Naringin Ameliorates Skeletal Muscle Atrophy and Improves Insulin Resistance in High-Fat-Diet-Induced Insulin Resistance in Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. In Vitro
2.2.1. The α-Glucosidase Inhibition Assay
2.2.2. The Cholesterol Esterase Inhibition
2.2.3. The Pancreatic Lipase Inhibition Assay
2.3. Animal Groups and Treatments
2.4. Oral Glucose Tolerance Test
2.5. Biochemical Analyses
2.6. Muscle Homogenization
2.7. The Level of Superoxide Dismutase
2.8. The Level of Catalase
2.9. The Level of Lipid Peroxidation
2.10. Measurement of Glucose Uptake in the Muscle Tissues
2.11. Measurement of Glycogen in the Skeletal Muscle
2.12. RT-PCR Analysis
2.13. Western Blotting Analysis
2.14. Hematoxylin and Eosin (H&E) Staining
2.15. Statistical Analysis
3. Results
3.1. Effect of Naringin on In Vitro Study
3.2. Effect of Naringin on the Body Weight of High-Fat-Diet-Fed Rats
3.3. The Effect of Naringin on Lipid Accumulation in the Muscle of High-Fat-Diet Rats
3.4. Effect of Naringin on Markers of Muscle Injury in Serum of High-Fat-Diet-Fed Rats
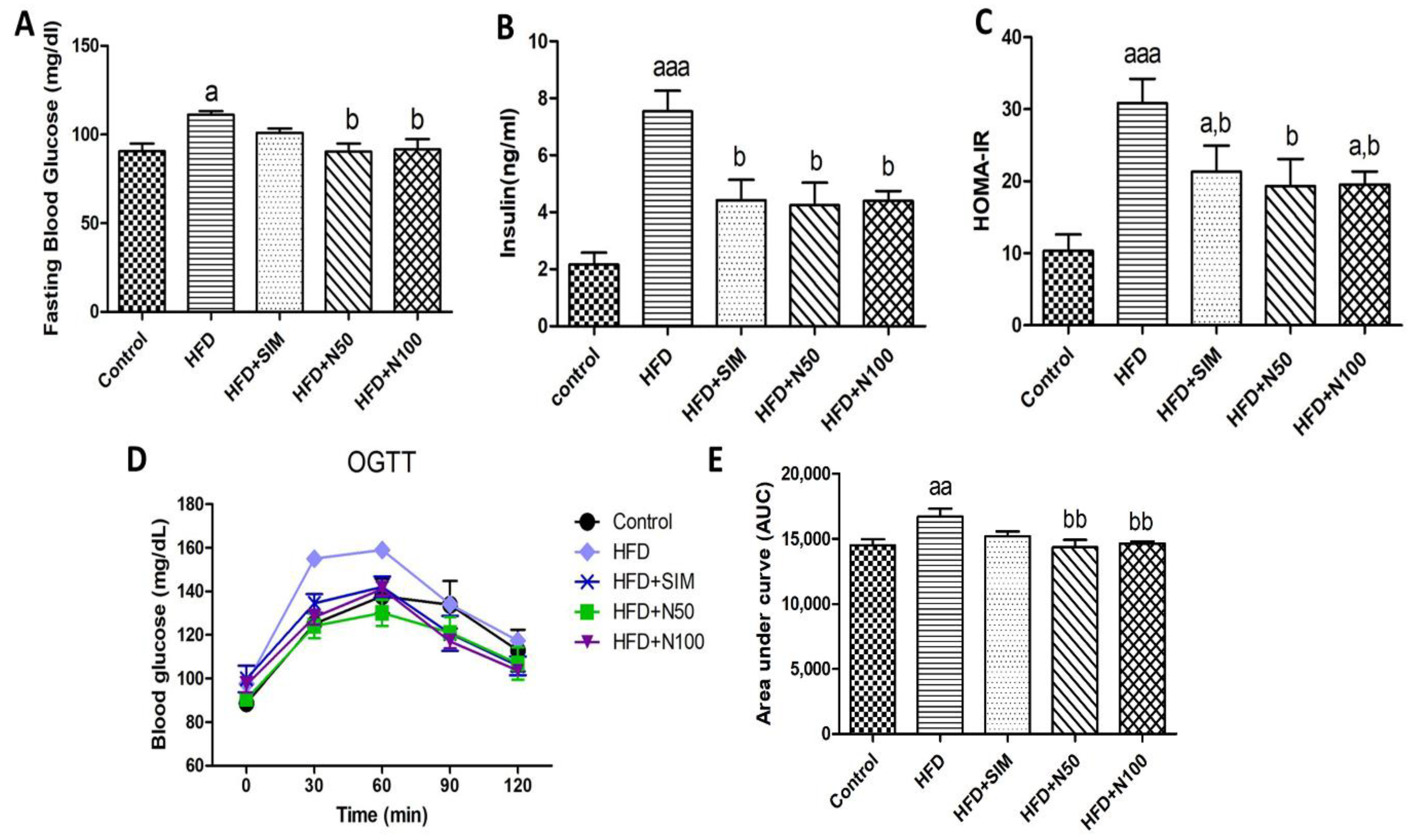
3.5. Effect of Naringin on Blood Glucose and Insulin Resistance in High-Fat-Diet-Fed Rats
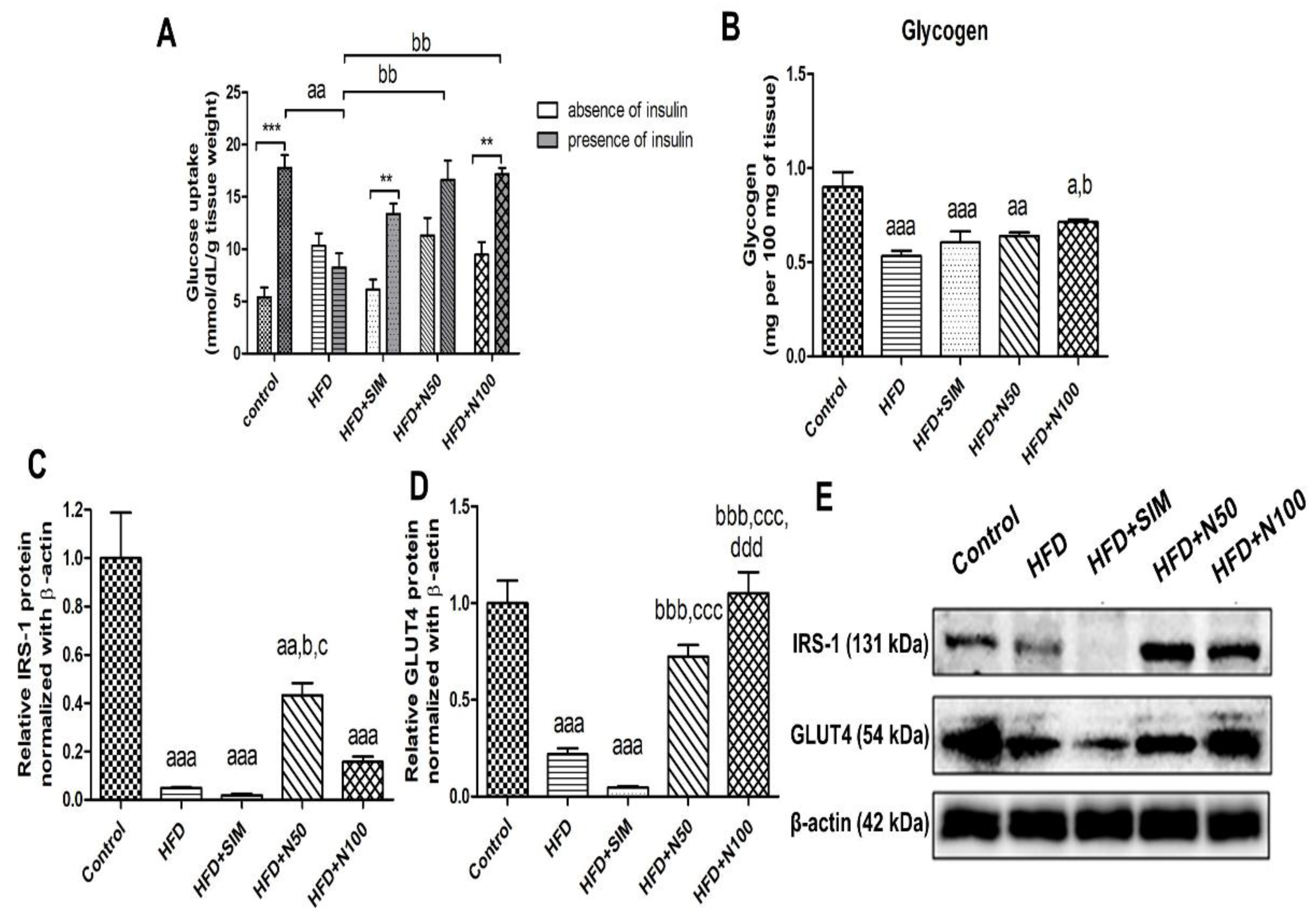
3.6. Effect of Naringin on Glucose Uptake, Protein Expressions, and Glycogen Content of Skeletal Muscles in High-Fat-Diet-Fed Rats
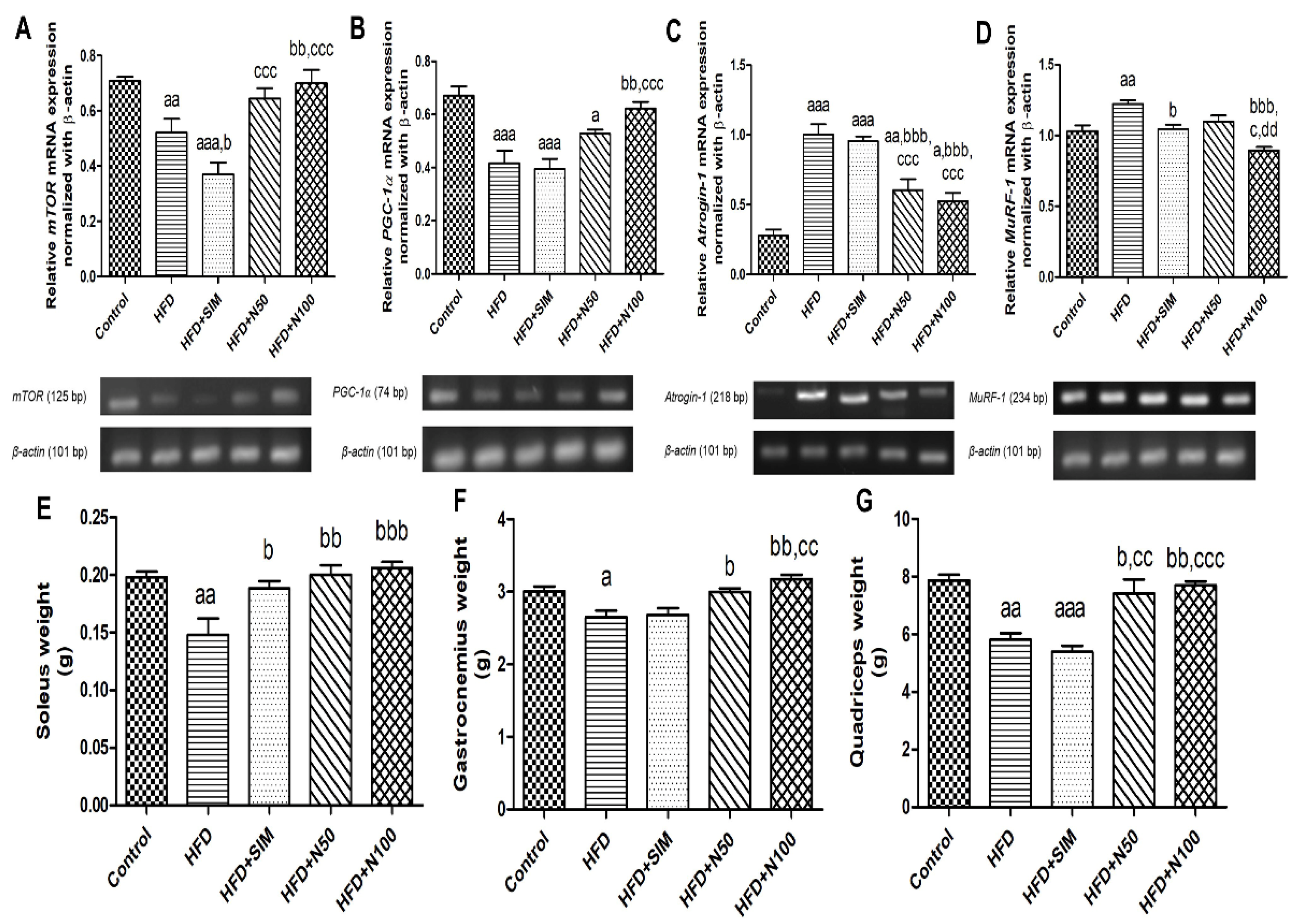
3.7. Effect of Naringin on the Expression of Antioxidant Enzymes, Protein Metabolism mRNA in Quadriceps Muscle Tissues
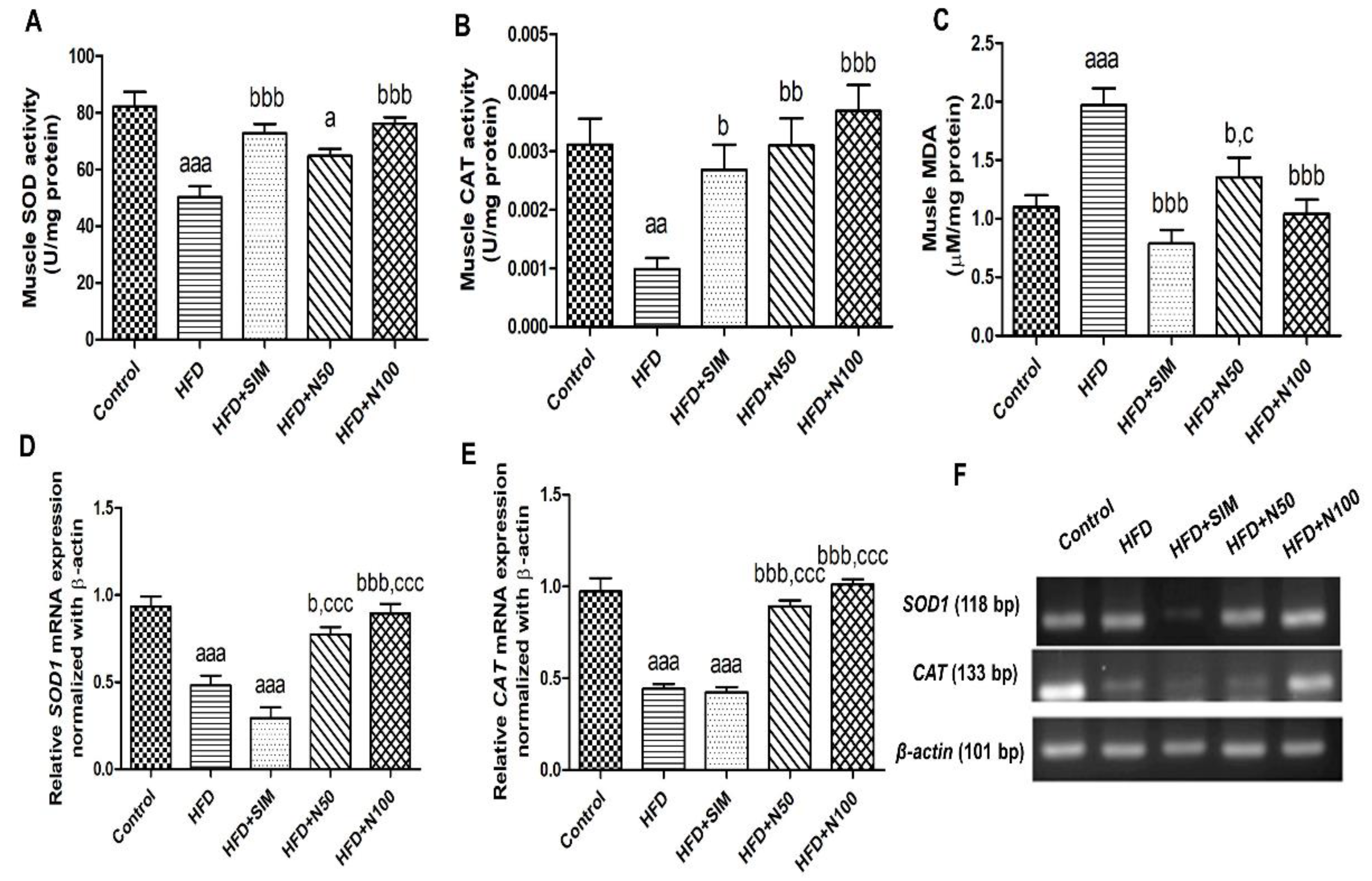
3.8. Effect of Naringin on Antioxidants and Lipid Peroxidation in High-Fat-Diet-Fed Rats
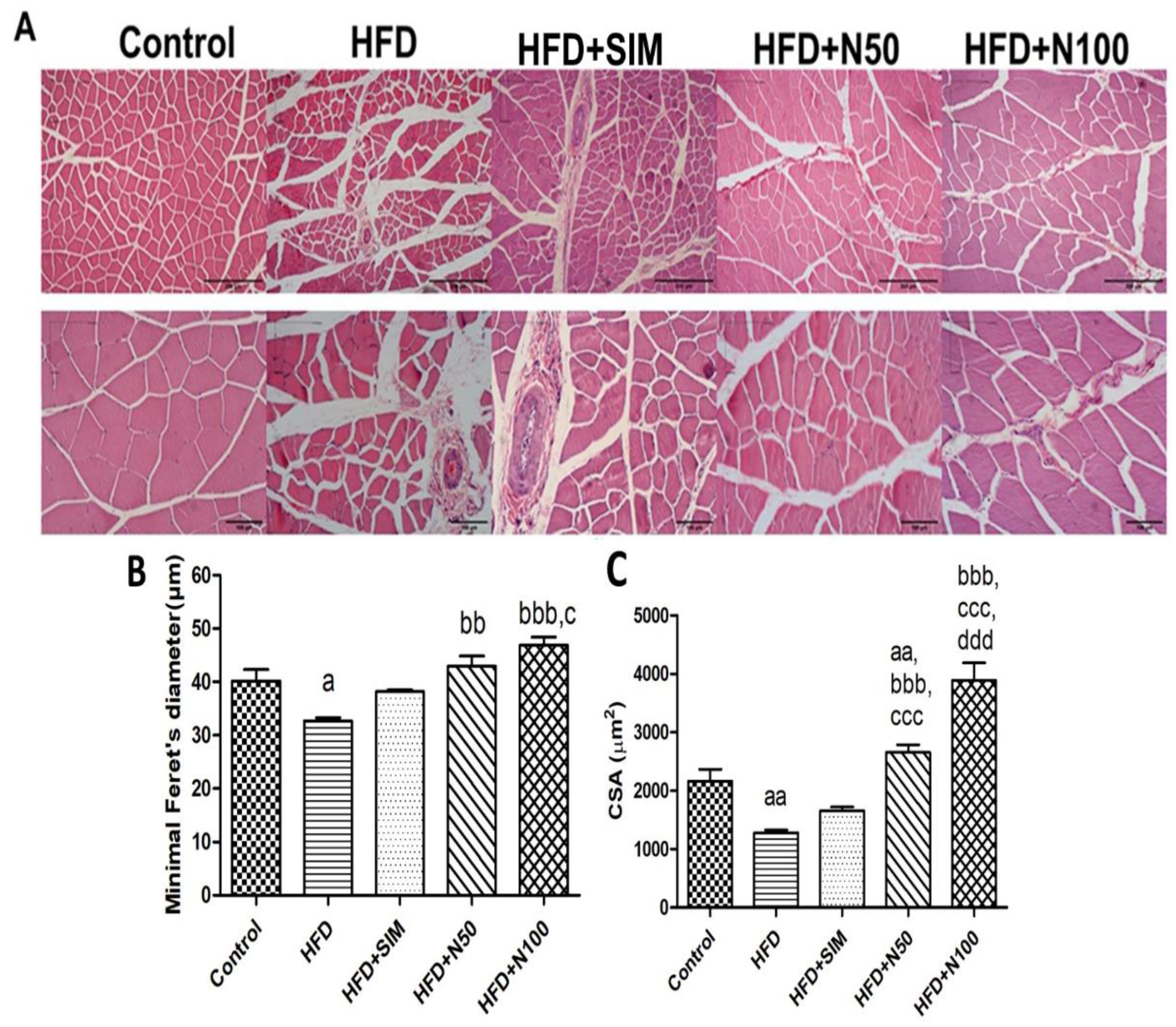
3.9. Histopathology of Quadriceps Muscles in High-FatDiet-Fed Rats
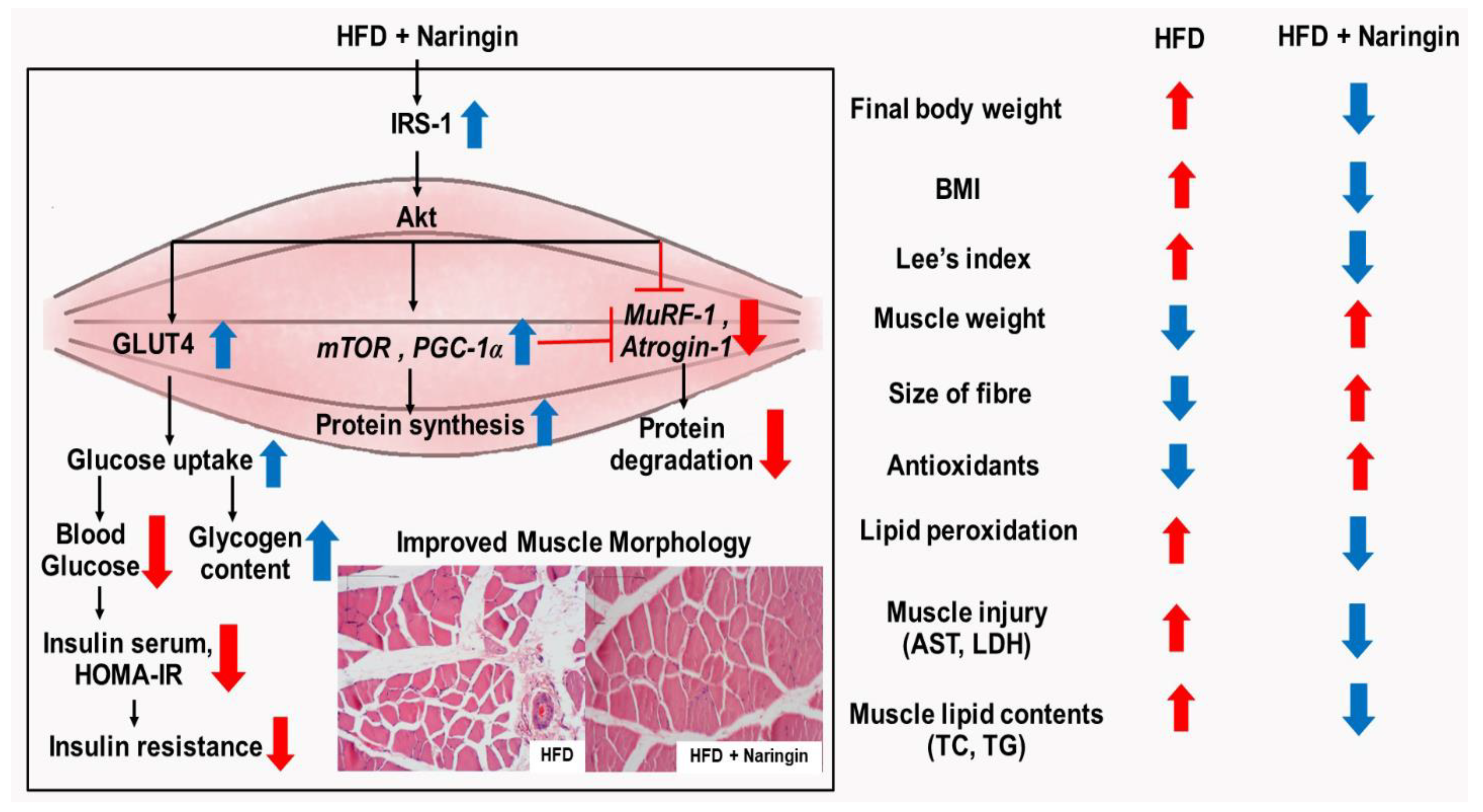
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3′-ethylbenzthiazoline-6-sulphonic acid) |
| Atrogin-1/MAFbx | muscle-specific F-box |
| CAT | catalase |
| CSA | cross-sectional area |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FBG | Fasting blood glucose |
| FOXO | Forkhead Box O |
| GLUT4 | Glucose transporter type 4 |
| HFD | high-fat diet |
| IRS-1 | insulin receptor substrate 1 |
| MDA | malondialdehyde |
| mTOR | mechanistic target of rapamycin |
| MuRF-1 | muscle ring-finger protein-1 |
| OGTT | Oral glucose tolerance test |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TC | total cholesterol |
| TG | triglyceride |
| TPC | total phenolic content |
References
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front. Physiol. 2013, 4, 371. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef]
- Rasool, S.; Geetha, T.; Broderick, T.L.; Babu, J.R. High Fat with High Sucrose Diet Leads to Obesity and Induces Myodegeneration. Front. Physiol. 2018, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’Sullivan, J.F. Cardio-Metabolic Effects of High-Fat Diets and Their Underlying Mechanisms—A Narrative Review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I.H.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and Cell Dysfunction. Oxid. Med. Cell. Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef]
- Turner, R.M.; Pirmohamed, M. Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Corsini, A.; Roden, M. The role of mitochondria in statin-induced myopathy. Eur. J. Clin. Investig. 2015, 45, 745–754. [Google Scholar] [CrossRef]
- Soliman, M.; Atteia, S.; Kefafy, M.; Ali, A.; Radwan, E. Histological study on the effect of rosuvastatin (Crestor) on the skeletal muscle of adult male albino rats and the possible protective effect of coenzyme Q10. Menoufia Med. J. 2017, 30, 1117–1124. [Google Scholar]
- Rengo, J.L.; Callahan, D.M.; Savage, P.D.; Ades, P.A.; Toth, M.J. Skeletal muscle ultrastructure and function in statin-tolerant individuals. Muscle Nerve 2016, 53, 242–251. [Google Scholar] [CrossRef][Green Version]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Bharti, S.; Rani, N.; Krishnamurthy, B.; Arya, D.S. Preclinical evidence for the pharmacological actions of naringin: A review. Planta Med. 2014, 80, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Kauter, K.; Brown, L. Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients 2013, 5, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem. Biophys. Res. Commun. 2010, 398, 178–183. [Google Scholar] [CrossRef]
- Janson, B.; Prasomthong, J.; Malakul, W.; Boonsong, T.; Tunsophon, S. Hibiscus sabdariffa L. calyx extract prevents the adipogenesis of 3T3-L1 adipocytes, and obesity-related insulin resistance in high-fat diet-induced obese rats. Biomed. Pharmacother. 2021, 138, 111438. [Google Scholar] [CrossRef]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar] [CrossRef]
- Makynen, K.; Jitsaardkul, S.; Tachasamran, P.; Sakai, N.; Puranachoti, S.; Nirojsinlapachai, N.; Chattapat, V.; Caengprasath, N.; Ngamukote, S.; Adisakwattana, S. Cultivar variations in antioxidant and antihyperlipidemic properties of pomelo pulp (Citrus grandis [L.] Osbeck) in Thailand. Food Chem. 2013, 139, 735–743. [Google Scholar] [CrossRef]
- Od-Ek, P.; Deenin, W.; Malakul, W.; Phoungpetchara, I.; Tunsophon, S. Anti-obesity effect of Carica papaya in high-fat diet fed rats. Biomed. Rep. 2020, 13, 30. [Google Scholar] [CrossRef]
- Chung, A.; Gurtu, S.; Chakravarthi, S.; Moorthy, M.; Palanisamy, U.D. Geraniin Protects High-Fat Diet-Induced Oxidative Stress in Sprague Dawley Rats. Front. Nutr. 2018, 5, 17. [Google Scholar] [CrossRef]
- Prasomthong, J.; Limpeanchob, N.; Daodee, S.; Chonpathompikunlert, P.; Tunsophon, S. Hibiscus sabdariffa extract improves hepatic steatosis, partially through IRS-1/Akt and Nrf2 signaling pathways in rats fed a high fat diet. Sci. Rep. 2022, 12, 7022. [Google Scholar] [CrossRef] [PubMed]
- Hadwan, M. New Method for Assessment of Serum Catalase Activity. Indian J. Sci. Technol. 2016, 9, 1. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Jia, X.-F.; Yu, B.; Gao, Y.; Bai, J.-G. Exogenous hydrogen peroxide influences antioxidant enzyme activity and lipid peroxidation in cucumber leaves at low light. Sci. Hortic. 2011, 129, 656–662. [Google Scholar] [CrossRef]
- Hassan, Z.; Yam, M.F.; Ahmad, M.; Yusof, A.P.M. Antidiabetic Properties and Mechanism of Action of Gynura procumbens Water Extract in Streptozotocin-Induced Diabetic Rats. Molecules 2010, 15, 9008–9023. [Google Scholar] [CrossRef]
- Nader, G.A.; Esser, K.A. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J. Appl. Physiol. 2001, 90, 1936–1942. [Google Scholar] [CrossRef]
- Tosolini, A.P.; Mohan, R.; Morris, R. Targeting the full length of the motor end plate regions in the mouse forelimb increases the uptake of fluoro-gold into corresponding spinal cord motor neurons. Front. Neurol. 2013, 4, 58. [Google Scholar] [CrossRef]
- Kusano, Y.; Tsujihara, N.; Masui, H.; Shibata, T.; Uchida, K.; Takeuchi, W. Diosgenin Supplementation Prevents Lipid Accumulation and Induces Skeletal Muscle-Fiber Hypertrophy in Rats. J. Nutr. Sci. Vitaminol. 2019, 65, 421–429. [Google Scholar] [CrossRef]
- Redman, L.M.; Ravussin, E. Caloric restriction in humans: Impact on physiological, psychological, and behavioral outcomes. Antioxid. Redox. Signal. 2011, 14, 275–287. [Google Scholar] [CrossRef]
- Mitchell, S.E.; Tang, Z.; Kerbois, C.; Delville, C.; Konstantopedos, P.; Bruel, A.; Derous, D.; Green, C.; Aspden, R.M.; Goodyear, S.R.; et al. The effects of graded levels of calorie restriction: I. impact of short term calorie and protein restriction on body composition in the C57BL/6 mouse. Oncotarget 2015, 6, 15902–15930. [Google Scholar] [CrossRef]
- Pengnet, S.; Prommaouan, S.; Sumarithum, P.; Malakul, W. Naringin Reverses High-Cholesterol Diet-Induced Vascular Dysfunction and Oxidative Stress in Rats via Regulating LOX-1 and NADPH Oxidase Subunit Expression. BioMed Res. Int. 2019, 2019, 3708497. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem.-Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; McDonald, K.S.; Schluter, J.M. The determinants of skeletal muscle force and power: Their adaptability with changes in activity pattern. J. Biomech. 1991, 24 (Suppl. S1), 111–122. [Google Scholar] [CrossRef]
- Eshima, H.; Tamura, Y.; Kakehi, S.; Kurebayashi, N.; Murayama, T.; Nakamura, K.; Kakigi, R.; Okada, T.; Sakurai, T.; Kawamori, R.; et al. Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol. Rep. 2017, 5, e13250. [Google Scholar] [CrossRef]
- Liu, S.H.; Chiu, C.Y.; Wang, L.P.; Chiang, M.T. Omega-3 Fatty Acids-Enriched Fish Oil Activates AMPK/PGC-1alpha Signaling and Prevents Obesity-Related Skeletal Muscle Wasting. Mar. Drugs 2019, 17, 380. [Google Scholar] [CrossRef]
- Janssen, L.; Allard, N.A.E.; Saris, C.G.J.; Keijer, J.; Hopman, M.T.E.; Timmers, S. Muscle Toxicity of Drugs: When Drugs Turn Physiology into Pathophysiology. Physiol. Rev. 2020, 100, 633–672. [Google Scholar] [CrossRef]
- Vallejo, W.A.; Diaz Uribe, C.D.; Oliveros, G.; Muñoz, A. Study of scavenging capacity of naringin extracted from citrus uranium peel against free radicals. Prospectiva 2016, 14, 31–35. [Google Scholar] [CrossRef]
- Attia, S.M. Abatement by naringin of lomefloxacin-induced genomic instability in mice. Mutagenesis 2008, 23, 515–521. [Google Scholar] [CrossRef]
- Beals, J.W.; Burd, N.A.; Moore, D.R.; van Vliet, S. Obesity Alters the Muscle Protein Synthetic Response to Nutrition and Exercise. Front. Nutr. 2019, 6, 87. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef]
- Jeong, H.; Koh, A.; Lee, J.; Park, D.; Lee, J.O.; Lee, M.N.; Jo, K.J.; Tran, H.N.K.; Kim, E.; Min, B.S.; et al. Inhibition of C1-Ten PTPase activity reduces insulin resistance through IRS-1 and AMPK pathways. Sci. Rep. 2017, 7, 17777. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.A.; McCurry, M.P.; Marino, A.; South, M.A.; Howell, M.E.; Layne, A.S.; Ramsey, M.W.; Stone, M.H. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J. Clin. Endocrinol. Metab. 2013, 98, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Kho, M.C.; Lee, Y.J.; Park, J.H.; Kim, H.Y.; Yoon, J.J.; Ahn, Y.M.; Tan, R.; Park, M.C.; Cha, J.D.; Choi, K.M.; et al. Fermented Red Ginseng Potentiates Improvement of Metabolic Dysfunction in Metabolic Syndrome Rat Models. Nutrients 2016, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Li, Q.; Song, W.J.; Yang, H.M.; Kim, S.Y.; Park, S.C.; Ahn, J.O.; Youn, H.Y. Fibroblast growth factor-1 as a mediator of paracrine effects of canine adipose tissue-derived mesenchymal stem cells on in vitro-induced insulin resistance models. BMC Vet. Res. 2018, 14, 351. [Google Scholar] [CrossRef]
- Naowaboot, J.; Piyabhan, P.; Tingpej, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Anti-insulin resistant effect of ferulic acid on high fat diet-induced obese mice. Asian Pac. J. Trop. Biomed. 2018, 8, 604–608. [Google Scholar] [CrossRef]
- Li, W.; Liang, X.; Zeng, Z.; Yu, K.; Zhan, S.; Su, Q.; Yan, Y.; Mansai, H.; Qiao, W.; Yang, Q.; et al. Simvastatin inhibits glucose uptake activity and GLUT4 translocation through suppression of the IR/IRS-1/Akt signaling in C2C12 myotubes. Biomed. Pharmacother. 2016, 83, 194–200. [Google Scholar] [CrossRef]
- Yaluri, N.; Modi, S.; Kokkola, T. Simvastatin induces insulin resistance in L6 skeletal muscle myotubes by suppressing insulin signaling, GLUT4 expression and GSK-3β phosphorylation. Biochem. Biophys. Res. Commun. 2016, 480, 194–200. [Google Scholar] [CrossRef]
- Kumar, P.; Bhandari, U.; Jamadagni, S. Fenugreek seed extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced obese rats. BioMed Res. Int. 2014, 2014, 606021. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Deval, C.; Potier, M.; Constans, J.; Mazur, A.; Bennetau-Pelissero, C.; Morand, C.; Bérard, A.M. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J. Nutr. Biochem. 2012, 23, 469–477. [Google Scholar] [CrossRef]
- Shin, K.O.; Bae, J.Y.; Woo, J.; Jang, K.S.; Kim, K.S.; Park, J.S.; Kim, I.K.; Kang, S. The effect of exercise on expression of myokine and angiogenesis mRNA in skeletal muscle of high fat diet induced obese rat. J. Exerc. Nutr. Biochem. 2015, 19, 91–98. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics 2022, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Song, W.; Liu, Y.; Chaudhuri, A.; Pieke-Dahl, S.; Strong, R.; Huang, T.T.; Epstein, C.J.; Roberts, L.J., 2nd; Csete, M.; et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic. Biol. Med. 2006, 40, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxid. Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef] [PubMed]
- Panajatovic, M.V.; Singh, F.; Krahenbuhl, S.; Bouitbir, J. Simvastatin Impairs Glucose Homeostasis in Mice Depending on PGC-1alpha Skeletal Muscle Expression. Biomedicines 2020, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Shalaby, S.M.; El-Gendy, J.; Abdelghany, E.M.A. Beneficial effects of metformin on muscle atrophy induced by obesity in rats. J. Cell. Biochem. 2019, 120, 5677–5686. [Google Scholar] [CrossRef]
- Abrigo, J.; Rivera, J.C.; Aravena, J.; Cabrera, D.; Simon, F.; Ezquer, F.; Ezquer, M.; Cabello-Verrugio, C. High Fat Diet-Induced Skeletal Muscle Wasting Is Decreased by Mesenchymal Stem Cells Administration: Implications on Oxidative Stress, Ubiquitin Proteasome Pathway Activation, and Myonuclear Apoptosis. Oxid. Med. Cell. Longev. 2016, 2016, 9047821. [Google Scholar] [CrossRef]
- Bouitbir, J.; Sanvee, G.M.; Panajatovic, M.V.; Singh, F.; Krähenbühl, S. Mechanisms of statin-associated skeletal muscle-associated symptoms. Pharmacol. Res. 2020, 154, 104201. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Novel Invasive and Noninvasive Cardiac-Specific Biomarkers in Obesity and Cardiovascular Diseases. Metab. Syndr. Relat. Disord. 2020, 18, 10–30. [Google Scholar] [CrossRef]
- Amin, K.A.; Nagy, M.A. Effect of Carnitine and herbal mixture extract on obesity induced by high fat diet in rats. Diabetol. Metab. Syndr. 2009, 1, 17. [Google Scholar] [CrossRef]
- Wang, X.X.; Ye, T.; Li, M.; Li, X.; Qiang, O.; Tang, C.W.; Liu, R. Effects of octreotide on hepatic glycogenesis in rats with high fat diet-induced obesity. Mol. Med. Rep. 2017, 16, 109–118. [Google Scholar] [CrossRef]
- Ma, Z.; Chu, L.; Liu, H.; Wang, W.; Li, J.; Yao, W.; Yi, J.; Gao, Y. Beneficial effects of paeoniflorin on non-alcoholic fatty liver disease induced by high-fat diet in rats. Sci. Rep. 2017, 7, 44819. [Google Scholar] [CrossRef]
- Reddy, T.; Nagaraju, I.; Kumar, K.; Lokanatha, V.; Reddy, C.; Jagetia, G. Cardioprotective Effect of Naringin in Mice Treated with Doxorubicin. Planta Med. 2008, 74, 49. [Google Scholar] [CrossRef]
- Ghalwash, M.; Elmasry, A.; El-Adeeb, N. Effect of L-carnitine on the skeletal muscle contractility in simvastatin-induced myopathy in rats. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rabearivony, A.; Zhang, W.; Chen, S.; An, X.; Liu, C. Chronopharmacology of simvastatin on hyperlipidaemia in high-fat diet-fed obese mice. J. Cell. Mol. Med. 2020, 24, 11024–11029. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073s–2085s. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′-3′) | Amplification Size (bp) | Annealing Tm (°C) | |
|---|---|---|---|---|
| SOD1 | F: | AATGTGTCCATTGAAGATCGTGTG | 118 | 60 |
| R: | GCTTCCAGCATTTCCAGTCTTTGTA | |||
| CAT | F: | GCAGGAAGACTTGCACACGGA | 133 | 58 |
| R: | ATGGGAAGGTTTCTGCCTCC | |||
| MuRF-1 | F: | GCCATCCTGGACGAGAAGAA | 234 | 55 |
| R: | CAGCTGGCAGCCCTTGGA | |||
| Atrogin-1 | F: | AGACCGGCTACTGTGGAAGAG | 218 | 60 |
| R: | CCGTGCATGGATGGTCAGTG | |||
| mTOR | F: | TCCACTGGAAGCACAGACCAAG | 125 | 58 |
| R: | GCTTATCAAGCAAGCGACATCTCA | |||
| PGC-1α | F: | GCGCCGTGTGATTTACGT | 74 | 56 |
| R: | AAAACTTCAAAGCGGTCTCTCAA | |||
| β-actin | F: | TGTCCACCTTCCAGCAGATGT | 101 | 58 |
| R: | AGCTCAGTAACAGTCGCGCTAGA |
| Assay | Naringin | Positive Control When Compared with Sample | ||
|---|---|---|---|---|
| Acarbose | Simvastatin | Orlistat | ||
| TPC (mgGAE/gDW) | 1.72 | N/A | N/A | N/A |
| DPPH (molAAE/gDW) | 3.30 | N/A | N/A | N/A |
| IC50 values of DPPH (mg/mL) | 24.82 | N/A | N/A | N/A |
| ABTS (molTE/gDW) | 0.17 | N/A | N/A | N/A |
| IC50 values of ABTS (mg/mL) | 2.67 | N/A | N/A | N/A |
| α-glucosidase | ||||
| IC50 value (mg/mL) | 0.259 | 1.025 | N/A | N/A |
| Maximum of inhibition (%) | 81.22 ± 0.35 | 88.27 ± 0.13 | N/A | N/A |
| Cholesterol esterase | ||||
| IC50 value (mg/mL) | 0.21 | N/A | 0.92 | N/A |
| Maximum of inhibition (%) | 97.26 ± 0.88 | N/A | 82.40 ± 0.38 | N/A |
| Pancreatic lipase | ||||
| IC50 value (mg/mL) | 0.009 | N/A | N/A | 0.0002 |
| Maximum of inhibition (%) | 71.21 ± 3.29 | N/A | N/A | 84.99 ± 1.05 |
| Parameters | Control | HFD | HFD + SIM | HFD + N50 | HFD + N100 |
|---|---|---|---|---|---|
| Initial weight (g) | 255.32 ± 19.57 | 257.79 ± 22.04 | 245.06 ± 30.69 | 249.62 ± 15.98 | 237.80 ± 14.66 |
| Final weight (g) | 516.20 ± 15.61 | 601.40 ± 2.71 aaa | 555.20 ± 7.79 a,bb | 568.60 ± 5.27 aa | 556.20 ± 7.83 a,b |
| Lee’s index (g/cm) | 308.38 ± 2.71 | 321.14 ± 2.5 aaa | 316.19 ± 3.49 | 313.54 ± 2.18 | 312.68 ± 0.89 b |
| BMI (kg/m2) | 0.69 ± 0.03 | 0.79 ± 0.03 aaa | 0.75 ± 0.03 aa,b | 0.74 ± 0.03 a,b | 0.74 ± 0.02 a,bb |
| Caloric intake (kacl/day) | 79.78 ± 1.41 | 90.78 ± 3.27 aa | 84.39 ± 2.19 | 84.04 ± 2.26 | 83.57 ± 2.50 |
| Muscle TC (mg/g of tissue) | 134.42 ± 10.63 | 218.76 ± 23.01 a | 120.07 ± 8.74 bb | 91.94 ± 7.56 bbb | 69.49 ± 12.74 bbb |
| Muscle TG (mg/g of tissue) | 410.28 ± 29.14 | 1217.18 ± 53.13 aaa | 418.40 ± 35.71 bbb | 877.05 ± 38.03 aaa,bbb,ccc | 626.39 ± 28.94 aaa,bbb,ccc,ddd |
| Serum CK (units/L) | 42.67 ± 2.68 | 56.08 ± 6.32 | 85.24 ± 12.19 a | 49.01 ± 4.74 c | 46.87 ± 6.94 c |
| Serum LDH (nmol) | 101.84 ± 15.50 | 147.65 ± 9.97 | 175.16 ± 43.07 | 118.9 ± 11.78 | 88.98 ± 20.31 b |
| Serum AST (U/L) | 92.4 ± 3.59 | 223.4 ± 21.08 aaa | 191.8 ± 14.65 aaa | 174.2 ± 21.24 aa,b | 173 ± 13.33 aa,b |
| Serum ALT (U/L) | 24.25 ± 1.25 | 89.5 ± 15.50 aa | 75 ± 15.07 aa | 58 ± 10.46 | 59.75 ± 11.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Termkwancharoen, C.; Malakul, W.; Phetrungnapha, A.; Tunsophon, S. Naringin Ameliorates Skeletal Muscle Atrophy and Improves Insulin Resistance in High-Fat-Diet-Induced Insulin Resistance in Obese Rats. Nutrients 2022, 14, 4120. https://doi.org/10.3390/nu14194120
Termkwancharoen C, Malakul W, Phetrungnapha A, Tunsophon S. Naringin Ameliorates Skeletal Muscle Atrophy and Improves Insulin Resistance in High-Fat-Diet-Induced Insulin Resistance in Obese Rats. Nutrients. 2022; 14(19):4120. https://doi.org/10.3390/nu14194120
Chicago/Turabian StyleTermkwancharoen, Chutimon, Wachirawadee Malakul, Amnat Phetrungnapha, and Sakara Tunsophon. 2022. "Naringin Ameliorates Skeletal Muscle Atrophy and Improves Insulin Resistance in High-Fat-Diet-Induced Insulin Resistance in Obese Rats" Nutrients 14, no. 19: 4120. https://doi.org/10.3390/nu14194120
APA StyleTermkwancharoen, C., Malakul, W., Phetrungnapha, A., & Tunsophon, S. (2022). Naringin Ameliorates Skeletal Muscle Atrophy and Improves Insulin Resistance in High-Fat-Diet-Induced Insulin Resistance in Obese Rats. Nutrients, 14(19), 4120. https://doi.org/10.3390/nu14194120





