The Effect of Exercise Training and Royal Jelly on Hippocampal Cannabinoid-1-Receptors and Pain Threshold in Experimental Autoimmune Encephalomyelitis in Rats as Animal Model of Multiple Sclerosis
Abstract
1. Introduction
2. Methods
2.1. Animals and Implementation of Research Design
2.2. Induction of EAE Disease
2.3. Training Protocol
2.4. Royal Jelly Supplementation
2.5. Pain Threshold (PT) Evaluation
2.6. Measurement of CB1R
2.7. Data Analysis Procedure
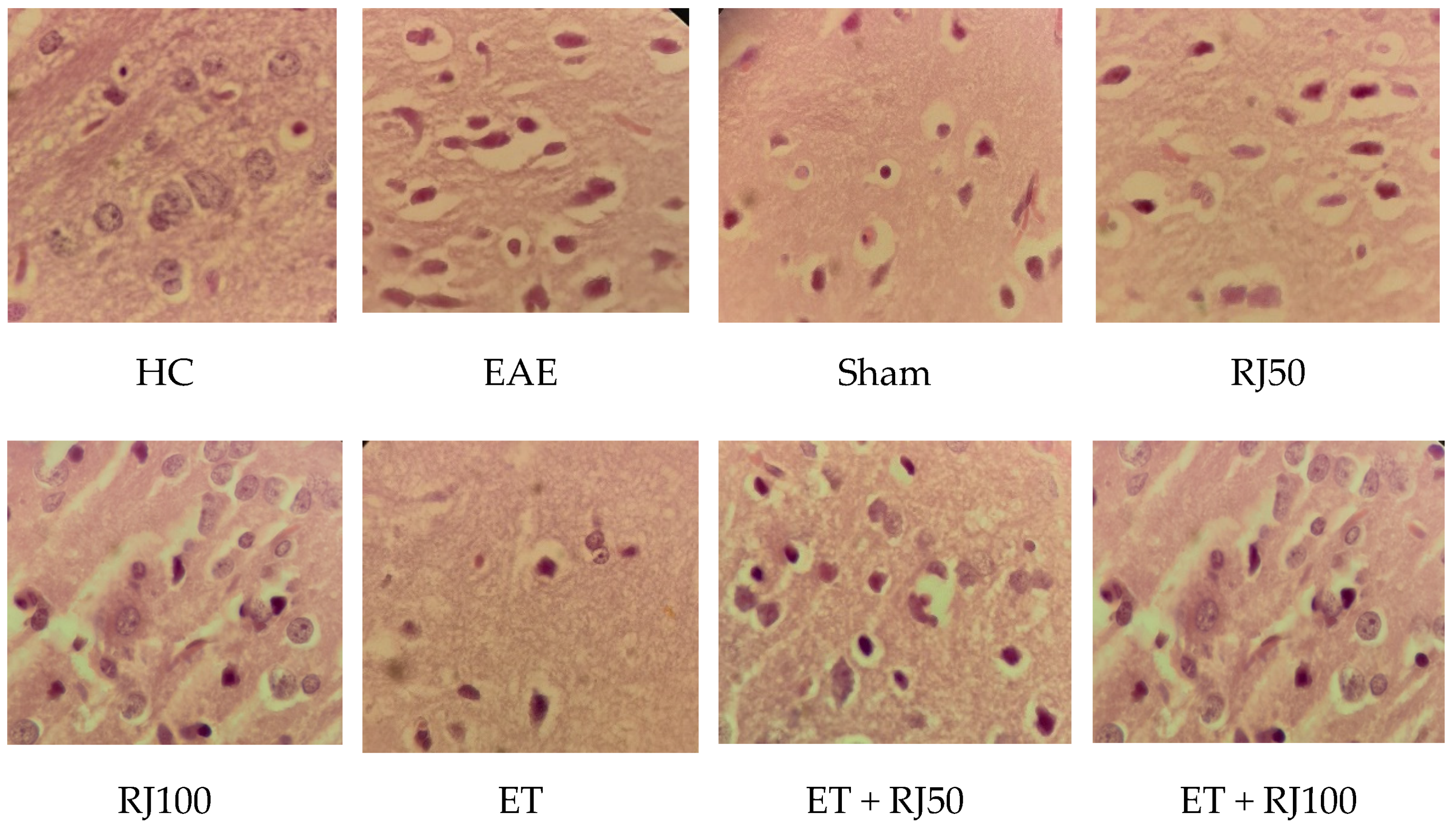
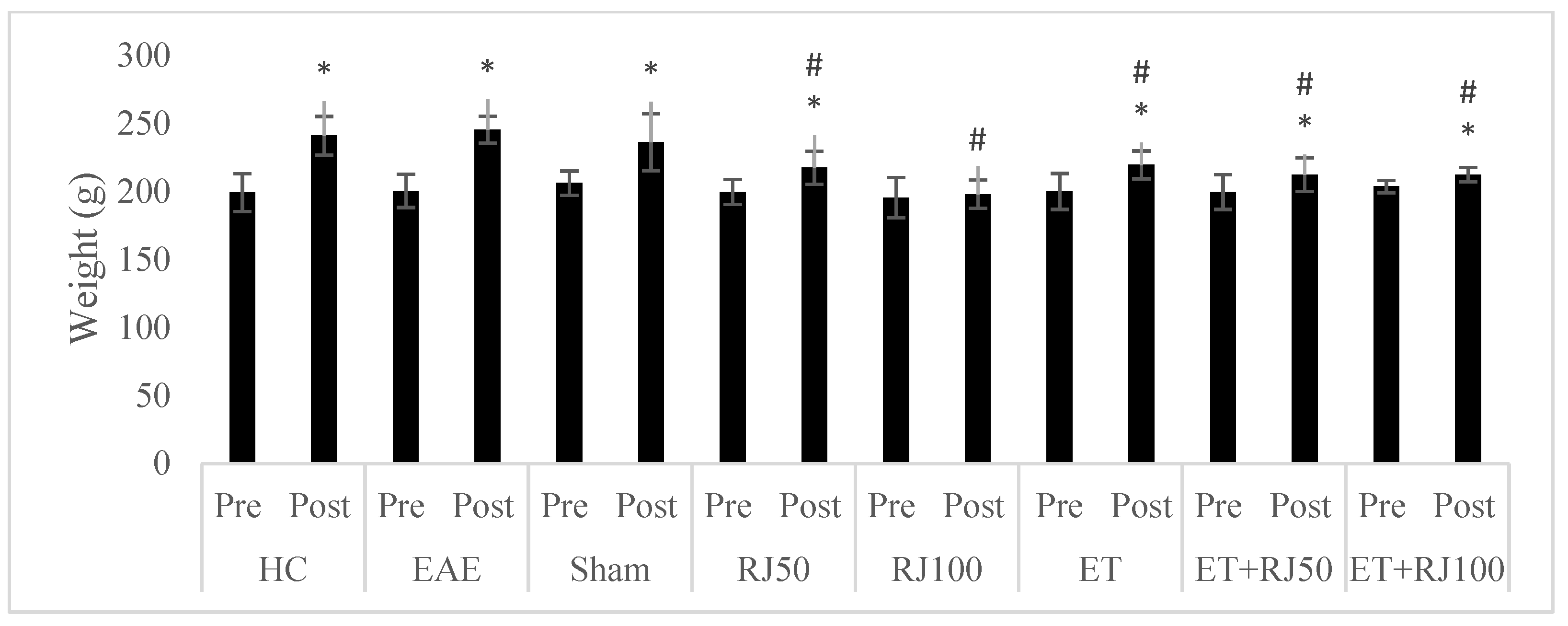
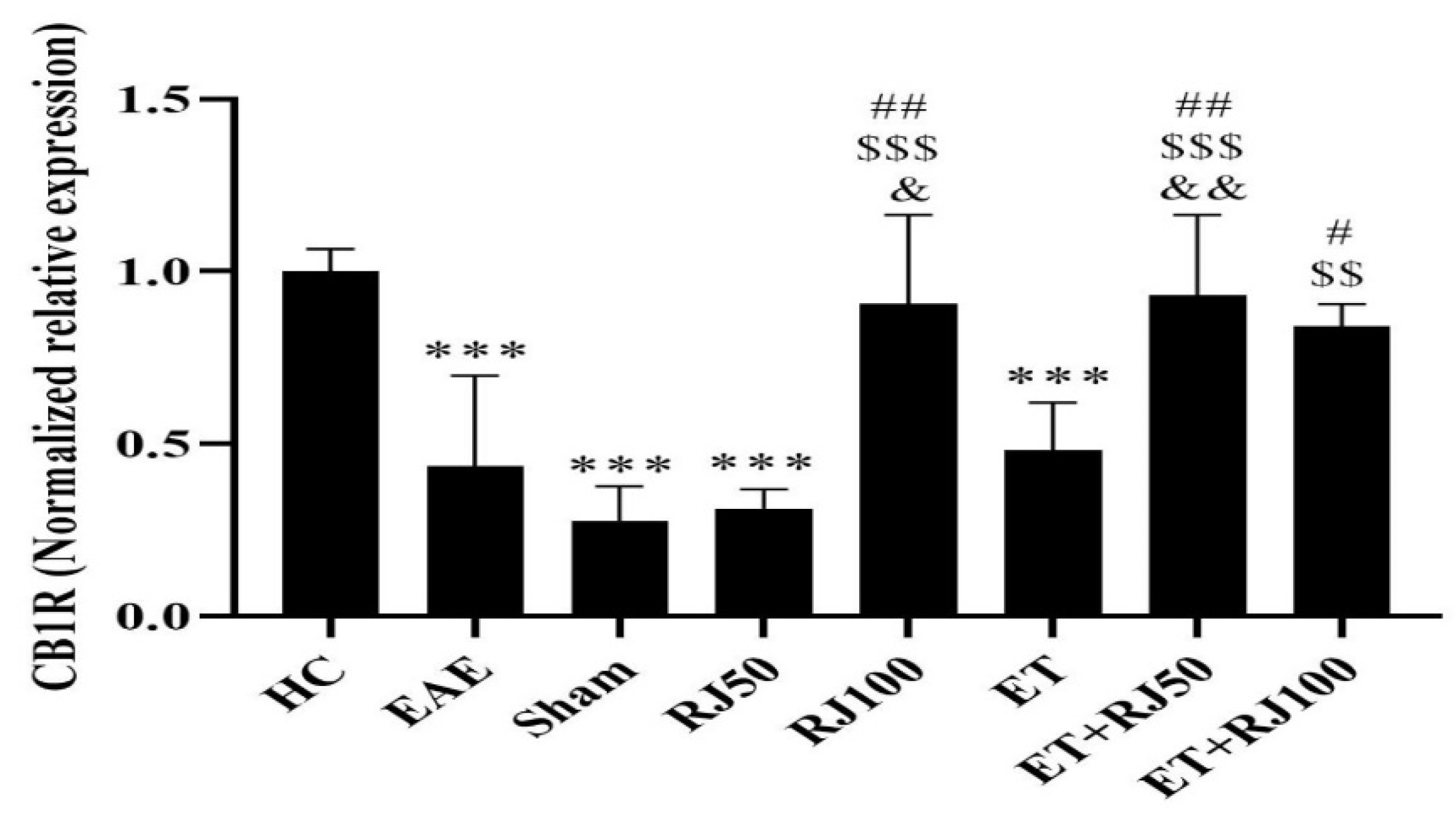
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of Multiple Sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, N.B.; Sant’Anna, M.; Giardini, A.C.; Araujo, L.; Fonseca, L.A.; Basso, A.; Cury, Y.; Picolo, G. Crotoxin down-modulates pro-inflammatory cells and alleviates pain on the MOG35-55-induced experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Brain. Behav. Immun. 2020, 84, 253–268. [Google Scholar] [CrossRef]
- Dworsky-Fried, Z.; Faig, C.A.; Vogel, H.A.; Kerr, B.J.; Taylor, A.M. Central amygdala inflammation drives pain hypersensitivity and attenuates morphine analgesia in experimental autoimmune encephalomyelitis. Pain 2022, 163, e49–e61. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front. Microbiol. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Depont, F.; Berenbaum, F.; Filippi, J.; Le Maitre, M.; Nataf, H.; Paul, C.; Peyrin-Biroulet, L.; Thibout, E. Interventions to Improve Adherence in Patients with Immune-Mediated Inflammatory Disorders: A Systematic Review. PLoS ONE 2015, 10, e0145076. [Google Scholar] [CrossRef]
- Evans, C.; Marrie, R.A.; Zhu, F.; Leung, S.; Lu, X.; Melesse, D.Y.; Kingwell, E.; Zhao, Y.; Tremlett, H. Adherence and persistence to drug therapies for multiple sclerosis: A population-based study. Mult. Scler. Relat. Disord. 2016, 8, 78–85. [Google Scholar] [CrossRef]
- Miller, E.; Morel, A.; Redlicka, J.; Miller, I.; Saluk, J. Pharmacological and non-pharmacological therapies of cognitive impairment in multiple sclerosis. Curr. Neuropharmacol. 2018, 16, 475–483. [Google Scholar] [CrossRef]
- Coote, S.; Uszynski, M.; Herring, M.P.; Hayes, S.; Scarrott, C.; Newell, J.; Gallagher, S.; Larkin, A.; Motl, R.W. Effect of exercising at minimum recommendations of the multiple sclerosis exercise guideline combined with structured education or attention control education-secondary results of the step it up randomised controlled trial. BMC Neurol. 2017, 17, 119. [Google Scholar] [CrossRef]
- Kalb, R.; Brown, T.R.; Coote, S.; Costello, K.; Dalgas, U.; Garmon, E.; Giesser, B.; Halper, J.; Karpatkin, H.; Keller, J.; et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult. Scler. 2020, 26, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Sandroff, B.M. Physical exercise in multiple sclerosis is not just a symptomatic therapy: It has a disease-modifying effect-Yes. Mult. Scler. 2022, 28, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Parks, N.E.; Jackson-Tarlton, C.S.; Vacchi, L.; Merdad, R.; Johnston, B.C. Dietary interventions for multiple sclerosis-related outcomes. Cochrane. Database. Syst. Rev. 2020, 5, CD004192. [Google Scholar] [CrossRef] [PubMed]
- Lohrasbi, M.; Taghian, F.; Jalali Dehkordi, K.; Hosseini, S.A. The functional mechanisms of synchronizing royal jelly consumption and physical activity on rat with multiple sclerosis-like behaviors hallmarks based on bioinformatics analysis, and experimental survey. BMC Neurosci. 2022, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Razazian, N.; Yavari, Z.; Farnia, V.; Azizi, A.; Kordavani, L.; Bahmani, D.S.; Holsboer-Trachsler, E.; Brand, S. Exercising Impacts on Fatigue, Depression, and Paresthesia in Female Patients with Multiple Sclerosis. Med. Sci. Sports. Exerc. 2016, 48, 796–803. [Google Scholar] [CrossRef]
- Sadeghi Bahmani, D.; Kesselring, J.; Papadimitriou, M.; Bansi, J.; Puhse, U.; Gerber, M.; Shaygannejad, V.; Holsboer-Trachsler, E.; Brand, S. In Patients With Multiple Sclerosis, Both Objective and Subjective Sleep, Depression, Fatigue, and Paresthesia Improved After 3 Weeks of Regular Exercise. Front. Psychiatry 2019, 10, 265. [Google Scholar] [CrossRef]
- Sadeghi Bahmani, D.; Razazian, N.; Farnia, V.; Alikhani, M.; Tatari, F.; Brand, S. Compared to an active control condition, in persons with multiple sclerosis two different types of exercise training improved sleep and depression, but not fatigue, paresthesia, and intolerance of uncertainty. Mult. Scler. Relat. Disord. 2019, 36, 101356. [Google Scholar] [CrossRef]
- Farì, G.; Lunetti, P.; Pignatelli, G.; Raele, M.V.; Cera, A.; Mintrone, G.; Ranieri, M.; Megna, M.; Capobianco, L. The Effect of Physical Exercise on Cognitive Impairment in Neurodegenerative Disease: From Pathophysiology to Clinical and Rehabilitative Aspects. Int. J. Mol. Sci. 2021, 22, 11632. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Salehi, O.; Keikhosravi, F.; Hassanpour, G.; Ardakani, H.D.; Farkhaie, F.; Shadmehri, S.; Azarbayjani, M.A. Mental health benefits of exercise and genistein in elderly rats. Exp. Aging. Res. 2022, 48, 42–57. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can physical activity support the endocannabinoid system in the preventive and therapeutic approach to neurological disorders? Int. J. Mol. Sci. 2020, 21, 4221. [Google Scholar] [CrossRef]
- Dąbrowska-Bouta, B.; Strużyńska, L.; Sidoryk-Węgrzynowicz, M.; Sulkowski, G. Memantine Modulates Oxidative Stress in the Rat Brain following Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2021, 22, 11330. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Amor, S. Experimental autoimmune encephalomyelitis is a good model of multiple sclerosis if used wisely. Mult. Scler. Relat. Disord. 2014, 3, 555–564. [Google Scholar] [CrossRef]
- El-Emam, M.A.; El Achy, S.; Abdallah, D.M.; El-Abhar, H.S.; Gowayed, M.A. Does physical exercise improve or deteriorate treatment of multiple sclerosis with mitoxantrone? Experimental autoimmune encephalomyelitis study in rats. BMC Neurosci. 2022, 23, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P.; Azari, H.B. Exercise Training Improves Memory Performance in Older Adults: A Narrative Review of Evidence and Possible Mechanisms. Front. Hum. Neurosci. 2021, 15, 771553. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Royal Jelly as an Intelligent Anti-Aging Agent—A Focus on Cognitive Aging and Alzheimer’s Disease: A Review. Antioxidants 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.A.L.; Rodrigues, C.M.; Mendes, B.F.; Improta-Caria, A.C.; Peixoto, M.F.D.; Cassilhas, R.C. Physical exercise protocols in animal models of Alzheimer’s disease: A systematic review. Metab. Brain Dis. 2021, 36, 85–95. [Google Scholar] [CrossRef]
- Weissert, R. Actively Induced Experimental Autoimmune Encephalomyelitis in Rats. Methods. Mol. Biol. 2016, 1304, 161–169. [Google Scholar] [CrossRef]
- Kalkowski, L.; Golubczyk, D.; Kwiatkowska, J.; Domzalska, M.; Walczak, P.; Malysz-Cymborska, I. Local autoimmune encephalomyelitis model in a rat brain with precise control over lesion placement. PLoS ONE 2022, 21, e0262677. [Google Scholar] [CrossRef]
- Malekinejad, H.; Ahsan, S.; Delkhosh-Kasmaie, F.; Cheraghi, H.; Rezaei-Golmisheh, A.; Janbaz-Acyabar, H. Cardioprotective effect of royal jelly on paclitaxel-induced cardio-toxicity in rats. Iran. J. Basic. Med. Sci. 2016, 19, 221. [Google Scholar]
- Tajiri, N.; Yasuhara, T.; Shingo, T.; Kondo, A.; Yuan, W.; Kadota, T.; Wang, F.; Baba, T.; Tayra, J.T.; Morimoto, T. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res. 2010, 1310, 200–207. [Google Scholar] [CrossRef]
- Bernardes, D.; Oliveira, A.L.R.d. Regular exercise modifies histopathological outcomes of pharmacological treatment in experimental autoimmune encephalomyelitis. Front. Neurol. 2018, 950, 950. [Google Scholar] [CrossRef] [PubMed]
- Polli, A.; Ickmans, K.; Godderis, L.; Nijs, J. When Environment Meets Genetics: A Clinical Review of the Epigenetics of Pain, Psychological Factors, and Physical Activity. Arch. Phys. Med. Rehabil. 2019, 100, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoogen, N.J.; Harding, E.K.; Davidson, C.E.; Trang, T. Cannabinoids in Chronic Pain: Therapeutic Potential Through Microglia Modulation. Front. Neural Circuits 2021, 15, 816747. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory effects of high and moderate intensity exercise—A systematic review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Farahani, Z.K.; Taherianfard, M.; Naderi, M.M.; Ferrero, H. Possible therapeutic effect of royal jelly on endometriotic lesion size, pain sensitivity, and neurotrophic factors in a rat model of endometriosis. Physiol. Rep. 2021, 9, e15117. [Google Scholar] [CrossRef]
- de Souza E Silva, T.G.; da Silva, J.R.M.; da Silva Alves, A.; Britto, L.R.G.; Xavier, G.F.; Sandoval, M.R.L. Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer’s disease. Heliyon 2020, 6, e03281. [Google Scholar] [CrossRef]
- Zamani, Z.; Reisi, P.; Alaei, H.; Pilehvarian, A.A. Effect of Royal Jelly on spatial learning and memory in rat model of streptozotocin-induced sporadic Alzheimer’s disease. Adv. Biomed. Res. 2012, 1, 26. [Google Scholar] [CrossRef]
- Ou, M.; Pan, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Si, J.; Wang, K.; Hu, F. Royal Jelly Alleviates Cognitive Deficits and β-Amyloid Accumulation in APP/PS1 Mouse Model Via Activation of the cAMP/PKA/CREB/BDNF Pathway and Inhibition of Neuronal Apoptosis. Front. Aging Neurosci. 2019, 10, 428. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hijikata, K.; Seike, K.; Nakano, S.; Banjo, M.; Sato, Y.; Takahashi, K.; Hatta, H. Effects of Royal Jelly Administration on Endurance Training-Induced Mitochondrial Adaptations in Skeletal Muscle. Nutrients 2018, 10, 1735. [Google Scholar] [CrossRef]
- Marrodan, M.; Farez, M.F.; Balbuena Aguirre, M.E.; Correale, J. Obesity and the risk of Multiple Sclerosis. The role of Leptin. Ann. Clin. Transl. Neurol. 2021, 8, 406–424. [Google Scholar] [CrossRef]

| Gene Name | Sequence of Primers | Product Size (bp) |
|---|---|---|
| TBP | Forward: 5′-GCGGGGTCATGAAATCCAGT-3′ | 147 |
| Reverse: 5′-AGTGATGTGGGGACAAAACGA-3′ | ||
| CB1 receptor | Forward: 5′-AGAACTTACTGTGAACAGGCTCT-3′ | 105 |
| Reverse: 5′-ACTGAGAAAGAGATGCCGGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheirdeh, M.; Koushkie Jahromi, M.; Brühl, A.B.; Brand, S. The Effect of Exercise Training and Royal Jelly on Hippocampal Cannabinoid-1-Receptors and Pain Threshold in Experimental Autoimmune Encephalomyelitis in Rats as Animal Model of Multiple Sclerosis. Nutrients 2022, 14, 4119. https://doi.org/10.3390/nu14194119
Kheirdeh M, Koushkie Jahromi M, Brühl AB, Brand S. The Effect of Exercise Training and Royal Jelly on Hippocampal Cannabinoid-1-Receptors and Pain Threshold in Experimental Autoimmune Encephalomyelitis in Rats as Animal Model of Multiple Sclerosis. Nutrients. 2022; 14(19):4119. https://doi.org/10.3390/nu14194119
Chicago/Turabian StyleKheirdeh, Maryam, Maryam Koushkie Jahromi, Annette Beatrix Brühl, and Serge Brand. 2022. "The Effect of Exercise Training and Royal Jelly on Hippocampal Cannabinoid-1-Receptors and Pain Threshold in Experimental Autoimmune Encephalomyelitis in Rats as Animal Model of Multiple Sclerosis" Nutrients 14, no. 19: 4119. https://doi.org/10.3390/nu14194119
APA StyleKheirdeh, M., Koushkie Jahromi, M., Brühl, A. B., & Brand, S. (2022). The Effect of Exercise Training and Royal Jelly on Hippocampal Cannabinoid-1-Receptors and Pain Threshold in Experimental Autoimmune Encephalomyelitis in Rats as Animal Model of Multiple Sclerosis. Nutrients, 14(19), 4119. https://doi.org/10.3390/nu14194119








