; Goran, M.I.; Spruijt-Metz, D. Eating breakfast more frequently is cross-sectionally associated with greater physical activity and lower levels of adiposity in overweight Latina and African American girls. Am. J. Clin. Nutr. 2013, 98, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, E.A.; Richardson, J.D.; Holman, G.D.; Tsintzas, K.; Thompson, D.; Betts, J.A. The causal role of breakfast in energy balance and health: A randomized controlled trial in obese adults. Am. J. Clin. Nutr. 2014, 100, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, E.; Hatamoto, Y.; Yonekura, S.; Tanaka, H. Skipping breakfast reduces energy intake and physical activity in healthy women who are habitual breakfast eaters: A randomized crossover trial. Physiol. Behav. 2017, 174, 89–94. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Phillips, A.J.K.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef]
- Morris, C.J.; Garcia, J.I.; Myers, S.; Yang, J.N.; Trienekens, N.; Scheer, F.A.J.L. The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity 2015, 23, 2053–2058. [Google Scholar] [CrossRef] [Green Version]
- Bo, S.; Fadda, M.; Castiglione, A.; Ciccone, G.; De Francesco, A.; Fedele, D.; Guggino, A.; Parasiliti Caprino, M.; Ferrara, S.; Vezio Boggio, M.; et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int. J. Obes. 2015, 39, 1689–1695. [Google Scholar] [CrossRef] [Green Version]
- Romon, M.; Boulenguez, C.; Frimat, P. Circadian variation of diet-induced thermogenesis. Am. J. Clin. Nutr. 1993, 57, 476–480. [Google Scholar] [CrossRef]
- Kelly, K.P.; McGuinness, O.P.; Buchowski, M.; Hughey, J.J.; Chen, H.; Powers, J.; Page, T.; Johnson, C.H. Eating breakfast and avoiding late-evening snacking sustains lipid oxidation. PLoS Biol. 2020, 18, e3000622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Health Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Shubhroz Gill, S.P. A Smartphone App Reveals erratic diurnal eating patterns in humans. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]


| Total (n = 97) | Men (n = 51) | Women (n = 46) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | ES | Pre | Post | ES | Pre | Post | ES | |
| Age (years) | 47.2 ± 8.4 | - | - | 47.6 ± 8.7 | - | - | 46.9 ± 8.2 | - | - |
| Height (cm) | 165.5 ± 7.9 | - | - | 171.7 ± 3.8 | - | - | 158.6 ± 5.1 | - | - |
| Body weight (kg) | 70 ± 12.9 | 67.9 ± 12.8 ** | 0.163 | 77.6 ± 11.6 | 75 ± 11.8 ** | 0.222 | 61.5 ± 8.3 | 59.9 ± 8.3 ** | 0.193 |
| BMI (kg/m2) | 25.4 ± 3.7 | 24.7 ± 3.7 ** | 0.189 | 26.3 ± 3.7 | 25.4 ± 3.8 ** | 0.240 | 24.5 ± 3.4 | 23.8 ± 3.5 ** | 0.203 |
| Body weight change (kg) | - | −2.1 ± 2 | - | - | −2.6 ± 2.1 | - | - | −1.6 ± 1.7 | - |
| Body weight change rate (%) | - | −3.0 ± 2.7 | - | - | −3.4 ± 2.7 | - | - | −2.5 ± 2.6 | - |
| Dietary intake (BDHQ) | |||||||||
| Total energy intake (kcal/day) | 1900 ± 568 | 1732 ± 550 ** | 0.300 | 2122 ± 574 | 1910 ± 604 * | 0.360 | 1654 ± 452 | 1533 ± 404 * | 0.282 |
| Total energy intake change (kcal/day) | - | −169 ± 442 | - | - | −211 ± 553 | - | - | −121 ± 269 | - |
| Protein (%) | 15.1 ± 2.6 | 16 ± 3.1 * | 0.315 | 14.7 ± 2.7 | 15.6 ± 3.5 * | 0.288 | 15.6 ± 2.6 | 16.4 ± 2.6 * | 0.308 |
| Fat (%) | 28.1 ± 5.4 | 29.1 ± 6 | 0.175 | 26.1 ± 5.5 | 27.3 ± 6.5 | 0.199 | 30.3 ± 4.2 | 31.1 ± 4.6 | 0.182 |
| Carbohydrate (%) | 50.5 ± 7.3 | 48.5 ± 7.7 * | 0.267 | 50.3 ± 8.1 | 47.8 ± 8.8 | 0.296 | 50.7 ± 6.4 | 49.3 ± 6.2 | 0.222 |
| Physical activity | |||||||||
| Wearing time (min/day) | 943 ± 105 | 917 ± 111 * | 0.241 | 956.6 ± 116.8 | 908.6 ± 114.4 ** | 0.415 | 928.9 ± 88.3 | 927.1 ± 106.7 | 0.018 |
| Step counts (steps/day) | 7633 ± 3051 | 7876 ± 3234 | 0.045 | 8398 ± 3579 | 8823 ± 3729 | 0.055 | 6786 ± 2056 | 6827 ± 2172 | 0.011 |
| ≤1.5 METs (min/day) | 590 ± 104 | 575 ± 94 | 0.013 | 620 ± 112 | 584 ± 93 | 0.013 | 557 ± 83 | 564 ± 95 | 0.055 |
| 1.6–2.9 METs (min/day) | 295 ± 72 | 284 ± 74 | 0.022 | 276 ± 67 | 262 ± 68 | 0.007 | 317 ± 73 | 309 ± 74 | 0.055 |
| 3.0 METs ≤ (min/day) | 58 ± 24 | 58 ± 24 | 0.022 | 61 ± 28 | 63 ± 28 | 0.045 | 54 ± 19 | 53 ± 19 | 0.032 |
| ≤1.5 METs (%) | 62.3 ± 7.4 | 62.5 ± 7.4 | 0.027 | 64.4 ± 7 | 64.1 ± 6.6 | 0.044 | 60 ± 7.3 | 60.7 ± 7.9 | 0.092 |
| 1.6–2.9 METs (%) | 31.5 ± 6.9 | 31.1 ± 7 | 0.058 | 29.1 ± 6.4 | 28.9 ± 6.3 | 0.031 | 34.1 ± 6.6 | 33.5 ± 7.1 | 0.088 |
| 3.0 METs ≤ (%) | 6.2 ± 2.7 | 6.4 ± 2.7 | 0.074 | 6.5 ± 3.1 | 7 ± 3.2 | 0.159 | 5.9 ± 2.1 | 5.8 ± 1.9 | 0.050 |
| Step counts (steps/h wear time) | 492 ± 206 | 520 ± 221 * | 0.131 | 536 ± 242 | 589 ± 260 * | 0.211 | 443 ± 145 | 443 ± 134 | 0.000 |
| ≤1.5 METs (min/h wear time) | 37.5 ± 4.5 | 37.6 ± 4.5 | 0.022 | 38.8 ± 4.2 | 38.6 ± 4 | 0.049 | 36 ± 4.4 | 36.6 ± 4.7 | 0.132 |
| 1.6–2.9 METs (min/h wear time) | 18.8 ± 4.2 | 18.5 ± 4.2 | 0.071 | 17.4 ± 3.9 | 17.2 ± 3.8 | 0.052 | 20.4 ± 4 | 20 ± 4.2 | 0.098 |
| 3.0 METs ≤ (min/h wear time) | 3.7 ± 1.6 | 3.8 ± 1.6 | 0.063 | 3.9 ± 1.9 | 4.2 ± 1.9 | 0.158 | 3.5 ± 1.3 | 3.4 ± 1.1 | 0.083 |
| Early Group (n = 47, Men: 25/Women: 22) | Late Group (n = 50, Men: 26/Women: 24) | Early Group vs. Late Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | |||||||
| Pre | Post | ES | Pre | Post | ES | ES | ES | |
| Start of eating window (h:mm) | 6:48 ± 0:22 | 7:00 ± 0:30 * | 0.429 | 8:09 ± 1:05 †† | 8:19 ± 1:27 †† | 0.127 | 1.649 | 1.214 |
| Age (years) | 48.8 ± 6.6 | - | - | 45.8 ± 9.7 | - | - | 0.362 | - |
| Height (cm) | 165.4 ± 7.1 | - | - | 165.5 ± 8.7 | - | - | 0.013 | - |
| Body weight (kg) | 69.3 ± 12.1 | 66.7 ± 11.6 ** | 0.219 | 70.6 ± 13.7 | 68.9 ± 13.8 ** | 0.124 | 0.101 | 0.173 |
| BMI (kg/m2) | 25.2 ± 3.6 | 24.3 ± 3.4 ** | 0.257 | 25.6 ± 3.8 | 25.0 ± 4.0 ** | 0.154 | 0.108 | 0.189 |
| Body weight change (kg) | - | −2.6 ± 2.0 | - | - | −1.6 ± 1.9 † | - | - | 0.513 |
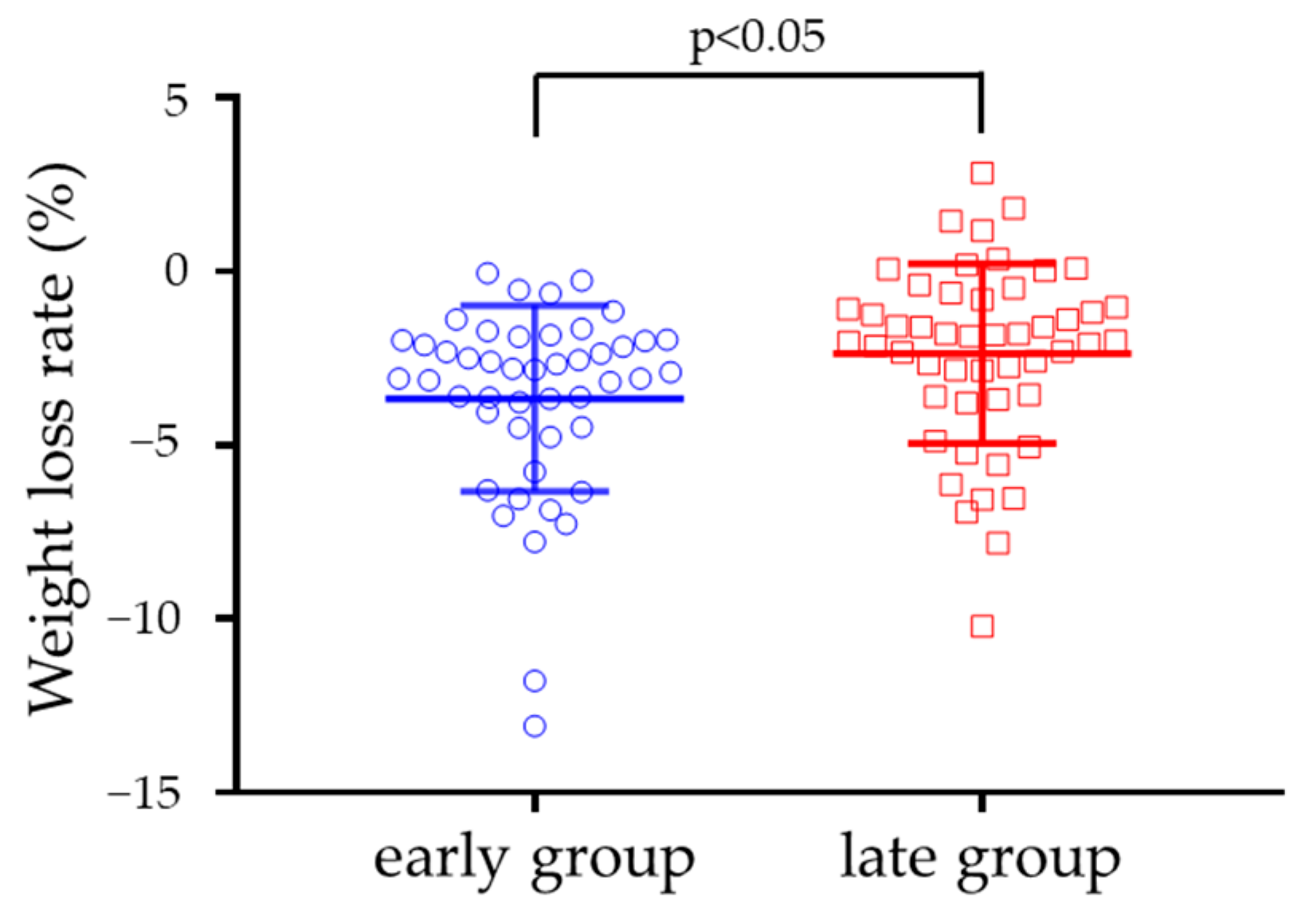
| Body weight change (%) | - | −3.7 ± 2.7 | - | - | −2.4 ± 2.6 † | - | - | 0.490 |
| Meal and sleep timing | ||||||||
| Wake time (h:mm) | 5:54 ± 0:33 | 6:05 ± 0:36 ** | 0.306 | 6:40 ± 0:42 †† | 6:46 ± 0:50 †† | 0.124 | 1.212 | 0.937 |
| Bedtime (h:mm) | 23:17 ± 0:55 | 23:15 ± 0:56 | 0.030 | 23:49 ± 1:00 † | 23:57 ± 0:55 †† | 0.135 | 0.549 | 0.744 |
| Sleep duration (min) | 396 ± 50 | 409 ± 49 * | 0.263 | 410 ± 50 | 408 ± 43 | 0.053 | 0.281 | 0.031 |
| Midpoint of sleep (h:mm) | 26:35 ± 0:38 | 26:40 ± 0:40 | 0.121 | 27:15 ± 0:46 †† | 27:22 ± 0:48 * †† | 0.143 | 0.919 | 0.927 |
| End of eating window (h:mm) | 19:27 ± 1:14 | 19:22 ± 1:00 | 0.075 | 19:48 ± 1:05 | 19:37 ± 1:05 * | 0.168 | 0.306 | 0.247 |
| Midpoint of eating window (h:mm) | 13:23 ± 0:50 | 13:11 ± 0:36 * | 0.276 | 13:47 ± 0:55 † | 13:59 ± 1:01 †† | 0.194 | 0.467 | 0.944 |
| Time elapsed between sleep offset and start of eating window (min) | 57 ± 25 | 56 ± 26 | 0.039 | 91 ± 62 † | 93 ± 78 † | 0.028 | 0.719 | 0.636 |
| Time elapsed between end of eating window and sleep onset (min) | 231 ± 71 | 231 ± 61 | 0.000 | 240 ± 59 | 259 ± 60 * † | 0.319 | 0.138 | 0.463 |
| Fasting duration (min) | 681 ± 65 | 696 ± 60* | 0.240 | 741 ± 86 †† | 759 ± 92 * †† | 0.202 | 0.787 | 0.811 |
| Dietary intake | ||||||||
| Total energy intake (kcal/day) | 1818 ± 542 | 1622 ± 390 * | 0.415 | 1977 ± 586 | 1834 ± 654 * | 0.230 | 0.282 | 0.394 |
| Total energy intake change (kcal/day) | - | −196 ± 389 | - | - | −143 ± 489 | - | - | 0.120 |
| Protein (%) | 15.4 ± 2.4 | 16.1 ± 3.3 | 0.243 | 14.9 ± 2.9 | 15.9 ± 3.0 * | 0.339 | 0.188 | 0.063 |
| Fat (%) | 28.2 ± 5.4 | 28.0 ± 6.2 | 0.034 | 28.0 ± 5.4 | 30.2 ± 5.6 * | 0.400 | 0.037 | 0.372 |
| Carbohydrate (%) | 50.5 ± 7.6 | 49.5 ± 8.5 | 0.124 | 50.5 ± 7.1 | 47.6 ± 6.8 * | 0.417 | 0.000 | 0.128 |
| Physical activity | ||||||||
| Wearing time (min/day) | 987 ± 105 | 955 ± 112 * | 0.295 | 903 ± 88 †† | 882 ± 98 * † | 0.225 | 0.867 | 0.200 |
| Step counts (steps/day) | 7607 ± 2749 | 8233 ± 2873 | 0.132 | 7658 ± 3337 | 7541 ± 3536 | 0.032 | 0.000 | 0.084 |
| ≤1.5 METs (min/day) | 603 ± 116 | 579 ± 101 | 0.007 | 578 ± 91 † | 570 ± 88 † | 0.063 | 0.279 | 0.291 |
| 1.6–2.9 METs (min/day) | 324 ± 73 | 313 ± 69 | 0.032 | 269 ± 61 † | 257 ± 69 † | 0.045 | 0.281 | 0.261 |
| 3.0 METs ≤ (min/day) | 59 ± 24 | 62 ± 23 | 0.095 | 56 ± 25 | 54 ± 26 | 0.045 | 0.045 | 0.115 |
| ≤1.5 METs (%) | 60.8 ± 8.2 | 60.4 ± 7.2 | 0.052 | 63.7 ± 6.4 | 64.4 ± 7.1 † | 0.104 | 0.394 | 0.559 |
| 1.6–2.9 METs (%) | 33.1 ± 7.3 | 33.0 ± 6.4 | 0.015 | 29.9 ± 6.3 † | 29.3 ± 7.1 † | 0.089 | 0.469 | 1.248 |
| 3.0 METs ≤ (%) | 6.1 ± 2.5 | 6.6 ± 2.4 | 0.204 | 6.4 ± 2.9 | 6.3 ± 3.1 | 0.033 | 0.111 | 0.108 |
| Step counts (steps/h wear time) | 467 ± 173 | 520 ± 188 * | 0.293 | 515 ± 232 | 520 ± 250 | 0.021 | 0.235 | 0.000 |
| ≤1.5 METs (min/h wear time) | 36.6 ± 4.9 | 36.4 ± 4.4 | 0.043 | 38.3 ± 3.9 | 38.8 ± 4.3 † | 0.122 | 0.384 | 0.552 |
| 1.6–2.9 METs (min/h wear time) | 19.8 ± 4.4 | 19.7 ± 3.9 | 0.024 | 17.9 ± 3.8 † | 17.4 ± 4.2 † | 0.125 | 0.462 | 0.568 |
| 3.0 METs ≤ (min/h wear time) | 3.6 ± 1.5 | 3.9 ± 1.4 | 0.207 | 3.8 ± 1.7 | 3.7 ± 1.8 | 0.057 | 0.125 | 0.124 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatanaka, M.; Hatamoto, Y.; Tajiri, E.; Matsumoto, N.; Tanaka, S.; Yoshimura, E. An Earlier First Meal Timing Associates with Weight Loss Effectiveness in A 12-Week Weight Loss Support Program. Nutrients 2022, 14, 249. https://doi.org/10.3390/nu14020249
Hatanaka M, Hatamoto Y, Tajiri E, Matsumoto N, Tanaka S, Yoshimura E. An Earlier First Meal Timing Associates with Weight Loss Effectiveness in A 12-Week Weight Loss Support Program. Nutrients. 2022; 14(2):249. https://doi.org/10.3390/nu14020249
Chicago/Turabian StyleHatanaka, Mana, Yoichi Hatamoto, Eri Tajiri, Naoyuki Matsumoto, Shigeho Tanaka, and Eiichi Yoshimura. 2022. "An Earlier First Meal Timing Associates with Weight Loss Effectiveness in A 12-Week Weight Loss Support Program" Nutrients 14, no. 2: 249. https://doi.org/10.3390/nu14020249
APA StyleHatanaka, M., Hatamoto, Y., Tajiri, E., Matsumoto, N., Tanaka, S., & Yoshimura, E. (2022). An Earlier First Meal Timing Associates with Weight Loss Effectiveness in A 12-Week Weight Loss Support Program. Nutrients, 14(2), 249. https://doi.org/10.3390/nu14020249





