Mediterranean Diet Adherence and Risk of Depressive Symptomatology in a French Population-Based Cohort of Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Study Sample
2.3. Adherence to the Mediterranean Diet
2.4. Depressive Symptomatology
2.5. Covariates
2.6. Statistical Analysis
2.7. Association between MeDi Adherence and Risk of DS
2.8. Additional Analyses
2.8.1. CES-D Score ≥ 16
2.8.2. Sex-Specific Cutoffs for Identifying DS
2.8.3. Mediterranean Diet Adherence Assessed by Two Alternative Scores
2.9. Missing Data
3. Results
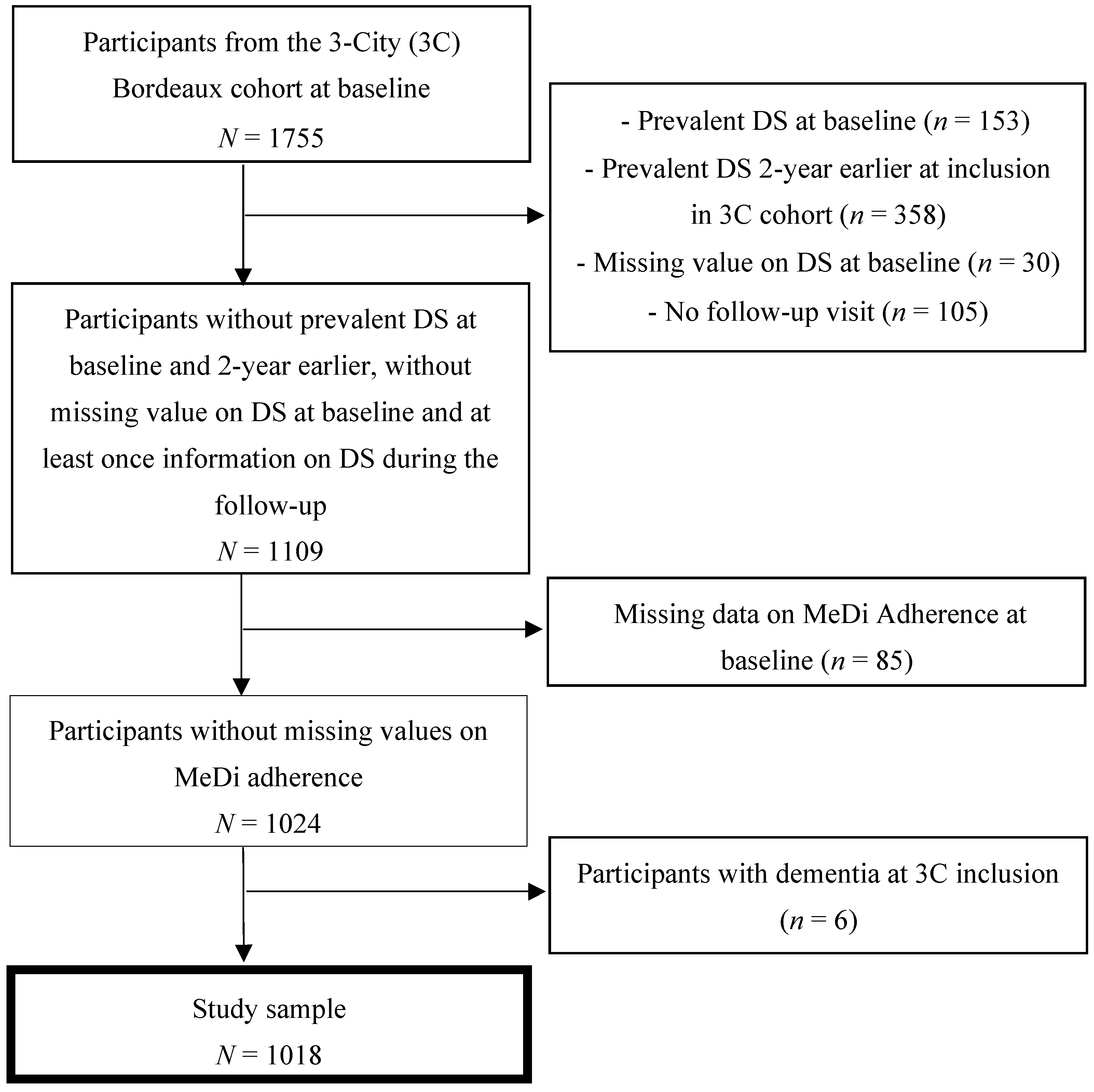
3.1. Sample Selection
3.2. Descriptive Characteristics of the Study Sample
3.3. Adherence to the Mediterranean Diet
3.4. Incidence of DS over Time
3.5. Association between MeDi Adherence and Risk of DS
3.6. Additional Analyses
3.6.1. CES-D Score ≥ 16
3.6.2. Sex-Specific Cutoffs for Identifying DS
3.6.3. Mediterranean Diet Adherence Assessed with Two Alternative Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Léon, C.; Chan Chee, C.; du Roscoät, E. La dépression en france chez les 18–75 ans: Résultats du baromètre santé 2017//depression in france among the 18–75 year-olds: Results from the 2017 health barometer. Bull Epidemiol Hebd. 2018, 32–33, 637–644. Available online: http://invs.santepubliquefrance.fr/beh/2018/32-33/2018_32-33_1.html (accessed on 30 March 2021).
- GBD. 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Prevalence of Depression by Age. Our World in Data. Available online: https://ourworldindata.org/grapher/prevalence-of-depression-by-age (accessed on 30 March 2021). [CrossRef]
- Perez-Wehbe, A.I.; Perestelo-Pérez, L.; Bethencourt-Pérez, J.M.; Cuellar-Pompa, L.; Peñate-Castro, W. Treatment-resistant depression: A systematic review of systematic reviews. Int. J. Clin. Health Psychol. 2014, 14, 145–153. [Google Scholar] [CrossRef][Green Version]
- Buchalter, E.L.F.; Oughli, H.A.; Lenze, E.J.; Dixon, D.; Miller, J.P.; Blumberger, D.M.; Karp, J.F.; Reynolds, C.F., 3rd; Mulsant, B.H. Predicting Remission in Late-Life Major Depression: A Clinical Algorithm Based Upon Past Treatment History. J. Clin. Psychiatry 2019, 80, 18m12483. [Google Scholar] [CrossRef] [PubMed]
- Worrall, C.; Jongenelis, M.; Pettigrew, S. Modifiable Protective and Risk Factors for Depressive Symptoms among Older Community-dwelling Adults: A Systematic Review. J. Affect. Disord. 2020, 272, 305–317. [Google Scholar] [CrossRef]
- Farioli Vecchioli, S.; Sacchetti, S.; Nicolis di Robilant, V.; Cutuli, D. The Role of Physical Exercise and Omega-3 Fatty Acids in Depressive Illness in the Elderly. Curr. Neuropharmacol. 2018, 16, 308–326. [Google Scholar] [CrossRef]
- Kovess-Masfety, V.; Alonso, J.; Brugha, T.S.; Angermeyer, M.C.; Haro, J.M.; Sevilla-Dedieu, C.; ESEMeD/MHEDEA 2000 Investigators. Differences in lifetime use of services for mental health problems in six European countries. Psychiatr. Serv. Wash. DC 2007, 58, 213–220. [Google Scholar] [CrossRef]
- Chishti, P.; Stone, D.H.; Corcoran, P.; Williamson, E.; Petridou, E.; EUROSAVE Working Group. Suicide mortality in the European Union. Eur. J. Public Health 2003, 13, 108–114. [Google Scholar] [CrossRef]
- Shafiei, F.; Moghaddam, A.S.; Larijani, B.; Esmaillzadeh, A. Adherence to the Mediterranean diet and risk of depression: A systematic review and updated meta-analysis of observational studies. Nutr. Rev. 2019, 77, 230–239. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2013, 17, 2769–2782. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean Diet and Mild Cognitive Impairment. Arch Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef]
- Féart, C. Adherence to a Mediterranean Diet, Cognitive Decline, and Risk of Dementia. JAMA 2009, 302, 638. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Delgado-Rodríguez, M.; Alonso, A.; Schlatter, J.; Lahortiga, F.; Majem, L.S.; Martínez-González, M.A. Association of the Mediterranean Dietary Pattern With the Incidence of Depression: The Seguimiento Universidad de Navarra/University of Navarra Follow-up (SUN) Cohort. Arch Gen. Psychiatry 2009, 66, 1090. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef]
- Skarupski, K.A.; Tangney, C.C.; Li, H.; Evans, D.A.; Morris, M.C. Mediterranean diet and depressive symptoms among older adults over time. J. Nutr. Health Aging 2013, 17, 441–445. [Google Scholar] [CrossRef]
- Rienks, J.; Dobson, A.J.; Mishra, G.D. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: Results from a large community-based prospective study. Eur. J. Clin. Nutr. 2012, 67, 75–82. [Google Scholar] [CrossRef]
- Das, A.; Cumming, R.G.; Naganathan, V.; Ribeiro, R.V.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Hirani, V. The association between antioxidant intake, dietary pattern and depressive symptoms in older Australian men: The Concord Health and Ageing in Men Project. Eur. J. Nutr. 2020, 60, 443–454. [Google Scholar] [CrossRef]
- Matison, A.P.; Mather, K.A.; Flood, V.M.; Reppermund, S. Associations between nutrition and the incidence of depression in middle-aged and older adults: A systematic review and meta-analysis of prospective observational population-based studies. Ageing Res. Rev. 2021, 70, 101403. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, M.; Pugliatti, M.; Hubbard, R.; Britton, J.; Sotgiu, S.; Sadovnick, A.D.; Yee, I.M.L.; Cumsille, M.A.; Bevilacqua, J.A.; Burdett, S.; et al. Vascular Factors and Risk of Dementia: Design of the Three-City Study and Baseline Characteristics of the Study Population. Neuroepidemiology 2003, 22, 316–325. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a literature-based adherence score to Mediterranean diet: The MEDI-LITE score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef] [PubMed]
- André, P.; de Barros, J.-P.P.; Merle, B.M.; Samieri, C.; Helmer, C.; Delcourt, C.; Féart, C. Mediterranean diet and prudent diet are both associated with low circulating esterified 3-hydroxy fatty acids, a proxy of LPS burden, among older adults. Am. J. Clin. Nutr. 2021, 114, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- The CES-D Scale: A Self-Report Depression Scale for Research in the General Population—Lenore Sawyer Radloff. 1977. Available online: https://journals.sagepub.com/doi/abs/10.1177/014662167700100306 (accessed on 30 March 2021).
- Vilagut, G.; Forero, C.G.; Barbaglia, M.G.; Alonso, J. Screening for Depression in the General Population with the Center for Epidemiologic Studies Depression (CES-D): A Systematic Review with Meta-Analysis. PLoS ONE 2016, 11, e0155431. [Google Scholar] [CrossRef]
- Classification ATC Des Médicaments. VIDAL. Available online: https://www.vidal.fr/medicaments/classification/atc.html (accessed on 12 July 2021).
- Boyd, C.M.; Fortin, M. Future of Multimorbidity Research: How Should Understanding of Multimorbidity Inform Health System Design? Public Health Rev. 2010, 32, 451–474. [Google Scholar] [CrossRef]
- Fuhrer, R.; Rouillon, F. La version française de l’échelle CES-D (Center for Epidemiologic Studies-Depression Scale). Description et traduction de l’échelle d’autoévaluation. Psychiatry Psychobiol. 1989, 4, 163–166. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-De-Mesquita, B.; Ocké, M.C.; Peeters, P.H.; Van Der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005, 330, 991. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K.; Vink, G.; Schouten, R.; Robitzsch, A.; Rockenschaub, P.; Doove, L.; Jolani, S.; Moreno-Betancur, M.; White, I.; et al. Mice: Multivariate Imputation by Chained Equation. 2021. Available online: https://CRAN.R-project.org/package=mice (accessed on 21 March 2022).
- Greenland, S.; Finkle, W.D. A Critical Look at Methods for Handling Missing Covariates in Epidemiologic Regression Analyses. Am. J. Epidemiol. 1995, 142, 1255–1264. [Google Scholar] [CrossRef]
- Adjibade, M.; Assmann, K.; Andreeva, V.; Lemogne, C.; Hercberg, S.; Galan, P.; Kesse-Guyot, E. Prospective association between adherence to the Mediterranean diet and risk of depressive symptoms in the French SU.VI.MAX cohort. Eur. J. Nutr. 2017, 57, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Henríquez-Sánchez, P.; Ruiz-Canela, M.; Lahortiga, F.; Molero, P.; Toledo, E.; Martínez-González, M.A. A longitudinal analysis of diet quality scores and the risk of incident depression in the SUN Project. BMC Med. 2015, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- CNFS. Atteintes Neurocognitives (DSM V). Available online: https://cnfs.ca/pathologies/atteintes-neurocognitives-dsm-v (accessed on 4 February 2022).
- Leong, I.Y.; Farrell, M.J.; Helme, R.D.; Gibson, S.J. The relationship between medical comorbidity and self-rated pain, mood disturbance, and function in older people with chronic pain. J. Gerontol. Ser. A 2007, 62, 550–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, P.; Hazif-Thomas, C. Les nouvelles approches de la dépression de la personne âgée. Gerontol. Soc. 2008, 126, 141–155. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, F.; Brombo, G.; Zuliani, G. Nootropics, Functional Foods, and Dietary Patterns for Prevention of Cognitive Decline. Nutr. Funct. Foods Healthy Aging 2017, 19, 211–232. [Google Scholar] [CrossRef]
- Moludi, J.; Moradinazar, M.; Hamzeh, B.; Najafi, F.; Pasdar, Y. Depression Relationship with Dietary Patterns and Dietary Inflammatory Index in Women: Result from Ravansar Cohort Study. Neuropsychiatr. Dis. Treat. 2020, 16, 1595–1603. [Google Scholar] [CrossRef]
- Adjibade, M.; Lemogne, C.; Touvier, M.; Hercberg, S.; Galan, P.; E Assmann, K.; Julia, C.; Kesse-Guyot, E. The Inflammatory Potential of the Diet is Directly Associated with Incident Depressive Symptoms Among French Adults. J. Nutr. 2019, 149, 1198–1207. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2020, 26, 134–150. [Google Scholar] [CrossRef]
- Lucas, M.; Chocano-Bedoya, P.O.; Schulze, M.B.; Mirzaei, F.; O’Reilly, J.; Okereke, O.I.; Hu, F.B.; Willett, W.C.; Ascherio, A. Inflammatory dietary pattern and risk of depression among women. Brain Behav. Immun. 2013, 36, 46–53. [Google Scholar] [CrossRef]
- Tabue-Teguo, M.; Grasset, L.; Avila-Funes, J.A.; Genuer, R.; Proust-Lima, C.; Peres, K.; Féart, C.; Amieva, H.; Harmand, M.G.-C.; Helmer, C.; et al. Prevalence and Co-Occurrence of Geriatric Syndromes in People Aged 75 Years and Older in France: Results From the Bordeaux Three-city Study. J. Gerontol. Ser. A 2017, 73, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, A.; Barul, C.; Féart, C.; Helmer, C.; Bernard, C.; Periot, O.; Dilharreguy, B.; Dartigues, J.; Allard, M.; Barberger-Gateau, P.; et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimer’s Dement. 2015, 11, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Féart, C.; Samieri, C.; Allès, B.; Barberger-Gateau, P. Potential benefits of adherence to the Mediterranean diet on cognitive health. Proc. Nutr. Soc. 2012, 72, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Lôo, H.; Olié, J.P. Effets thérapeutiques et indications des antidépresseurs. EMC—Psychiatr. 2004, 1, 273–283. [Google Scholar] [CrossRef]
| Mediterranean Diet Adherence 1 | |||||
|---|---|---|---|---|---|
| Overall (n = 1018) | Low Score ≤ 9 (n = 257) | Moderate Score = [10, 11] (n = 389) | High Score ≥ 12 (n = 372) | p-Value 4 | |
| N (%)/ Mean ± SD | N (%)/ Mean ± SD | N (%)/ Mean ± SD | N (%)/ Mean ± SD | ||
| Women | 582 (57.2) | 130 (50.6) | 239 (61.4) | 213 (57.3) | 0.024 |
| Age (years) | 75.6 ± 4.8 | 75.5 ± 5.0 | 75.7 ± 4.7 | 75.7 ± 4.8 | 0.543 |
| Living conditions | 0.521 | ||||
| 358 (35.2) | 100 (38.9) | 131 (33.7) | 127 (34.1) | |
| 600 (58.9) | 140 (54.5) | 234 (60.2) | 226 (60.8) | |
| 60 (5.9) | 17 (6.6) | 24 (6.2) | 19 (5.1) | |
| Educational level | 0.086 | ||||
| 296 (29.1) | 83 (32.3) | 112 (28.8) | 101 (27.2) | |
| 280 (27.5) | 84 (32.7) | 95 (24.4) | 101 (27.2) | |
| 112 (11.0) | 22 (8.6) | 48 (12.3) | 42 (11.3) | |
| 330 (32.4) | 68 (26.5) | 134 (34.4) | 128 (34.4) | |
| Monthly income, EUR a | 0.129 | ||||
| 373 (36.6) | 113 (44.0) | 136 (35.0) | 124 (33.3) | |
| 267 (26.2) | 62 (24.1) | 109 (28.0) | 96 (25.8) | |
| 311 (30.6) | 69 (26.8) | 116 (29.8) | 126 (33.9) | |
| 67 (6.6) | 13 (5.1) | 28 (7.2) | 26 (7.0) | |
| Number of pack-years smoked b | 9.5 ± 18.1 | 12.8 ± 22.0 | 9.0 ± 17.0 | 7.7 ± 15.9 | 0.001 |
| Body mass index (in kg/m2) c | 0.208 | ||||
| 379 (37.6) | 90 (35.6) | 137 (35.6) | 152 (41.1) | |
| 463 (45.9) | 113 (44.7) | 182 (47.3) | 168 (45.4) | |
| 166 (16.5) | 50 (19.8) | 66 (17.1) | 50 (13.5) | |
| Regular physical activity d | 319 (31.3) | 62 (24.1) | 133 (34.2) | 124 (33.3) | 0.023 |
| Total energy intake (kcal/day) e | 1741 ± 540 | 1752 ± 580 | 1729 ± 538 | 1746 ± 513 | 0.931 |
| Mini Mental State Examination score f | 27.8 ± 1.9 | 27.7 ± 2.0 | 27.8 ± 1.8 | 27.8 ± 2.0 | 0.508 |
| Multimorbidity 2 | 550 (54.0) | 120 (46.7) | 216 (55.5) | 214 (57.5) | 0.021 |
| CES-D score at baseline | 4.9 ± 4.0 | 4.9 ± 3.9 | 4.8 ± 4.1 | 5.0 ± 3.9 | |
| DS 3 during the follow-up | 400 (39.3) | 104 (40.5) | 155 (39.8) | 141 (37.9) | 0.779 |
| 297 (29.2) | 84 (32.7) | 111 (28.5) | 102 (27.4) | |
| 182 (17.9) | 37 (14.4) | 73 (18.8) | 72 (19.4) | |
| Mediterranean Diet Adherence 1 | ||||
|---|---|---|---|---|
| Food Groups | Overall (n = 1018) | Low Score ≤ 9 (n = 257) | Moderate Score = [10, 11] (n = 389) | High Score ≥ 12 (n = 372) |
| N (%) Mean ± SD | N (%) Mean ± SD | N (%) Mean ± SD | N (%) Mean ± SD | |
| Fruits | 13.6 ± 6.6 | 9.3 ± 6.5 | 13.8 ± 6.1 | 16.2 ± 5.6 |
| Vegetables | 19.5 ± 7.2 | 15.7 ± 6.8 | 19.5 ± 6.8 | 22.3 ± 6.6 |
| Legumes | 0.6 ± 0.7 | 0.5 ± 0.7 | 0.6 ± 0.7 | 0.8 ± 0.6 |
| Cereals | 22.3 ± 5.9 | 20.8 ± 6.9 | 22.2 ± 5.9 | 23.4 ± 4.9 |
| Fish | 3.0 ± 1.8 | 2.0 ± 1.4 | 3.0 ± 1.7 | 3.6 ± 1.8 |
| Dairy products | 16.0 ± 7.2 | 17.4 ± 7.4 | 15.7 ± 6.7 | 15.4 ± 7.5 |
| Alcohol | 11.1 ± 12.6 | 15.3 ± 16.4 | 10.6 ± 12.2 | 8.8 ± 8.8 |
| Meat | 4.9 ± 2.5 | 5.9 ± 2.9 | 4.8 ± 2.4 | 4.3 ± 1.9 |
| Olive oil | ||||
| 393 (38.6) | 175 (68.1) | 164 (42.2) | 54 (14.5) |
| 353 (34.7) | 59 (23.0) | 152 (39.1) | 142 (38.2) |
| 272 (26.7) | 23 (8.9) | 73 (18.8) | 176 (47.3) |
| Incident DS/Total | OR [95% CI] ** | p-Value *** | |
|---|---|---|---|
| Mediterranean diet adherence * | 0.404 | ||
| 104/257 | 1 | |
| 155/389 | 0.82 [0.52; 1.30] | |
| 141/372 | 0.72 [0.45; 1.16] |
| Incident DS/Total | OR [95% CI] ** | p-Value *** | |
|---|---|---|---|
| Mediterranean diet adherence * | 0.053 | ||
| 84/257 | 1 | |
| 111/389 | 0.72 [0.46; 1.11] | |
| 102/372 | 0.57 [0.36; 0.90] |
| Incident DS/Total | OR [95% CI] ** | p-Value *** | |
|---|---|---|---|
| Mediterranean diet adherence * | |||
| 0.301 | ||
| 103/251 | 1 | |
| 184/441 | 0.86 [0.55; 1.35] | |
| 113/326 | 0.69 [0.42; 1.12] | |
| 0.560 | ||
| 128/332 | 1 | |
| 151/373 | 0.87 [0.53; 1.44] | |
| 121/313 | 0.75 [0.44; 1.27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardinet, J.; Chuy, V.; Carriere, I.; Galéra, C.; Pouchieu, C.; Samieri, C.; Helmer, C.; Cougnard-Grégoire, A.; Féart, C. Mediterranean Diet Adherence and Risk of Depressive Symptomatology in a French Population-Based Cohort of Older Adults. Nutrients 2022, 14, 4121. https://doi.org/10.3390/nu14194121
Bardinet J, Chuy V, Carriere I, Galéra C, Pouchieu C, Samieri C, Helmer C, Cougnard-Grégoire A, Féart C. Mediterranean Diet Adherence and Risk of Depressive Symptomatology in a French Population-Based Cohort of Older Adults. Nutrients. 2022; 14(19):4121. https://doi.org/10.3390/nu14194121
Chicago/Turabian StyleBardinet, Jeanne, Virginie Chuy, Isabelle Carriere, Cédric Galéra, Camille Pouchieu, Cécilia Samieri, Catherine Helmer, Audrey Cougnard-Grégoire, and Catherine Féart. 2022. "Mediterranean Diet Adherence and Risk of Depressive Symptomatology in a French Population-Based Cohort of Older Adults" Nutrients 14, no. 19: 4121. https://doi.org/10.3390/nu14194121
APA StyleBardinet, J., Chuy, V., Carriere, I., Galéra, C., Pouchieu, C., Samieri, C., Helmer, C., Cougnard-Grégoire, A., & Féart, C. (2022). Mediterranean Diet Adherence and Risk of Depressive Symptomatology in a French Population-Based Cohort of Older Adults. Nutrients, 14(19), 4121. https://doi.org/10.3390/nu14194121








