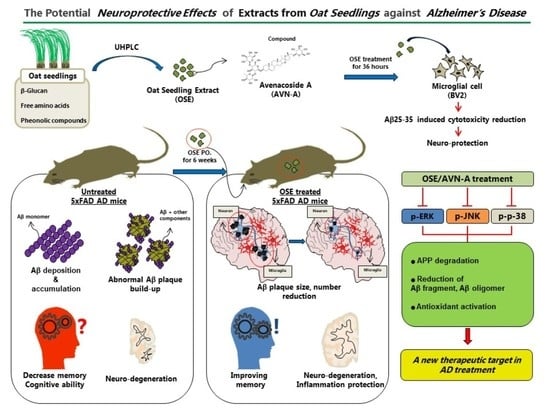
The Potential Neuroprotective Effects of Extracts from Oat Seedlings against Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Plant Materials, Extract Chemical Isolation Method and Ultra-High Performance Liquid Chromatography (UHPLC) Analysis
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Cell Death Detection Assay
2.6. Fluorescent Measurement of Intracellular Reactive Oxygen Species (ROS)
2.7. Animals
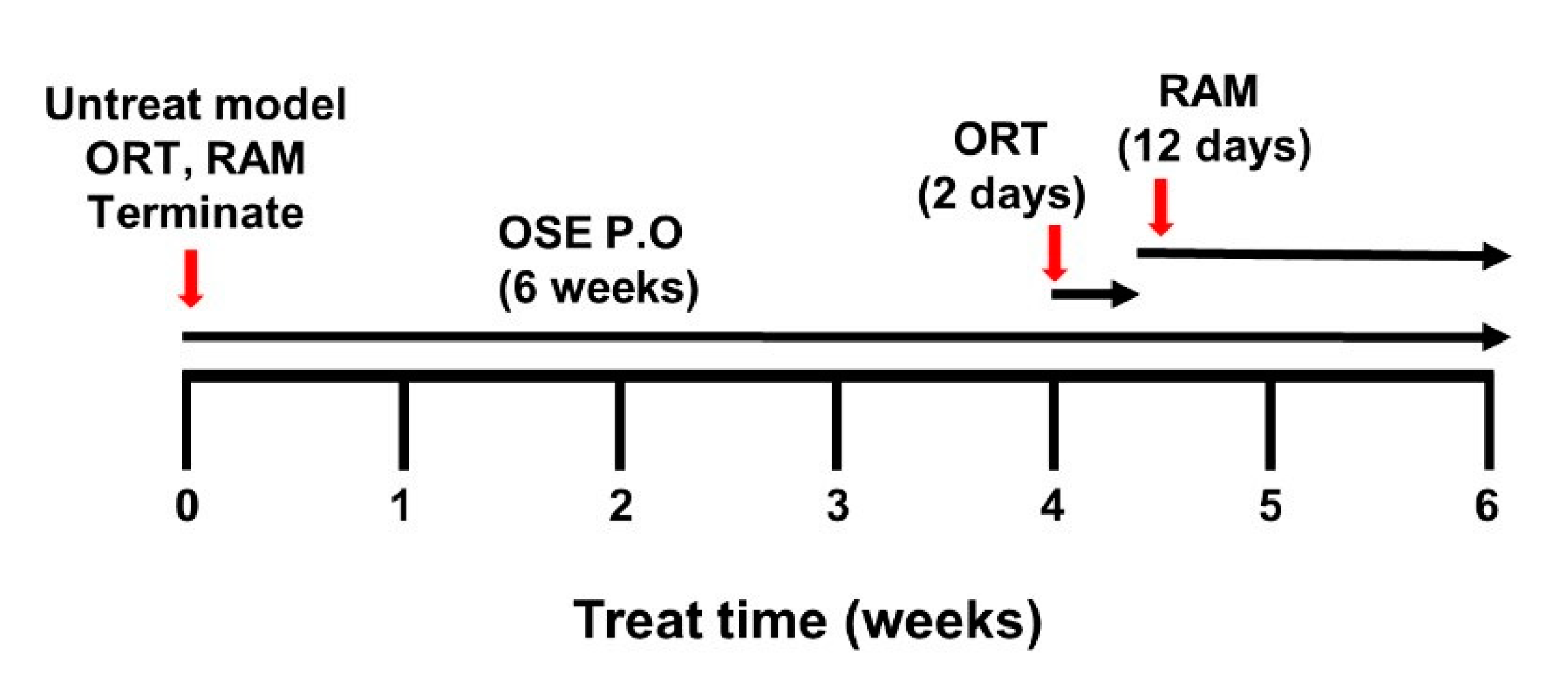
2.8. Object Recognition Test (ORT)
2.9. Radial Arm Maze Test (RAM)
2.10. Tissue Preparation
2.11. Immunohistochemistry
2.12. Analysis of Western Blot
2.13. Real-Time Polymerase Chain Reaction (qRT-PCR)
2.14. Statistical Analysis
3. Results
3.1. Effect of OSE on Cell Viability and Treatment with OSE Protects against LPS-Induced Cytotoxicity in BV2 Cells
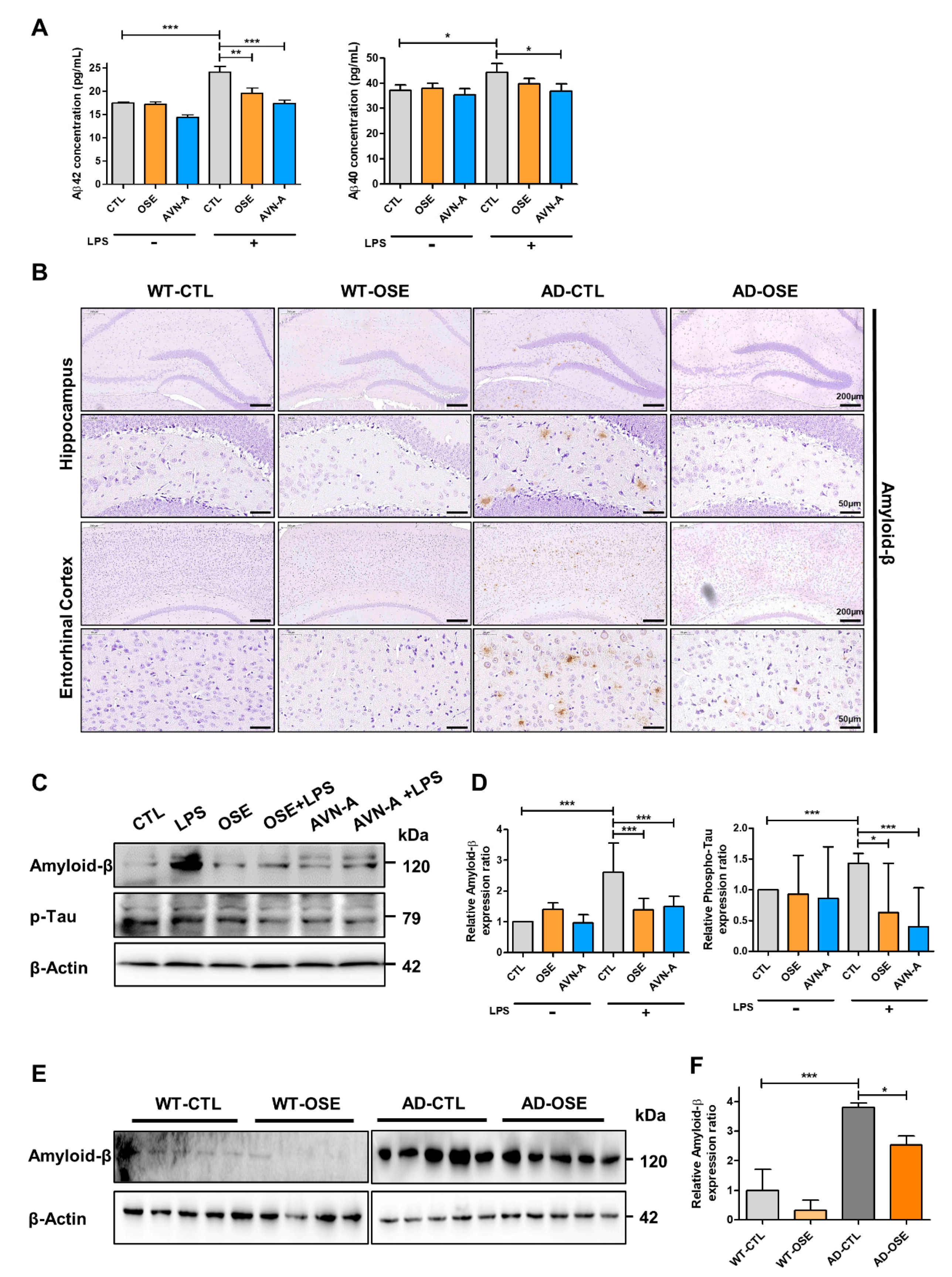
3.2. OSE and AVN-A Exposure Effectively Inhibits Brain Aβ Deposition and Protein Expression
3.3. BACE1 Expression in the Brain after OSE and AVN-A Exposure
3.4. OSE and AVN-A Exposure Decreases Neuroinflammatory Cells
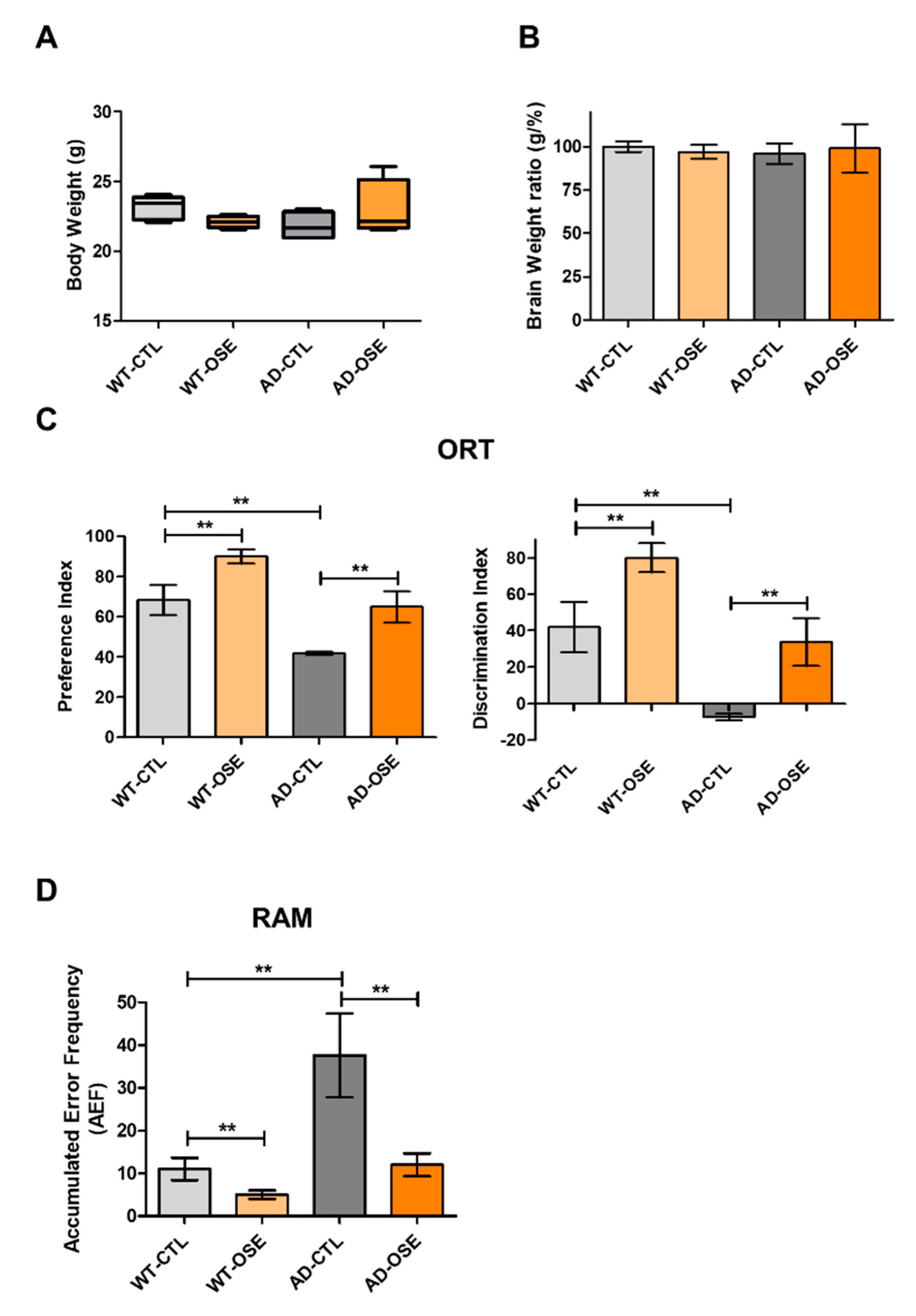
3.5. OSE Exposure Ameliorates Memory Impairment
3.6. Mechanism of OSE and AVN-A Effects on AD-Related Phenotypes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byun, A.R.; Chun, H.; Lee, J.; Lee, S.W.; Lee, H.S.; Shim, K.W. Effects of a Dietary Supplement with Barley Sprout Extract on Blood Cholesterol Metabolism. Evid. Based Complement. Altern. Med. 2015, 2015, 473056. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Pu, X.; Yang, J.; Du, J.; Yang, X.; Li, X.; Li, L.; Zhou, Y.; Yang, T. Preventive and Therapeutic Role of Functional Ingredients of Barley Grass for Chronic Diseases in Human Beings. Oxid. Med. Cell Longev. 2018, 2018, 3232080. [Google Scholar] [CrossRef]
- Lee, J.H.; Jia, Y.; Thach, T.T.; Han, Y.; Kim, B.; Wu, C.; Kim, Y.; Seo, W.D.; Lee, S.J. Hexacosanol reduces plasma and hepatic cholesterol by activation of AMP-activated protein kinase and suppression of sterol regulatory element-binding protein-2 in HepG2 and C57BL/6J mice. Nutr. Res. 2017, 43, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, S.H.; Lee, S.; Kim, K.M.; Jung, J.C.; Son, T.G.; Ki, S.H.; Seo, W.D.; Kwak, J.H.; Hong, J.T.; et al. Antioxidant Effect of Barley Sprout Extract via Enhancement of Nuclear Factor-Erythroid 2 Related Factor 2 Activity and Glutathione Synthesis. Nutrients 2017, 9, 1252. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Hwang, S.H.; Jia, Y.; Seo, W.D.; Lee, S.J. Barley sprout extracts reduce hepatic lipid accumulation in ethanol-fed mice by activating hepatic AMP-activated protein kinase. Food Res. Int. 2017, 101, 209–217. [Google Scholar] [CrossRef]
- Seo, K.H.; Park, M.J.; Ra, J.E.; Han, S.I.; Nam, M.H.; Kim, J.H.; Lee, J.H.; Seo, W.D. Saponarin from barley sprouts inhibits NF-kappaB and MAPK on LPS-induced RAW 264.7 cells. Food Funct. 2014, 5, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.D.; Lee, J.H.; Jia, Y.; Wu, C.; Lee, S.J. Saponarin activates AMPK in a calcium-dependent manner and suppresses gluconeogenesis and increases glucose uptake via phosphorylation of CRTC2 and HDAC5. Bioorg. Med. Chem. Lett. 2015, 25, 5237–5242. [Google Scholar] [CrossRef]
- Lee, H.; Woo, S.Y.; Ra, J.E.; Lee, K.S.; Seo, W.D.; Lee, J.H. Saponarin content and biosynthesis-related gene expression in young barley (Hordeum vulgare L.) seedlings. J. Plant Biotechnol. 2019, 46, 247–254. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Le, K.A.; Van den Broeck, H.C.; Brouns, F.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Penas, E.; Garcia, M.D.C.; Martinez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martin-Diana, A.B. Sprouted Barley Flour as a Nutritious and Functional Ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Aborus, N.E.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T.; Vulić, J.; Ilić, N. Powdered barley sprouts: Composition, functionality andpolyphenol digestibility. Int. J. Food Sci. Technol. 2017, 52, 231–238. [Google Scholar] [CrossRef]
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.D.; Wang, Y.D. beta-Amyloid: The key peptide in the pathogenesis of Alzheimer’s disease. Front. Pharm. 2015, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Wood, P.L.; Romani, A.; Valacchi, G.; Squerzanti, M.; Sanz, J.M.; Ortolani, B.; Zuliani, G. Oxidative challenge in Alzheimer’s disease: State of knowledge and future needs. J. Investig. Med. 2016, 64, 21–32. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Nelson, P.T.; Braak, H.; Markesbery, W.R. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009, 68, 1–14. [Google Scholar] [CrossRef]
- Gravina, S.A.; Ho, L.; Eckman, C.B.; Long, K.E.; Otvos, L., Jr.; Younkin, L.H.; Suzuki, N.; Younkin, S.G. Amyloid beta protein (A beta) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43). J. Biol. Chem. 1995, 270, 7013–7016. [Google Scholar] [CrossRef] [PubMed]
- Iwatsubo, T.; Mann, D.M.; Odaka, A.; Suzuki, N.; Ihara, Y. Amyloid beta protein (A beta) deposition: A beta 42(43) precedes A beta 40 in Down syndrome. Ann. Neurol. 1995, 37, 294–299. [Google Scholar] [CrossRef]
- Tran, T.A.; McCoy, M.K.; Sporn, M.B.; Tansey, M.G. The synthetic triterpenoid CDDO-methyl ester modulates microglial activities, inhibits TNF production, and provides dopaminergic neuroprotection. J. Neuroinflamm. 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, O.; Coleman, M.P.; Durrant, C.S. Lipopolysaccharide-induced neuroinflammation induces presynaptic disruption through a direct action on brain tissue involving microglia-derived interleukin 1 beta. J. Neuroinflamm. 2019, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zhang, L.; Shi, D.L.; Song, X.H.; Shen, Y.L.; Zheng, M.Z.; Wang, L.L. Resveratrol Attenuates Subacute Systemic Inflammation-Induced Spatial Memory Impairment via Inhibition of Astrocyte Activation and Enhancement of Synaptophysin Expression in the Hippocampus. Ann. Clin. Lab. Sci. 2017, 47, 17–24. [Google Scholar] [PubMed]
- Rao, J.S.; Kellom, M.; Kim, H.W.; Rapoport, S.I.; Reese, E.A. Neuroinflammation and synaptic loss. Neurochem. Res. 2012, 37, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.G.; Bora, S.H.; Xu, G.; Borchelt, D.R.; Price, D.L.; Koliatsos, V.E. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol. Dis. 2003, 14, 133–145. [Google Scholar] [CrossRef]
- Neniskyte, U.; Neher, J.J.; Brown, G.C. Neuronal death induced by nanomolar amyloid beta is mediated by primary phagocytosis of neurons by microglia. J. Biol. Chem. 2011, 286, 39904–39913. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers. Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-Y.; Yang, J.Y.; Lee, H.; Ahn, H.J.; Lee, Y.B.; Do, S.H.; Kim, J.Y.; Seo, W.D. Changes in metabolites with harvest times of seedlings of various Korean oat (Avena sativa L.) cultivars and their neuraminidase inhibitory effects. Food Chem. 2022, 373, 131429. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Bui, B.P.; Lee, H.; Cho, J. A Novel 1,8-Naphthyridine-2-Carboxamide Derivative Attenuates Inflammatory Responses and Cell Migration in LPS-Treated BV2 Cells via the Suppression of ROS Generation and TLR4/Myd88/NF-kappaB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 2527. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, Y.; Lou, W.; Cui, Y.; Evans, C.P.; Gao, A.C. Inhibition of constitutively active Stat3 reverses enzalutamide resistance in LNCaP derivative prostate cancer cells. Prostate 2014, 74, 201–209. [Google Scholar] [CrossRef]
- Lee, W.S.; Seo, S.J.; Chung, H.K.; Park, J.W.; Kim, J.K.; Kim, E.H. Tumor-treating fields as a proton beam-sensitizer for glioblastoma therapy. Am. J. Cancer. Res. 2021, 11, 4582–4594. [Google Scholar]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Broadbent, N.J.; Gaskin, S.; Squire, L.R.; Clark, R.E. Object recognition memory and the rodent hippocampus. Learn. Mem. 2010, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.K.; Furgerson, M.; Crystal, J.D.; Fechheimer, M.; Furukawa, R.; Wagner, J.J. Alterations in synaptic plasticity coincide with deficits in spatial working memory in presymptomatic 3xTg-AD mice. Neurobiol. Learn. Mem. 2015, 125, 152–162. [Google Scholar] [CrossRef]
- Penley, S.C.; Gaudet, C.M.; Threlkeld, S.W. Use of an eight-arm radial water maze to assess working and reference memory following neonatal brain injury. J. Vis. Exp. 2013, 82, e50940. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.; Bleibaum, R.; Thomas, H.A. Sensory Evaluation Practices; Stone, H., Bleibaum, R., Thomas, H.A., Eds.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Kim, E.H.; Jo, Y.; Sai, S.; Park, M.J.; Kim, J.Y.; Kim, J.S.; Lee, Y.J.; Cho, J.M.; Kwak, S.Y.; Baek, J.H.; et al. Tumor-treating fields induce autophagy by blocking the Akt2/miR29b axis in glioblastoma cells. Oncogene 2019, 38, 6630–6646. [Google Scholar] [CrossRef]
- Wong, M.L.; Medrano, J.F. Real-time PCR for mRNA quantitation. Biotechniques 2005, 39, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Lucena, D.; Kruse, N.; Thune, K.; Schmitz, M.; Villar-Pique, A.; da Cunha, J.E.G.; Hermann, P.; Lopez-Perez, O.; Andres-Benito, P.; Ladogana, A.; et al. TREM2 expression in the brain and biological fluids in prion diseases. Acta Neuropathol. 2021, 141, 841–859. [Google Scholar] [CrossRef] [PubMed]
- 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020, 16, 391–460. [CrossRef] [PubMed]
- Summers, W.K.; Koehler, A.L.; Marsh, G.M.; Tachiki, K.; Kling, A. Long-term hepatotoxicity of tacrine. Lancet 1989, 1, 729. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Bastone, A.; Ploia, C.; Sclip, A.; Salmona, M.; Forloni, G.; Borsello, T. JNK regulates APP cleavage and degradation in a model of Alzheimer’s disease. Neurobiol. Dis. 2009, 33, 518–525. [Google Scholar] [CrossRef]
- Webber, K.M.; Smith, M.A.; Lee, H.G.; Harris, P.L.; Moreira, P.; Perry, G.; Zhu, X. Mitogen- and stress-activated protein kinase 1: Convergence of the ERK and p38 pathways in Alzheimer’s disease. J. Neurosci. Res. 2005, 79, 554–560. [Google Scholar] [CrossRef]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef] [PubMed]
- Sarina, Y.; Nakano, O.; Hashimoto, T.; Kimura, K.; Asakawa, Y.; Zhong, M.; Narimatsu, S.; Gohda, E. Induction of neurite outgrowth in PC12 cells by artemisinin through activation of ERK and p38 MAPK signaling pathways. Brain Res. 2013, 1490, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Munoz, L.; Ammit, A.J. Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology 2010, 58, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences, T.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.Q.; Tang, X.C.; Zhang, H.Y. Retrospect and prospect of active principles from Chinese herbs in the treatment of dementia. Acta Pharm. Sin. 2010, 31, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Beutler, J.A. Natural Products as a Foundation for Drug Discovery. Curr Protoc Pharm. 2009, 46, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharm. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.S.; Lee, H.-J.; Yang, J.Y.; Shin, H.-L.; Choi, S.-W.; Kim, J.-K.; Seo, W.D.; Kim, E.H. The Potential Neuroprotective Effects of Extracts from Oat Seedlings against Alzheimer’s Disease. Nutrients 2022, 14, 4103. https://doi.org/10.3390/nu14194103
Lee WS, Lee H-J, Yang JY, Shin H-L, Choi S-W, Kim J-K, Seo WD, Kim EH. The Potential Neuroprotective Effects of Extracts from Oat Seedlings against Alzheimer’s Disease. Nutrients. 2022; 14(19):4103. https://doi.org/10.3390/nu14194103
Chicago/Turabian StyleLee, Won Seok, Hae-June Lee, Ji Yeong Yang, Hye-Lim Shin, Sik-Won Choi, Jong-Ki Kim, Woo Duck Seo, and Eun Ho Kim. 2022. "The Potential Neuroprotective Effects of Extracts from Oat Seedlings against Alzheimer’s Disease" Nutrients 14, no. 19: 4103. https://doi.org/10.3390/nu14194103
APA StyleLee, W. S., Lee, H.-J., Yang, J. Y., Shin, H.-L., Choi, S.-W., Kim, J.-K., Seo, W. D., & Kim, E. H. (2022). The Potential Neuroprotective Effects of Extracts from Oat Seedlings against Alzheimer’s Disease. Nutrients, 14(19), 4103. https://doi.org/10.3390/nu14194103