The Applicability of the ESPEN and EASO-Defined Diagnostic Criteria for Sarcopenic Obesity in Japanese Patients after Stroke: Prevalence and Association with Outcomes
Abstract
1. Introduction
2. Methods
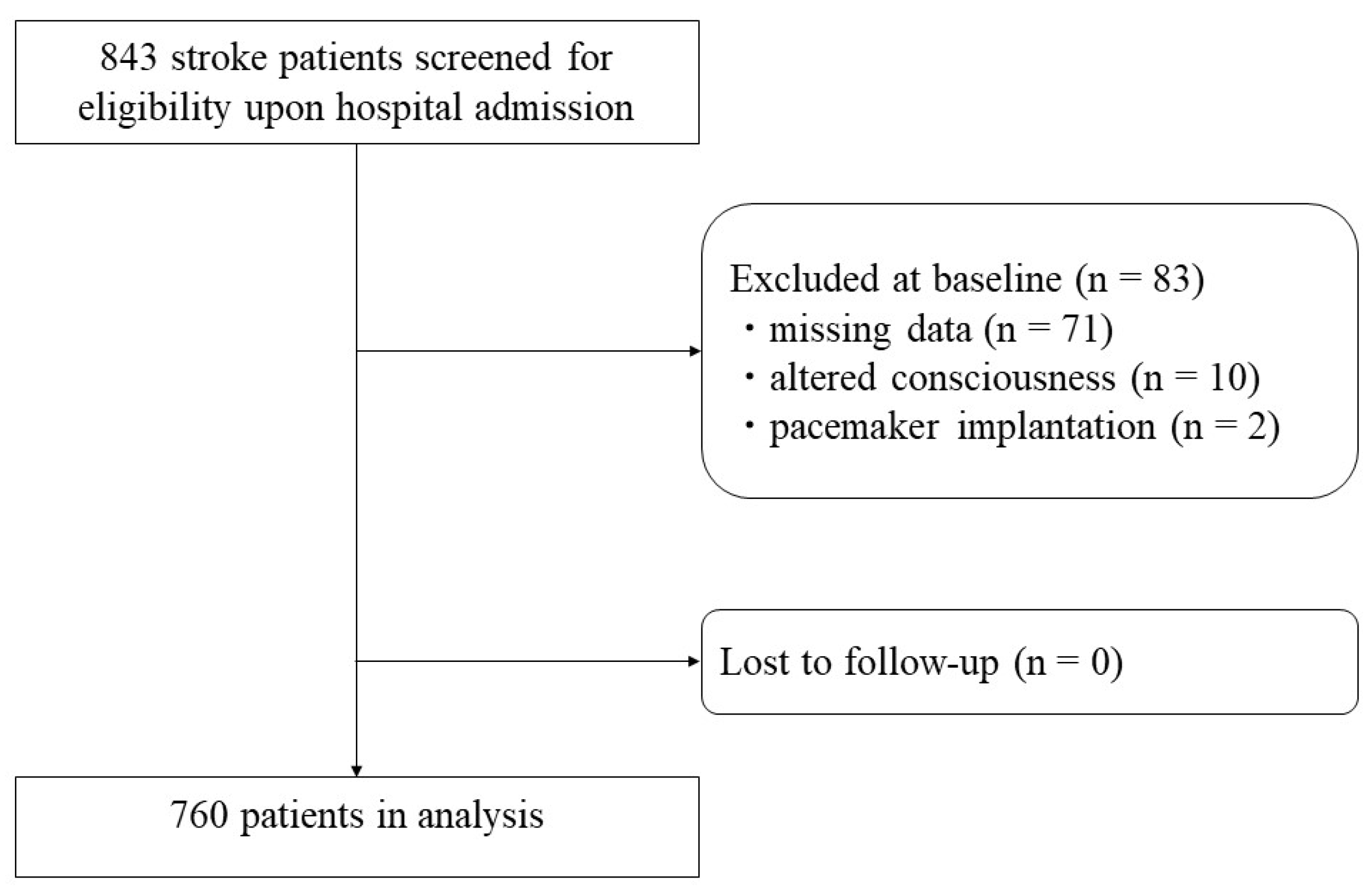
2.1. Participants and Setting
2.2. Convalescent Rehabilitation Program
2.3. Data Collection
2.4. Diagnosis of Sarcopenic Obesity
2.4.1. Screening
2.4.2. Diagnosis
2.4.3. Staging
2.5. Outcomes
2.6. Sample Size Calculation
2.7. Statistical Analysis
2.8. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barazzoni, R.; Bischoff, S.C.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic Obesity: Time to Meet the Challenge. Clin. Nutr. 2018, 37, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic Obesity Predicts Instrumental Activities of Daily Living Disability in the Elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.C.; Chen, W.L.; Chen, Y.Y.; Chao, Y.P.; Wu, L.W.; Kao, T.W. Associations between Different Measurements of Sarcopenic Obesity and Health Outcomes among Non-Frail Community-Dwelling Older Adults in Taiwan. Br. J. Nutr. 2021, 126, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Wannamathee, S.G. Sarcopenic Obesity in Ageing: Cardiovascular Outcomes and Mortality. Br. J. Nutr. 2020, 124, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Bise, T.; Shimazu, S.; Kudo, M.; Shiraishi, A. Sarcopenic Obesity Is Associated with Activities of Daily Living and Home Discharge in Post-Acute Rehabilitation. J. Am. Med. Dir. Assoc. 2020, 21, 1475–1480. [Google Scholar] [CrossRef]
- Altman, K.W.; Yu, G.P.; Schaefer, S.D. Consequence of Dysphagia in the Hospitalized Patient: Impact on Prognosis and Hospital Resources. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.Q.C.; Prvubettger, J.; Guerrier, T.; Hirsch, M.A.; Thomas, J.G.; Pugh, T.M.; Rhoads, C.F. Factors Associated with Discharge to Home versus Discharge to Institutional Care after Inpatient Stroke Rehabilitation. Arch. Phys. Med. Rehabil. 2015, 96, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Everink, I.H.J.; van Haastregt, J.C.M.; van Hoof, S.J.M.; Schols, J.M.G.A.; Kempen, G.I.J.M. Factors Influencing Home Discharge after Inpatient Rehabilitation of Older Patients: A Systematic Review Health Services Research. BMC Geriatr. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Setia Ningrum, D.A.; Abdul-Mumin, K.H.; Yi-Li, C. Assessing Quality of Life and Associated Factors in Post-Stroke Patients Using the World Health Organization Abbreviated Generic Quality of Life Questionnaire (WHOQOL-BREF). Clin. Epidemiol. Glob. Health 2022, 13, 100941. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Tanoue, M. Prevalence of Sarcopenia and Its Association with Activities of Daily Living and Dysphagia in Convalescent Rehabilitation Ward Inpatients. Clin. Nutr. 2018, 37, 2022–2028. [Google Scholar] [CrossRef]
- Kido, Y.; Yoshimura, Y.; Wakabayashi, H.; Momosaki, R.; Nagano, F.; Bise, T.; Shimazu, S.; Shiraishi, A. Sarcopenia Is Associated with Incontinence and Recovery of Independence in Urination and Defecation in Post-Acute Rehabilitation Patients. Nutrition 2021, 91–92, 111397. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Sarcopenia Is Associated with Worse Recovery of Physical Function and Dysphagia and a Lower Rate of Home Discharge in Japanese Hospitalized Adults Undergoing Convalescent Rehabilitation. Nutrition 2019, 61, 111–118. [Google Scholar] [CrossRef]
- De Sire, A.; Ferrillo, M.; Lippi, L.; Agostini, F.; de Sire, R.; Ferrara, P.E.; Raguso, G.; Riso, S.; Roccuzzo, A.; Ronconi, G.; et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients 2022, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Nishioka, S.; Taguchi, S.; Yamanouchi, A.; Nakashima, R.; Wakabayashi, H. Sarcopenic Obesity and Activities of Daily Living in Stroke Rehabilitation Patients: A Cross-Sectional Study. Healthcare 2020, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Wakabayashi, H.; Maeda, K.; Shamoto, H.; Taketani, Y.; Kayashita, J.; Momosaki, R. Body Mass Index and Recovery of Activities of Daily Living in Older Patients with Femoral Fracture: An Analysis of a National Inpatient Database in Japan. Arch. Gerontol. Geriatr. 2020, 87, 255. [Google Scholar] [CrossRef]
- Nishioka, S.; Wakabayashi, H.; Yoshida, T.; Mori, N.; Watanabe, R.; Nishioka, E. Obese Japanese Patients with Stroke Have Higher Functional Recovery in Convalescent Rehabilitation Wards: A Retrospective Cohort Study. J. Stroke Cereb. Dis. 2016, 25, 26–33. [Google Scholar] [CrossRef]
- Hsu, K.J.; de Liao, C.; Tsai, M.W.; Chen, C.N. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef]
- Petroni, M.L.; Caletti, M.T.; Grave, R.D.; Bazzocchi, A.; Aparisi Gómez, M.P.; Marchesini, G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients 2019, 11, 1302. [Google Scholar] [CrossRef]
- Binay Safer, V.; Geler Kulcu, D. Bioimpedance Analysis and Frailty. J. Am. Geriatr. Soc. 2015, 63, 1050. [Google Scholar] [CrossRef]
- Dietzel, R.; Reisshauer, A.; Jahr, S.; Calafiore, D.; Armbrecht, G. Body Composition in Lipoedema of the Legs Using Dual-Energy X-Ray Absorptiometry: A Case-Control Study. Br. J. Dermatol. 2015, 173, 594–596. [Google Scholar] [CrossRef]
- Batsis, J.A.; Barre, L.K.; Mackenzie, T.A.; Pratt, S.I.; Lopez-Jimenez, F.; Bartels, S.J. Variation in the Prevalence of Sarcopenia and Sarcopenic Obesity in Older Adults Associated with Different Research Definitions: Dual-Energy X-Ray Absorptiometry Data from the National Health and Nutrition Examination Survey 1999–2004. J. Am. Geriatr. Soc. 2013, 61, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Clin. Nutr. 2022, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Bise, T.; Shimazu, S.; Shiraishi, A.; Kido, Y.; Matsumoto, A. Chair-Stand Exercise Improves Sarcopenia in Rehabilitation Patients after Stroke. Nutrients 2022, 14, 461. [Google Scholar] [CrossRef]
- Shimazu, S.; Yoshimura, Y.; Kudo, M.; Nagano, F.; Bise, T.; Shiraishi, A.; Sunahara, M. Frequent and Personalized Nutritional Support Leads to Improved Nutritional Status, Activities of Daily Living, and Dysphagia after Stroke. Nutrition 2021, 83, 111091. [Google Scholar] [CrossRef]
- Shiraishi, A.; Wakabayashi, H.; Yoshimura, Y. Oral Management in Rehabilitation Medicine: Oral Frailty, Oral Sarcopenia, and Hospital-Associated Oral Problems. J. Nutr. Health Aging 2020, 24, 1094–1099. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Shiraishi, A.; Tsuji, Y.; Momosaki, R. Oral Management and the Role of Dental Hygienists in Convalescent Rehabilitation. Prog. Rehabil. Med. 2022, 7, 20220019. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, A.; Yoshimura, Y.; Wakabayashi, H.; Tsuji, Y.; Yamaga, M.; Koga, H. Hospital Dental Hygienist Intervention Improves Activities of Daily Living, Home Discharge and Mortality in Post-Acute Rehabilitation. Geriatr. Gerontol. Int. 2019, 19, 189–196. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Matsumoto, A.; Momosaki, R. Pharmacotherapy and the Role of Pharmacists in Rehabilitation Medicine. Prog. Rehabil. Med. 2022, 7, 20220025. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yoshimura, Y.; Wakabayashi, H.; Kose, E.; Nagano, F.; Bise, T.; Kido, Y.; Shimazu, S.; Shiraishi, A. Deprescribing Leads to Improved Energy Intake among Hospitalized Older Sarcopenic Adults with Polypharmacy after Stroke. Nutrients 2022, 14, 443. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yoshimura, Y.; Nagano, F.; Bise, T.; Kido, Y.; Shimazu, S.; Shiraishi, A. Polypharmacy and Potentially Inappropriate Medications in Stroke Rehabilitation: Prevalence and Association with Outcomes. Int. J. Clin. Pharm. 2022, 44, 749–761. [Google Scholar] [CrossRef]
- Banks, J.L.; Marotta, C.A. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials: A Literature Review and Synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Momosaki, R.; Nagano, F.; Shimazu, S.; Shiraishi, A. Shorter Interval between Onset and Admission to Convalescent Rehabilitation Wards Is Associated with Improved Outcomes in Ischemic Stroke Patients. Tohoku J. Exp. Med. 2020, 252, 15–22. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lin, G.H.; Huang, Y.J.; Song, C.Y.; Lee, Y.C.; How, M.J.; Chen, Y.M.; Hsueh, I.P.; Chen, M.H.; Hsieh, C.L. Improving the Utility of the Brunnstrom Recovery Stages in Patients with Stroke: Validation and Quantification. Medicine 2016, 95, e4508. [Google Scholar] [CrossRef] [PubMed]
- Dodds, T.A.; Martin, D.P.; Stolov, W.C.; Deyo, R.A. A Validation of the Functional Independence Measurement and Its Performance among Rehabilitation Inpatients. Arch. Phys. Med. Rehabil. 1993, 74, 531–536. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366-72. [Google Scholar] [CrossRef]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and Validity of a Tool to Measure the Severity of Dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom Manag. 2013, 46, 201–206. [Google Scholar] [CrossRef]
- Ottenbacher, K.J.; Hsu, Y.; Granger, C.V.; Fiedler, R.C. The Reliability of the Functional Independence Measure: A Quantitative Review. Arch. Phys. Med. Rehabil. 1996, 77, 1226–1232. [Google Scholar] [CrossRef]
- Bise, T.; Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Kido, Y.; Shimazu, S.; Shiraishi, A.; Matsumoto, A. Association between BIA-Derived Phase Angle and Sarcopenia and Improvement in Activities of Daily Living and Dysphagia in Patients Undergoing Post-Stroke Rehabilitation. J. Nutr. Health Aging 2022, 26, 590–597. [Google Scholar] [CrossRef]
- Nishida, C.; Barba, C.; Cavalli-Sforza, T.; Cutter, J.; Deurenberg, P.; Darnton-Hill, I.; Deurenberg-Yap, M.; Gill, T.; James, P.; Ko, G.; et al. Appropriate Body-Mass Index for Asian Populations and Its Implications for Policy and Intervention Strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Examination Committee of Criteria for “Obesity Disease” in Japan; Japan Society for the Study of Obesity New Criteria for “obesity Disease” in Japan. Circ. J. 2002, 66, 987–992.
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Chang, C.; Tanaka, T.; Kuroda, A.; Tsuji, T.; Akishita, M.; Iijima, K. The Association between Sarcopenic Obesity and Depressive Symptoms in Older Japanese Adults. PLoS ONE 2016, 11, e0162898. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Iwata, K.; Kitai, T.; Yoshimura, Y.; Honda, A.; Shimogai, T.; Otsuka, S.; Takimoto, R.; Yamada, K.; Furukawa, Y.; Kohara, N.; et al. Clinical Impact of Functional Independent Measure (FIM) on 180-Day Readmission and Mortality in Elderly Patients Hospitalized with Acute Decompensated Heart Failure. Heart Vessels 2021, 36, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Oyanagi, K.; Kitai, T.; Yoshimura, Y.; Yokoi, Y.; Ohara, N.; Kohara, N.; Sakai, N.; Honda, A.; Onishi, H.; Iwata, K. Effect of Early Intensive Rehabilitation on the Clinical Outcomes of Patients with Acute Stroke. Geriatr. Gerontol. Int. 2021, 21, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Cournan, M. Use of the Functional Independence Measure for Outcomes Measurement in Acute Inpatient Rehabilitation. Rehabil. Nurs. J. 2011, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Beninato, M.; Gill-Body, K.M.; Salles, S.; Stark, P.C.; Black-Schaffer, R.M.; Stein, J. Determination of the Minimal Clinically Important Difference in the FIM Instrument in Patients with Stroke. Arch. Phys. Med. Rehabil. 2006, 87, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Systemic Inflammation in the Recovery Stage of Stroke: Its Association with Sarcopenia and Poor Functional Rehabilitation Outcomes. Prog. Rehabil. Med. 2018, 3, 20180011. [Google Scholar] [CrossRef]
- Nagano, F.; Yoshimura, Y.; Matsumoto, A.; Bise, T.; Kido, Y.; Shimazu, S.; Shiraishi, A. Muscle Strength Gain Is Positively Associated with Functional Recovery in Patients with Sarcopenic Obesity After Stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106429. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Momosaki, R.; Nagano, F.; Bise, T.; Shimazu, S.; Shiraishi, A. Stored Energy Increases Body Weight and Skeletal Muscle Mass in Older, Underweight Patients after Stroke. Nutrients 2021, 13, 3274. [Google Scholar] [CrossRef]
- World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Available online: https://apps.who.int/iris/handle/10665/206936 (accessed on 9 March 2022).
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Mori, T.; Nishioka, S.; Oshima, F.; Ogawa, S.; et al. Sarcopenia and Dysphagia: Position Paper by Four Professional Organizations. Geriatr. Gerontol. Int. 2019, 19, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-T.; Yang, M.; Wu, H.-M.; Yang, L.; Zhang, X.-M.; Huang, Y. Systematic Review and Meta-Analysis of the Association between Sarcopenia and Dysphagia. J. Nutr. Health Aging 2018, 22, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Wakabayashi, H.; Wada, F. Rehabilitation Nutrition for Individuals with Frailty, Disability, Sarcopenic Dysphagia, or Sarcopenic Respiratory Disability. Curr. Opin. Clin. Nutr. Metab. Care 2021, 25, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, A.; Yoshimura, Y.; Wakabayashi, H.; Tsuji, Y. Prevalence of Stroke-Related Sarcopenia and Its Association with Poor Oral Status in Post-Acute Stroke Patients: Implications for Oral Sarcopenia. Clin. Nutr. 2018, 37, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic Obesity in Older Adults: Aetiology, Epidemiology and Treatment Strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Dalamaga, M.; Kokkinos, A. Sarcopenic Obesity: Epidemiologic Evidence, Pathophysiology, and Therapeutic Perspectives. Curr. Obes. Rep. 2019, 8, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Chung, W.; Moon, S.; Ryu, O.H.; Kim, M.K.; Kang, J.G. Effect of Sarcopenia and Body Shape on Cardiovascular Disease According to Obesity Phenotypes. Diabetes Metab. J. 2021, 45, 209–2018. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Ruiz, J.G.; Dent, E.; Morley, J.E.; Merchant, R.A.; Beilby, J.; Beard, J.; Tripathy, C.; Sorin, M.; Andrieu, S.; Aprahamian, I.; et al. Screening for and Managing the Person with Frailty in Primary Care: ICFSR Consensus Guidelines. J. Nutr. Health Aging 2020, 24, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, N.Y.; Krakauer, J.C. Association of Body Shape Index (ABSI) with Hand Grip Strength. Int. J. Environ. Res. Public Health 2020, 17, 6797. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, N.Y.; Krakauer, J.C. Association of X-Ray Absorptiometry Body Composition Measurements with Basic Anthropometrics and Mortality Hazard. In.t J. Environ. Res. Public Health 2021, 18, 7927. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 760) | Female (n = 352) | Male (n = 408) | |||||
|---|---|---|---|---|---|---|---|
| Without Sarcopenic Obesity (n = 330) | With Sarcopenic Obesity (n = 22) | p Value | Without Sarcopenic Obesity (n = 370) | With Sarcopenic Obesity (n = 38) | p Value | ||
| Age, year | 73 (63, 81) | 76 (67, 83) | 76 (74, 81) | 0.640 | 68 (60, 79) | 76 (62, 82) | 0.048 |
| Stroke type, n (%) | |||||||
| -Cerebral infarction | 480 (63.2) | 190 (57.6) | 12 (54.5) | 0.826 | 246 (66.5) | 32 (84.2) | 0.028 |
| -Cerebral hemorrhage | 222 (29.2) | 100 (30.3) | 10 (45.5) | 0.156 | 106 (28.6) | 6 (15.8) | 0.125 |
| -SAH | 58 (7.6) | 38 (11.5) | 0 (0.0) | 0.149 | 20 (5.4) | 0 (0.0) | 0.239 |
| Stroke history, n (%) | 174 (22.9) | 84 (25.5) | 2 (9.1) | 0.121 | 72 (19.5) | 16 (42.1) | 0.003 |
| Premorbid mRS, score | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0.874 | 0 (0, 1) | 1 (0, 2) | 0.042 |
| CCI, score | 3 (1, 4) | 3 (1, 4) | 3 (1, 4) | 0.591 | 3 (1, 4) | 3 (1, 5) | 0.231 |
| Days from onset, day | 13 (10, 22) | 13 (10, 24) | 17 (12 23) | 0.076 | 14 (11, 21) | 14 (12, 22) | 0.804 |
| Paralysis | |||||||
| Right/Left/Both | 336 (44.2)/282 (37.1)/32 (4.2) | 148 (44.8)/120 (36.4)/10 (3.0) | 10 (45.5)/10 (45.5)/0 (0.0) | 0.494 | 158 (42.7)/138 (37.3)/18 (4.9) | 20 (52.6)/14 (36.8)/4 (10.5) | 0.303 |
| BRS, score UL/HF/LL | 5 (3, 6)/5 (3, 6)/5 (3, 6) | 5 (3, 6)/5 (3, 6)/5 (4 6) | 5 (2 6)/5 (2 6)/5 (2 6) | 0.202 | 5 (3, 6)/5 (3, 6)/5 (4 6) | 4 (1, 5)/4 (2, 5)/5 (1, 5) | 0.001 |
| FIM, score | |||||||
| -total | 71 (37, 96) | 77 (36, 94) | 41 (19, 55) | 0.001 | 72 (41, 97) | 47 (27, 63) | <0.001 |
| -motor | 49 (21, 70) | 54 (20, 70) | 16 (13, 40) | 0.001 | 52 (25, 71) | 31 (13, 42) | <0.001 |
| -cognitive | 22 (14, 28) | 22 (15, 28) | 20 (7 22) | 0.009 | 22 (14, 30) | 15 (12, 25) | <0.001 |
| FILS, score | 8 (7, 10) | 8 (7, 10) | 7 (2, 9) | <0.001 | 8 (7, 10) | 7 (6, 9) | 0.075 |
| MNA-SF, score | 7 (5, 9) | 8 (6, 9) | 7 (5, 9) | 0.117 | 8 (6, 9) | 7 (5, 9) | 0.170 |
| BMI, kg/m2 | 22.5 (20.2, 25.1) | 21.4 (19.2, 24.1) | 28.4 (27.7, 30.8) | <0.001 | 22.7 (21.1, 24.5) | 26.7 (25.9, 28.0) | <0.001 |
| -High BMI (> 25.0), n (%) | 200 (26.3) | 64 (19.4) | 22 (100.0) | <0.001 | 76 (20.5) | 38 (100.0) | <0.001 |
| -High BMI (> 27.5), n (%) | 106 (13.9) | 34 (10.3) | 18 (81.8) | <0.001 | 38 (10.3) | 16 (42.1) | <0.001 |
| SMM/W, % | 27.9 (24.0, 31.0) | 25.1 (22.5, 27.5) | 20.1 (18.5, 21.1) | <0.001 | 30.8 (28.5, 33.4) | 26.5 (23.6, 28.5) | <0.001 |
| -Low SMM/W, n (%) | 344 (45.3) | 62 (18.8) | 22 (100.0) | <0.001 | 222 (60.0) | 38 (100.0) | <0.001 |
| FM, % | 30.6 (23.8, 36.0) | 33.2 (27.6, 38.4) | 46.9 (45.8, 48.4) | <0.001 | 25.8 (20.6, 31.3) | 36.9 (33.0, 40.7) | <0.001 |
| -High FM, n (%) | 280 (36.8) | 94 (28.5) | 22 (100.0) | <0.001 | 126 (34.1) | 38 (100.0) | <0.001 |
| HG, kg | 19.2 (12.5, 28.6) | 15.3 (9.2, 19.4) | 6.5 (1.4, 15.4) | 0.002 | 28.3 (19.6, 34.6) | 16.6 (9.2, 22.9) | <0.001 |
| -Low HG, n (%) | 456 (60.0) | 214 (64.8) | 22 (100.0) | <0.001 | 182 (49.2) | 38 (100.0) | <0.001 |
| Number of total drugs | 5 (3, 7) | 4 (3, 6) | 5 (4, 7) | 0.154 | 5 (3, 7) | 5 (3, 8) | 0.058 |
| Length of hospital stay | 95 (56, 145) | 88 (52, 145) | 157 (109, 163) | <0.001 | 92 (57, 141) | 112 (73, 143) | 0.051 |
| Rehabilitation a | 8.3 (7.8, 8.6) | 8.2 (7.6, 8.5) | 8.3 (7.7, 8.6) | 0.637 | 8.3 (7.8, 8.5) | 8.3 (8.0, 8.4) | 0.346 |
| Total (n = 760) | Female (n = 352) | Male (n = 408) | |||||
|---|---|---|---|---|---|---|---|
| Without Sarcopenic Obesity (n = 334) | With Sarcopenic Obesity (n = 18) | p Value | Without Sarcopenic Obesity (n = 392) | With Sarcopenic Obesity (n = 16) | p Value | ||
| Age, year | 73 (63, 81]) | 76 (67, 83) | 76 (74, 81) | 0.644 | 72 (60, 80) | 72 (61, 79) | 0.579 |
| Sex (male), n (%) | 408 (53.7) | - | - | - | - | - | - |
| Stroke type, n (%) | |||||||
| -Cerebral infarction | 480 (63.2) | 192 (57.5) | 10 (55.6) | 0.999 | 266 (67.9) | 12 (75.0) | 0.785 |
| -Cerebral hemorrhage | 222 (29.2) | 102 (30.5) | 8 (44.4) | 0.295 | 108 (27.6) | 4 (25.0) | 0.989 |
| -SAH | 58 (7.6) | 38 (11.4) | 0 (0.0) | 0.238 | 20 (5.1) | 0 (0.0) | 0.971 |
| Stroke history, n (%) | 174 (22.9) | 84 (25.1) | 2 (11.1) | 0.261 | 80 (20.4) | 8 (50.0) | 0.088 |
| Premorbid mRS, score | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0.390 | 0 (0, 1) | 0 (0, 1) | 0.452 |
| CCI, score | 3 (1, 4) | 3 (1, 4) | 3 (1, 4) | 0.946 | 3 (1, 4) | 3 (1, 4) | 0.758 |
| Days from onset, day | 13 (10, 22) | 13 (10, 23) | 17 (12, 23) | 0.149 | 12 (11, 21) | 13 (12, 16) | 0.729 |
| Paralysis | |||||||
| Right/Left/Both | 336 (44.2)/282 (37.1)/32 (4.2) | 150 (44.9)/122 (36.5)/10 (3.0) | 8 (44.4)/8 (44.4)/0 (0.0) | 0.617 | 170 (43.4)/146 (37.2)/20 (5.1) | 8 (50.0)/6 (37.5)/2 (12.5) | 0.617 |
| BRS, score UL/HF/LL | 5 (3, 6)/5 (3, 6)/5 (3, 6) | 5 (3, 6)/5 (3, 6)/5 (3, 6) | 5 (3, 6)/5 (2, 6)/5 (2, 6) | 0.279 | 5 (3, 6)/5 (3, 6)/5 (3, 6) | 5 (3, 5)/5 (3, 5)/5 (2, 5) | 0.294 |
| FIM, score | |||||||
| -total | 71 (37, 96) | 77 (36, 94) | 41 (21, 46) | 0.001 | 71 (41, 96) | 53 (41, 72) | 0.191 |
| -motor | 49 (21, 70) | 54 (19, 70) | 16 (13, 22) | 0.001 | 49 (24, 70) | 32 (25, 54) | 0.194 |
| -cognitive | 22 (14, 28) | 22 (15, 28) | 20 (8, 22) | 0.025 | 22 (14, 29) | 17 (14, 28) | 0.142 |
| FILS, score | 8 (7, 10) | 8 (7, 10) | 7 (2, 9) | 0.131 | 8 (7, 10) | 7 (7, 10) | 0.308 |
| MNASF, score | 7 (5, 9) | 7 (6, 9) | 6 (4, 9) | 0.015 | 7 (5, 9) | 7 (4, 9) | 0.329 |
| BMI, kg/m2 | 22.5 (20.2, 25.1) | 21.4 (19.2, 24.3) | 28.5 (28.4, 31.0) | <0.001 | 22.8 (21.3, 24.9) | 28.2 (27.8, 29.8) | <0.001 |
| -High BMI (> 25.0), n (%) | 200 (26.3) | 68 (20.4) | 18 (100.0) | <0.001 | 98 (25.0) | 16 (100.0) | <0.001 |
| -High BMI (> 27.5), n (%) | 106 (13.9) | 34 (10.2) | 18 (100.0) | <0.001 | 38 (9.7) | 16 (100.0) | <0.001 |
| SMM/W, % | 27.9 (24.0, 31.0) | 25.1 (22.5, 27.5) | 20.1 (18.9, 21.1) | <0.001 | 30.6 (27.9, 33.3) | 26.6 (24.5, 28.5) | <0.001 |
| -Low SMM/W, n (%) | 344 (45.3) | 66 (19.8) | 18 (100.0) | <0.001 | 244 (62.2) | 16 (100.0) | <0.001 |
| FM, % | 30.6 (23.8, 36.0) | 33.4 (27.6, 38.7) | 46.9 (45.5, 48.7) | <0.001 | 26.3 (21.0, 32.2) | 36.4 (33.0, 39.7) | <0.001 |
| -High FM, n (%) | 280 (36.8) | 98 (29.3) | 18 (100.0) | <0.001 | 148 (37.8) | 16 (100.0) | <0.001 |
| HG, kg -Low HG, n (%) | 19.2 (12.5, 28.6) 456 (60.0) | 15.3 (9.1, 19.4) 218 (65.3) | 6.3 (0.0, 15.5) 18 (100.0) | 0.004 0.001 | 27.1 (17.5, 34.3) 204 (52.0) | 22.8 (15.8, 24.8) 16 (100.0) | 0.018 <0.001 |
| Sarcopenic obesity, n (%) | 34 (4.5) | - | - | - | - | - | - |
| Number of total drugs | 5 (3, 7) | 5 (3, 6) | 6 (4, 7) | 0.075 | 5 (3, 7) | 7 (6, 10) | 0.708 |
| Length of hospital stay | 95 (56, 145) | 88 (52, 145) | 131 (98, 141) | <0.001 | 94 (59, 143) | 112 (58, 139) | 0.849 |
| Rehabilitation a | 8.3 (7.8, 8.6) | 8.2 (7.7, 8.5) | 8.3 (7.6, 8.5) | 0.641 | 8.3 (7.9, 8.5) | 8.3 (8.2, 8.4) | 0.897 |
| Total (n = 760) | Female (n = 352) | Male (n = 408) | |||||
|---|---|---|---|---|---|---|---|
| Without Sarcopenic Obesity (n = 334) | With Sarcopenic Obesity (n = 18) | p Value | Without Sarcopenic Obesity (n = 392) | With Sarcopenic Obesity (n = 16) | p Value | ||
| FIM-motor at discharge | 83 (59, 89) | 83.0 (57.3, 88.8) | 77.1 (40.2, 80.4) | 0.019 | 87.8 (72.2, 90.5) | 80.0 (65.5, 88.7) | 0.117 |
| FILS at discharge | 10 (9, 10) | 10 (9, 10) | 9 (9, 10) | 0.129 | 10 (9, 10) | 10 (9, 10) | 0.334 |
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| FIM-Motor at Discharge | FILS at Discharge | FIM-Motor at Discharge | FILS at Discharge | |||||
| β | p | β | p | β | p | β | p | |
| Age | −0.094 | 0.018 | −0.079 | 0.127 | −0.157 | 0.000 | −0.085 | 0.093 |
| Stroke type | ||||||||
| Cerebral infarction | 0.184 | 0.009 | 0.080 | 0.365 | 0.040 | 0.674 | 0.024 | 0.862 |
| Cerebral hemorrhage | 0.221 | 0.003 | 0.198 | 0.030 | 0.067 | 469 | 0.013 | 0.921 |
| Subarachnoid hemorrhage | (reference) | - | (reference) | - | (reference) | - | (reference) | - |
| Stroke history | −0.074 | 0.043 | −0.074 | 0.128 | −0.034 | 0.350 | 0.089 | 0.088 |
| FIM-motor at admission | 0.430 | <0.001 | 0.365 | <0.001 | 0.410 | <0.001 | −0.130 | 0.165 |
| FIM-cognitive at admission | 0.192 | <0.001 | −0.049 | 0.484 | 0.194 | <0.001 | 0.280 | <0.001 |
| Premorbid mRS | −0.165 | <0.001 | −0.149 | 0.005 | −0.128 | 0.001 | −0.219 | <0.001 |
| CCI | 0.085 | 0.025 | 0.063 | 0.216 | −0.115 | 0.002 | −0.120 | 0.026 |
| MNASF at admission | −0.012 | 0.797 | −0.094 | 0.130 | 0.160 | <0.001 | 0.116 | 0.045 |
| Rehabilitation | 0.110 | 0.004 | 0.187 | <0.001 | 0.059 | 0.067 | 0.051 | 0.257 |
| BRS-lower | 0.404 | <0.001 | - | - | 0.214 | <0.001 | - | - |
| FILS at admission | - | - | 0.466 | <0.001 | - | - | 0.507 | <0.001 |
| LOS | 0.097 | 0.059 | 0.079 | 0.245 | 0.194 | <0.001 | 0.191 | 0.004 |
| Sarcopenic obesity | −0.048 | 0.031 | −0.095 | 0.046 | −0.117 | <0.001 | −0.004 | 0.323 |
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| FIM-Motor at Discharge | FILS at Discharge | FIM-Motor at Discharge | FILS at Discharge | |||||
| β | p | β | p | β | p | β | p | |
| Age | −0.094 | 0.018 | −0.111 | 0.033 | −0.166 | 0.000 | −0.099 | 0.052 |
| Stroke type | ||||||||
| Cerebral infarction | 0.186 | 0.009 | 0.183 | 0.165 | 0.048 | 0.624 | 0.021 | 0.876 |
| Cerebral hemorrhage | 0.224 | 0.003 | 0.326 | 0.060 | 0.079 | 412 | 0.017 | 0.961 |
| Subarachnoid hemorrhage | (reference) | - | (reference) | - | (reference) | - | (reference) | - |
| Stroke history | −0.076 | 0.039 | −0.095 | 0.048 | −0.019 | 0.607 | 0.086 | 0.098 |
| FIM-motor at admission | 0.434 | <0.001 | 0.242 | 0.015 | 0.398 | <0.001 | −0.161 | 0.107 |
| FIM-cognitive at admission | 0.187 | <0.001 | −0.034 | 0.618 | 0.193 | <0.001 | 0.296 | <0.001 |
| Premorbid mRS | −0.167 | <0.001 | −0.192 | <0.001 | −0.140 | <0.001 | −0.220 | <0.001 |
| CCI | 0.086 | 0.023 | 0.067 | 0.181 | −0.117 | 0.003 | −0.123 | 0.022 |
| MNASF at admission | −0.010 | 0.828 | −0.128 | 0.038 | 0.162 | <0.001 | 0.097 | 0.094 |
| Rehabilitation | 0.111 | 0.004 | 0.205 | <0.0010 | 0.059 | 0.073 | 0.061 | 0.182 |
| BRS-lower | 0.403 | <0.001 | 0.253 | 0.001 | 0.206 | <0.001 | 0.094 | 0.159 |
| FILS at admission | - | - | 0.449 | <0.001 | - | - | 0.471 | <0.001 |
| LOS | 0.098 | 0.055 | 0.125 | 0.066 | 0.177 | <0.001 | 0.206 | 0.002 |
| Sarcopenic obesity | −0.073 | 0.024 | −0.096 | 0.042 | −0.147 | 0.002 | −0.050 | 0.182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Matsumoto, A.; Shimazu, S.; Shiraishi, A.; Kido, Y.; Bise, T. The Applicability of the ESPEN and EASO-Defined Diagnostic Criteria for Sarcopenic Obesity in Japanese Patients after Stroke: Prevalence and Association with Outcomes. Nutrients 2022, 14, 4205. https://doi.org/10.3390/nu14194205
Yoshimura Y, Wakabayashi H, Nagano F, Matsumoto A, Shimazu S, Shiraishi A, Kido Y, Bise T. The Applicability of the ESPEN and EASO-Defined Diagnostic Criteria for Sarcopenic Obesity in Japanese Patients after Stroke: Prevalence and Association with Outcomes. Nutrients. 2022; 14(19):4205. https://doi.org/10.3390/nu14194205
Chicago/Turabian StyleYoshimura, Yoshihiro, Hidetaka Wakabayashi, Fumihiko Nagano, Ayaka Matsumoto, Sayuri Shimazu, Ai Shiraishi, Yoshifumi Kido, and Takahiro Bise. 2022. "The Applicability of the ESPEN and EASO-Defined Diagnostic Criteria for Sarcopenic Obesity in Japanese Patients after Stroke: Prevalence and Association with Outcomes" Nutrients 14, no. 19: 4205. https://doi.org/10.3390/nu14194205
APA StyleYoshimura, Y., Wakabayashi, H., Nagano, F., Matsumoto, A., Shimazu, S., Shiraishi, A., Kido, Y., & Bise, T. (2022). The Applicability of the ESPEN and EASO-Defined Diagnostic Criteria for Sarcopenic Obesity in Japanese Patients after Stroke: Prevalence and Association with Outcomes. Nutrients, 14(19), 4205. https://doi.org/10.3390/nu14194205







