Acute Fasting Modulates Food-Seeking Behavior and Neural Signaling in the Piriform Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Food-Search Task (FST)
2.3. Immunofluorescent Staining
2.4. Image Analysis
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
3.1. Acute Fasting Increases Food Search Behavior
3.2. Foraging Activates the Anterior Piriform Cortex
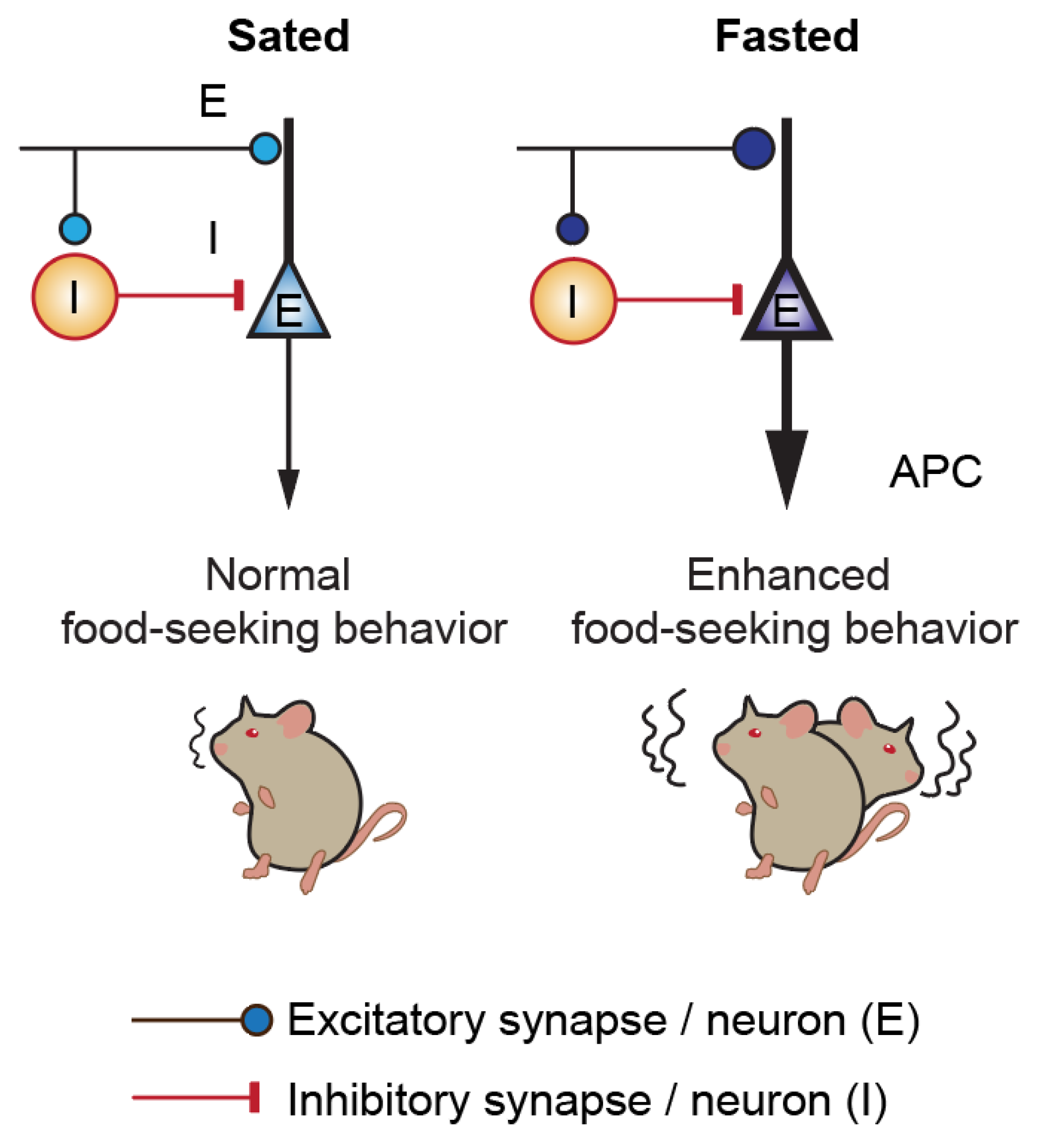
3.3. Acute Fasting Induces Plasticity of Excitatory and Inhibitory Synapses in the Anterior Piriform Cortex
3.4. Acute Fasting Enhances AMPK Phosphorylation and Decreases Akt Phosphorylation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gautron, L.; Elmquist, J.K.; Williams, K.W. Neural Control of Energy Balance: Translating Circuits to Therapies. Cell 2015, 161, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Andermann, M.L.; Lowell, B.B. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 2017, 95, 757–778. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef]
- Alcantara, I.C.; Tapia, A.P.M.; Aponte, Y.; Krashes, M.J. Acts of Appetite: Neural Circuits Governing the Appetitive, Consummatory, and Terminating Phases of Feeding. Nat. Metab. 2022, 4, 836–847. [Google Scholar] [CrossRef]
- Cone, R.D. Anatomy and Regulation of the Central Melanocortin System. Nat. Neurosci. 2005, 8, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.B.; Harlan, S.M.; Morgan, D.A.; Guo, D.-F.; Rahmouni, K. Differential Contribution of POMC and AgRP Neurons to the Regulation of Regional Autonomic Nerve Activity by Leptin. Mol. Metab. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Wu, Q.; Boyle, M.P.; Palmiter, R.D. Loss of GABAergic Signaling by AgRP Neurons to the Parabrachial Nucleus Leads to Starvation. Cell 2009, 137, 1225–1234. [Google Scholar] [CrossRef]
- Chen, Y.; Essner, R.A.; Kosar, S.; Miller, O.H.; Lin, Y.-C.; Mesgarzadeh, S.; Knight, Z.A. Sustained NPY Signaling Enables AgRP Neurons to Drive Feeding. eLife 2019, 8, e46348. [Google Scholar] [CrossRef]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, Reversible Activation of AgRP Neurons Drives Feeding Behavior in Mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, Y.-C.; Zimmerman, C.A.; Essner, R.A.; Knight, Z.A. Hunger Neurons Drive Feeding through a Sustained, Positive Reinforcement Signal. eLife 2016, 5, e18640. [Google Scholar] [CrossRef]
- Xu, Y.; Jones, J.E.; Kohno, D.; Williams, K.W.; Lee, C.E.; Choi, M.J.; Anderson, J.G.; Heisler, L.K.; Zigman, J.M.; Lowell, B.B.; et al. 5-HT2CRs Expressed by pro-Opiomelanocortin Neurons Regulate Energy Homeostasis. Neuron 2008, 60, 582–589. [Google Scholar] [CrossRef]
- Garfield, A.S.; Li, C.; Madara, J.C.; Shah, B.P.; Webber, E.; Steger, J.S.; Campbell, J.N.; Gavrilova, O.; Lee, C.E.; Olson, D.P.; et al. A Neural Basis for Melanocortin-4 Receptor-Regulated Appetite. Nat. Neurosci. 2015, 18, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Horio, N.; Liberles, S.D. Hunger Enhances Food-Odour Attraction through a Neuropeptide Y Spotlight. Nature 2021, 592, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Riera, C.E.; Tsaousidou, E.; Halloran, J.; Follett, P.; Hahn, O.; Pereira, M.M.A.; Ruud, L.E.; Alber, J.; Tharp, K.; Anderson, C.M.; et al. The Sense of Smell Impacts Metabolic Health and Obesity. Cell Metab. 2017, 26, 198–211.e5. [Google Scholar] [CrossRef] [PubMed]
- Soria-Gómez, E.; Bellocchio, L.; Reguero, L.; Lepousez, G.; Martin, C.; Bendahmane, M.; Ruehle, S.; Remmers, F.; Desprez, T.; Matias, I.; et al. The Endocannabinoid System Controls Food Intake via Olfactory Processes. Nat. Neurosci. 2014, 17, 407–415. [Google Scholar] [CrossRef]
- Wilson, D.A.; Sullivan, R.M. Cortical Processing of Odor Objects. Neuron 2011, 72, 506–519. [Google Scholar] [CrossRef]
- Illig, K.R.; Wilson, D.A. Olfactory Cortex: Comparative anatomy. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-801238-3. [Google Scholar]
- Wiegand, H.F.; Beed, P.; Bendels, M.H.K.; Leibold, C.; Schmitz, D.; Johenning, F.W. Complementary Sensory and Associative Microcircuitry in Primary Olfactory Cortex. J. Neurosci. 2011, 31, 12149–12158. [Google Scholar] [CrossRef] [PubMed]
- Diodato, A.; Ruinart de Brimont, M.; Yim, Y.S.; Derian, N.; Perrin, S.; Pouch, J.; Klatzmann, D.; Garel, S.; Choi, G.B.; Fleischmann, A. Molecular Signatures of Neural Connectivity in the Olfactory Cortex. Nat. Commun. 2016, 7, 12238. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, K.; Osakada, F.; Tarabrina, A.; Kizer, E.; Callaway, E.M.; Gage, F.H.; Sejnowski, T.J. Diverse Representations of Olfactory Information in Centrifugal Feedback Projections. J. Neurosci. 2016, 36, 7535–7545. [Google Scholar] [CrossRef]
- Mazo, C.; Grimaud, J.; Shima, Y.; Murthy, V.N.; Lau, C.G. Distinct Projection Patterns of Different Classes of Layer 2 Principal Neurons in the Olfactory Cortex. Sci. Rep. 2017, 7, 8282. [Google Scholar] [CrossRef]
- Jiang, H.-H.; Guo, A.; Chiu, A.; Li, H.; Lai, C.S.W.; Lau, C.G. Target-Specific Control of Piriform Cortical Output via Distinct Inhibitory Circuits. FASEB J. 2021, 35, e21944. [Google Scholar] [CrossRef]
- Boyd, A.M.; Sturgill, J.F.; Poo, C.; Isaacson, J.S. Cortical Feedback Control of Olfactory Bulb Circuits. Neuron 2012, 76, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Otazu, G.H.; Chae, H.; Davis, M.B.; Albeanu, D.F. Cortical Feedback Decorrelates Olfactory Bulb Output in Awake Mice. Neuron 2015, 86, 1461–1477. [Google Scholar] [CrossRef] [PubMed]
- Poo, C.; Agarwal, G.; Bonacchi, N.; Mainen, Z.F. Spatial Maps in Piriform Cortex during Olfactory Navigation. Nature 2022, 601, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Sharp, J.W.; Ross-Inta, C.M.; McDaniel, B.J.; Anthony, T.G.; Wek, R.C.; Cavener, D.R.; McGrath, B.C.; Rudell, J.B.; Koehnle, T.J.; et al. Uncharged TRNA and Sensing of Amino Acid Deficiency in Mammalian Piriform Cortex. Science 2005, 307, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Maurin, A.-C.; Jousse, C.; Averous, J.; Parry, L.; Bruhat, A.; Cherasse, Y.; Zeng, H.; Zhang, Y.; Harding, H.P.; Ron, D.; et al. The GCN2 Kinase Biases Feeding Behavior to Maintain Amino Acid Homeostasis in Omnivores. Cell Metab. 2005, 1, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Leib, D.E.; Knight, Z.A. Re-Examination of Dietary Amino Acid Sensing Reveals a GCN2-Independent Mechanism. Cell Rep. 2015, 13, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, Y.-C.; Kuo, T.-W.; Knight, Z.A. Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits. Cell 2015, 160, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Livneh, Y.; Ramesh, R.N.; Burgess, C.R.; Levandowski, K.M.; Madara, J.C.; Fenselau, H.; Goldey, G.J.; Diaz, V.E.; Jikomes, N.; Resch, J.M.; et al. Homeostatic Circuits Selectively Gate Food Cue Responses in Insular Cortex. Nature 2017, 546, 611–616. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Roy, R. AMPK Acts as a Molecular Trigger to Coordinate Glutamatergic Signals and Adaptive Behaviours during Acute Starvation. eLife 2016, 5, e16349. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Filipsson, K.; Abbott, C.R.; Woods, A.; Smith, K.; Bloom, S.R.; Carling, D.; Small, C.J. AMP-Activated Protein Kinase Plays a Role in the Control of Food Intake. J. Biol. Chem. 2004, 279, 12005–12008. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Atasoy, D.; Su, H.H.; Sternson, S.M. Hunger States Switch a Flip-Flop Memory Circuit via a Synaptic AMPK-Dependent Positive Feedback Loop. Cell 2011, 146, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M. Regulation and Function of AMPK in Physiology and Diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Sachse, S.; Beshel, J. The Good, the Bad, and the Hungry: How the Central Brain Codes Odor Valence to Facilitate Food Approach in Drosophila. Curr. Opin. Neurobiol. 2016, 40, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Betley, J.N.; Xu, S.; Cao, Z.F.H.; Gong, R.; Magnus, C.J.; Yu, Y.; Sternson, S.M. Neurons for Hunger and Thirst Transmit a Negative-Valence Teaching Signal. Nature 2015, 521, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Senapati, B.; Tsao, C.-H. Neural Basis of Hunger-Driven Behaviour in Drosophila. Open Biol. 2019, 9, 180259. [Google Scholar] [CrossRef] [PubMed]
- Bolding, K.A.; Franks, K.M. Complementary Codes for Odor Identity and Intensity in Olfactory Cortex. eLife 2017, 6, e22630. [Google Scholar] [CrossRef]
- Tyssowski, K.M.; Gray, J.M. The Neuronal Stimulation-Transcription Coupling Map. Curr. Opin. Neurobiol. 2019, 59, 87–94. [Google Scholar] [CrossRef]
- Lewis, S. Feast or Famine. Nat. Rev. Neurosci. 2016, 17, 466. [Google Scholar] [CrossRef]
- Mandelblat-Cerf, Y.; Ramesh, R.N.; Burgess, C.R.; Patella, P.; Yang, Z.; Lowell, B.B.; Andermann, M.L. Arcuate Hypothalamic AgRP and Putative POMC Neurons Show Opposite Changes in Spiking across Multiple Timescales. eLife 2015, 4, e07122. [Google Scholar] [CrossRef] [PubMed]
- Wicker, E.; Turchi, J.; Malkova, L.; Forcelli, P.A. Mediodorsal Thalamus Is Required for Discrete Phases of Goal-Directed Behavior in Macaques. eLife 2018, 7, e37325. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.P. The Hypothalamus, Hormones, and Hunger: Alterations in Human Obesity and Illness. Prog. Brain Res. 2006, 153, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Palouzier-Paulignan, B.; Lacroix, M.-C.; Aimé, P.; Baly, C.; Caillol, M.; Congar, P.; Julliard, A.K.; Tucker, K.; Fadool, D.A. Olfaction Under Metabolic Influences. Chem. Senses 2012, 37, 769–797. [Google Scholar] [CrossRef] [PubMed]
- Poessel, M.; Breuer, N.; Joshi, A.; Pampel, A.; Villringer, A.; Hummel, T.; Horstmann, A. Reduced Olfactory Bulb Volume in Obesity and Its Relation to Metabolic Health Status. Front. Hum. Neurosci. 2020, 14, 586998. [Google Scholar] [CrossRef]
- Boone, M.H.; Liang-Guallpa, J.; Krashes, M.J. Examining the Role of Olfaction in Dietary Choice. Cell Rep. 2021, 34, 108755. [Google Scholar] [CrossRef]
- Peris-Sampedro, F.; Stoltenborg, I.; Le May, M.V.; Sole-Navais, P.; Adan, R.A.H.; Dickson, S.L. The Orexigenic Force of Olfactory Palatable Food Cues in Rats. Nutrients 2021, 13, 3101. [Google Scholar] [CrossRef]

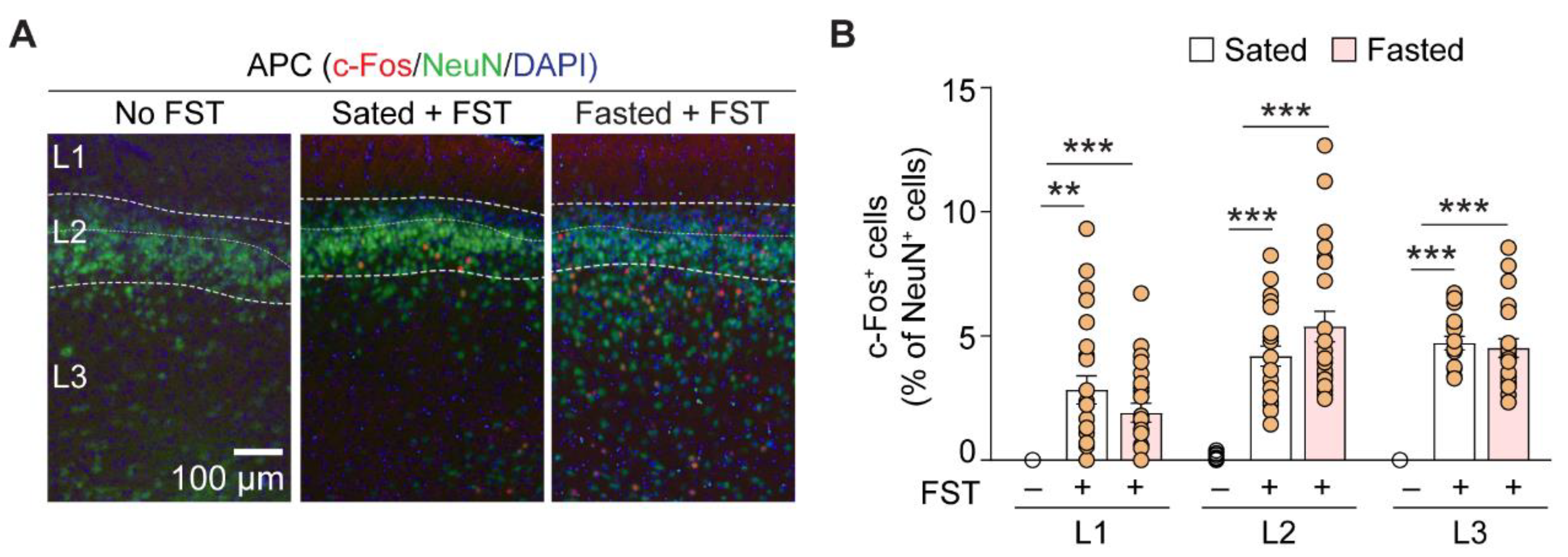

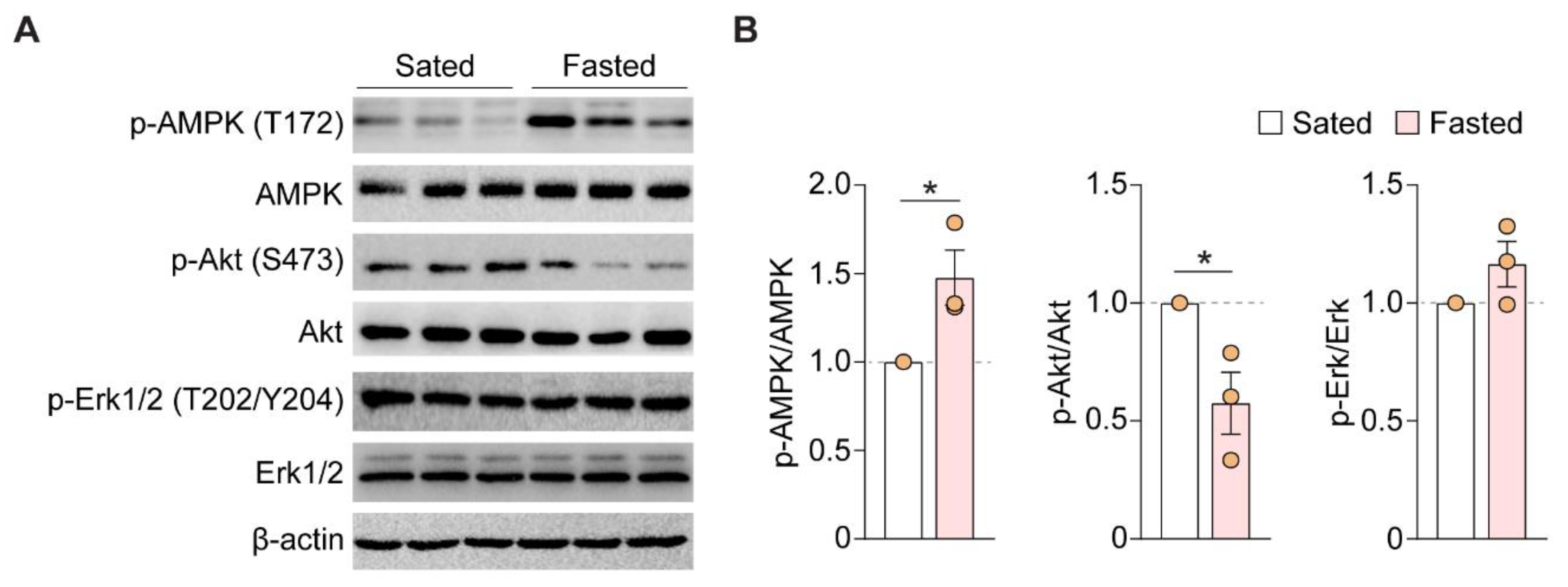
| Layer | Contrast | c-fos+ Neurons (% of NeuN+ Cells) | p-Value | n (APC Section) |
|---|---|---|---|---|
| L1 | Normal to sated | 0 to 2.8 ± 0.6 | <0.001 | 13, 24 |
| Normal to fasted | 0 to 1.9 ± 0.4 | 0.001 | 13, 23 | |
| Sated to fasted | 2.8 ± 0.6 to 1.9 ± 0.4 | 1.0 | 24, 23 | |
| L2 | Normal to sated | 0.1 ± 0.04 to 4.2 ± 0.4 | <0.001 | 13, 22 |
| Normal to fasted | 0.1 ± 0.04 to 5.4 ± 0.6 | <0.001 | 13, 23 | |
| Sated to fasted | 4.2 ± 0.4 to 5.4 ± 0.6 | 0.941 | 22, 23 | |
| L3 | Normal to sated | 0 to 4.7 ± 0.3 | <0.001 | 13, 20 |
| Normal to fasted | 0 to 4.5 ± 0.4 | <0.001 | 13, 21 | |
| Sated to fasted | 4.7 ± 0.3 to 4.5 ± 0.4 | 1.0 | 20, 21 |
| Layer | Contrast | Puncta Density (×104/mm2) | p-Value | n (APC Section) |
|---|---|---|---|---|
| L1 | Normal to sated | 8.0 ± 1.0 to 6.7 ± 1.8 | 1.0 | 19, 13 |
| Normal to fasted | 8.0 ± 1.0 to 16.3 ± 1.0 | 0.02 | 19, 10 | |
| Sated to fasted | 6.7 ± 1.8 to 16.3 ± 1.0 | 0.005 | 13, 10 | |
| L2 | Normal to sated | 8.0 ± 1.0 to 6.1 ± 1.1 | 0.617 | 19, 13 |
| Normal to fasted | 8.0 ± 0.8 to 13.2 ± 0.3 | 0.009 | 19, 10 | |
| Sated to fasted | 6.1 ± 1.1 to 13.2 ± 0.3 | <0.001 | 13, 10 | |
| L3 | Normal to sated | 9.6 ± 0.7 to 8.0 ± 0.4 | 0.757 | 19, 10 |
| Normal to fasted | 9.6 ± 0.7 to 15.7 ± 1.5 | 0.011 | 19, 10 | |
| Sated to fasted | 8.0 ± 0.4 to 15.7 ± 1.5 | 0.001 | 10 |
| Layer | Contrast | Puncta Density (×104/mm2) | p-Value | n (APC Section) |
|---|---|---|---|---|
| L1 | Normal to sated | 6.6 ± 1.2 to 6.8 ± 1.7 | 1.0 | 16, 13 |
| Normal to fasted | 6.6 ± 1.2 to 6.6 ± 1.1 | 1.0 | 16 | |
| Sated to fasted | 6.8 ± 1.7 to 6.6 ± 1.1 | 1.0 | 13, 16 | |
| L2 | Normal to sated | 7.3 ± 0.9 to 9.1 ± 1.2 | 0.50 | 15, 13 |
| Normal to fasted | 7.3 ± 0.9 to 6.8 ± 0.5 | 1.0 | 15, 16 | |
| Sated to fasted | 9.1 ± 1.2 to 6.8 ± 0.5 | 0.21 | 13, 16 | |
| L3 | Normal to sated | 5.9 ± 0.9 to 7.6 ± 1.3 | 0.67 | 15, 13 |
| Normal to fasted | 5.9 ± 0.9 to 5.5 ± 0.6 | 1.0 | 15, 13 | |
| Sated to fasted | 7.6 ± 1.3 to 5.5 ± 0.6 | 1.0 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, F.-Y.; Li, H.; Zhang, H.; Lau, C.-Y.G. Acute Fasting Modulates Food-Seeking Behavior and Neural Signaling in the Piriform Cortex. Nutrients 2022, 14, 4156. https://doi.org/10.3390/nu14194156
Ngo F-Y, Li H, Zhang H, Lau C-YG. Acute Fasting Modulates Food-Seeking Behavior and Neural Signaling in the Piriform Cortex. Nutrients. 2022; 14(19):4156. https://doi.org/10.3390/nu14194156
Chicago/Turabian StyleNgo, Fung-Yin, Huanhuan Li, Huiqi Zhang, and Chun-Yue Geoffrey Lau. 2022. "Acute Fasting Modulates Food-Seeking Behavior and Neural Signaling in the Piriform Cortex" Nutrients 14, no. 19: 4156. https://doi.org/10.3390/nu14194156
APA StyleNgo, F.-Y., Li, H., Zhang, H., & Lau, C.-Y. G. (2022). Acute Fasting Modulates Food-Seeking Behavior and Neural Signaling in the Piriform Cortex. Nutrients, 14(19), 4156. https://doi.org/10.3390/nu14194156





