A Healthful Plant-Based Diet Is Associated with Lower Odds of Nonalcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Dietary Assessment and Diet Indices
2.3. Ascertainments of NAFLD
2.4. Assessments of Covariates
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
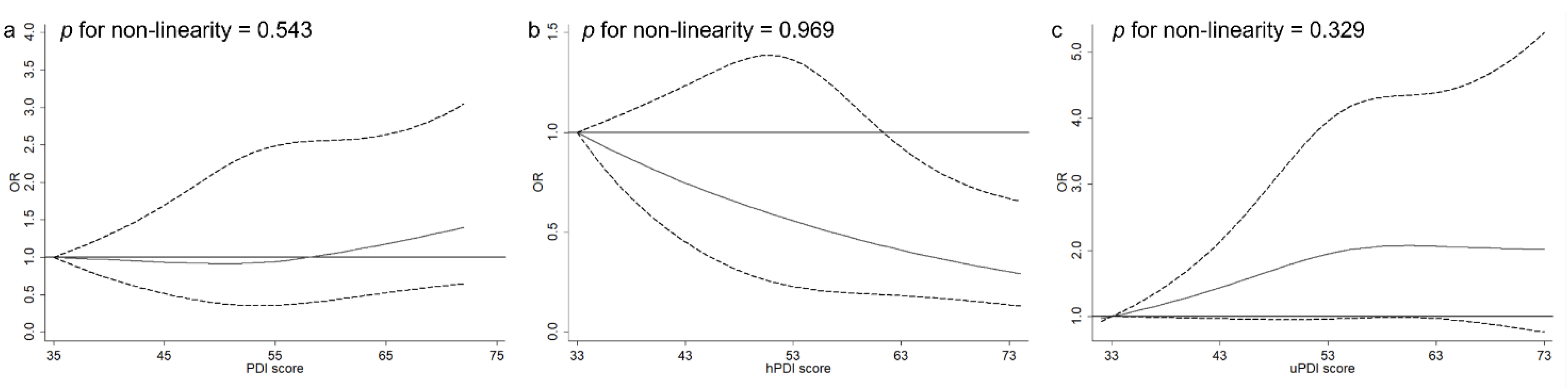
3.2. PDIs and NAFLD
3.3. Sensitivity and Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Nephew, L.D.; Vuppalanchi, R.; Gawrieh, S.; Mladenovic, A.; Pike, F.; Samala, N.; Chalasani, N. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 2021, 75, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: Expert review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef]
- Chiu, T.H.; Lin, M.N.; Pan, W.H.; Chen, Y.C.; Lin, C.L. Vegetarian diet, food substitution, and nonalcoholic fatty liver. Ci Ji Yi Xue Za Zhi 2018, 30, 102–109. [Google Scholar] [CrossRef]
- Choi, S.H.; Oh, D.J.; Kwon, K.H.; Lee, J.K.; Koh, M.S.; Lee, J.H.; Kang, H.W. A vegetarian diet does not protect against nonalcoholic fatty liver disease (NAFLD): A cross-sectional study between Buddhist priests and the general population. Turk. J. Gastroenterol. 2015, 26, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Garousi, N.; Tamizifar, B.; Pourmasoumi, M.; Feizi, A.; Askari, G.; Clark, C.C.T.; Entezari, M.H. Effects of lacto-ovo-vegetarian diet vs. standard-weight-loss diet on obese and overweight adults with non-alcoholic fatty liver disease: A randomised clinical trial. Arch. Physiol. Biochem. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P. Higher adherence to plant-based diets are associated with lower likelihood of fatty liver. Clin. Nutr. 2019, 38, 1672–1677. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.; Wang, Y.; Zhang, Z.; Zhu, Y.; Li, X.; Hu, A.; Zhao, Q.; Yang, W. A prospective study of healthful and unhealthful plant-based diet and risk of overall and cause-specific mortality. Eur. J. Nutr. 2022, 61, 387–398. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Li, H.; Zhou, Z.; Li, M.; Zeng, X.; Yang, H.; Zhang, M.; Huang, Y.; Zhu, Y.; et al. Associations between intake of starchy and non-starchy vegetables and risk of hepatic steatosis and fibrosis. Hepatol. Int. 2022, 16, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zeng, X.; Li, M.; Zhang, T.; Li, H.; Yang, H.; Huang, Y.; Zhu, Y.; Li, X.; Yang, W. A prospective study of fruit juice consumption and the risk of overall and cardiovascular disease mortality. Nutrients 2022, 14, 2127. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, I.; Morze, J.; Enderle, J.; Both, M.; Borggrefe, J.; Müller, H.-P.; Kassubek, J.; Koch, M.; Lieb, W. Adherence to a plant-based diet in relation to adipose tissue volumes and liver fat content. Am. J. Clin. Nutr. 2020, 112, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Tsiampalis, T.; Kosti, R.I.; Naumovski, N.; Chrysohoou, C.; Skoumas, J.; Pitsavos, C.S.; Panagiotakos, D.B.; Mantzoros, C.S. Quality of plant-based diets is associated with liver steatosis, which predicts type 2 diabetes incidence ten years later: Results from the ATTICA prospective epidemiological study. Clin. Nutr. 2022, 41, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Sawicki, C.M.; Goon, S.; Gujral, U.P.; Hu, F.B.; Kandula, N.R.; Kanaya, A.M. A healthy plant-based diet is favorably associated with cardiometabolic risk factors among participants of South Asian ancestry. Am. J. Clin. Nutr. 2022, 116, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- De Lédinghen, V.; Wong, G.L.-H.; Vergniol, J.; Chan, H.L.-Y.; Hiriart, J.-B.; Chan, A.W.-H.; Chermak, F.; Choi, P.C.-L.; Foucher, J.; Chan, C.K.-M.; et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2016, 31, 848–855. [Google Scholar] [CrossRef]
- Heidemann, C.; Schulze, M.B.; Franco, O.H.; van Dam, R.M.; Mantzoros, C.S.; Hu, F.B. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 2008, 118, 230–237. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). About the National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 11 March 2021).
- Kim, D.; Konyn, P.; Cholankeril, G.; Ahmed, A. Physical Activity Is Associated with Nonalcoholic Fatty Liver Disease and Significant Fibrosis Measured by FibroScan. Clin. Gastroenterol. Hepatol. 2022, 20, e1438–e1455. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES dietary data: Focus on collection, release, analytical considerations, and uses to inform public policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Hu, F.B. Are Fruit Juices Just as Unhealthy as Sugar-Sweetened Beverages? JAMA Netw. Open 2019, 2, e193109. [Google Scholar] [CrossRef]
- Yue, Y.; Yuan, C.; Wang, D.D.; Wang, M.; Song, M.; Shan, Z.; Hu, F.; Rosner, B.; Smith-Warner, S.A.; Willett, W.C. Reproducibility and validity of diet quality scores derived from food-frequency questionnaires. Am. J. Clin. Nutr. 2022, 115, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, S.V.; Sutherland, J.M.; Jessri, M. Adherence to emerging plant-based dietary patterns and its association with cardiovascular disease risk in a nationally representative sample of Canadian adults. Am. J. Clin. Nutr. 2022, 116, 57–73. [Google Scholar] [CrossRef]
- Ciardullo, S.; Monti, T.; Perseghin, G. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care 2021, 44, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). NHANES Procedure Manuals. 11 March 2021; Updated 4 August 2020. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?BeginYear=2019 (accessed on 11 March 2021).
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J.; et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018, 67, 1348–1359. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Everhart, J.E. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther. 2015, 41, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Dupont, W.D.; Plummer, W.D., Jr. Power and sample size calculations. A review and computer program. Control. Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Chang, S.-H.; Lin, Y.-H.; Ho, W.-C.; Wang, C.-Y.; Jeng, W.-J.; Wan, Y.-L.; Tsui, P.-H. Utility of quantitative ultrasound in community screening for hepatic steatosis. Ultrasonics 2021, 111, 106329. [Google Scholar] [CrossRef]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Savides, M.T.; Alquiraish, M.H.; et al. Magnetic Resonance Elastography vs. Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 598–607.e2. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Shin, S. Fruit and vegetable consumption and non-alcoholic fatty liver disease among Korean adults: A prospective cohort study. J. Epidemiol. Community Health 2020, 74, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, M.; Jafary Heidarloo, A.; Bakhshimoghaddam, F.; Alizadeh, M. Whole-grain consumption and its effects on hepatic steatosis and liver enzymes in patients with non-alcoholic fatty liver disease: A randomised controlled clinical trial. Br. J. Nutr. 2020, 123, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Tan, S.Y.; Daly, R.M.; Via, J.D.; Georgousopoulou, E.N.; George, E.S. Intake of Nuts and Seeds Is Associated with a Lower Prevalence of Nonalcoholic Fatty Liver Disease in US Adults: Findings from 2005–2018 NHANES. J. Nutr. 2021, 151, 3507–3515. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Zhao, X.; Wang, Y.; Lan, Y.; Jiang, X.; Gao, X.; Wu, S.; Wang, L. Adherence to the dietary approaches to stop hypertension diet and non-alcoholic fatty liver disease. Liver Int. 2022, 42, 809–819. [Google Scholar] [CrossRef]
- Park, S.Y.; Noureddin, M.; Boushey, C.; Wilkens, L.R.; Setiawan, V.W. Diet Quality Association with Nonalcoholic Fatty Liver Disease by Cirrhosis Status: The Multiethnic Cohort. Curr. Dev. Nutr. 2020, 4, nzaa024. [Google Scholar] [CrossRef]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Papamiltiadous, E.S.; Roberts, S.K.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Salim, A.; Tierney, A.C. A randomised controlled trial of a Mediterranean Dietary Intervention for Adults with Non Alcoholic Fatty Liver Disease (MEDINA): Study protocol. BMC Gastroenterol. 2016, 16, 14. [Google Scholar] [CrossRef]
- Khalatbari-Soltani, S.; Imamura, F.; Brage, S.; De Lucia Rolfe, E.; Griffin, S.J.; Wareham, N.J.; Marques-Vidal, P.; Forouhi, N.G. The association between adherence to the Mediterranean diet and hepatic steatosis: Cross-sectional analysis of two independent studies, the UK Fenland Study and the Swiss CoLaus Study. BMC Med. 2019, 17, 19. [Google Scholar] [CrossRef]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef]
- Tajima, R.; Kimura, T.; Enomoto, A.; Saito, A.; Kobayashi, S.; Masuda, K.; Iida, K. No association between fruits or vegetables and non-alcoholic fatty liver disease in middle-aged men and women. Nutrition 2019, 61, 119–124. [Google Scholar] [CrossRef]
- Leung, C.W.; Tapper, E.B. Sugar-sweetened Beverages Are Associated with Increased Liver Stiffness and Steatosis Among Apparently Healthy Adults in the United States. Clin. Gastroenterol. Hepatol. 2022, 20, 959–961.e1. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Godos, J.; Salomone, F. Lifestyle changes for the treatment of nonalcoholic fatty liver disease: A review of observational studies and intervention trials. Ther. Adv. Gastroenterol. 2016, 9, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Berna, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40 (Suppl. 1), 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Ip, S.; Bhanji, R.A.; Montano-Loza, A.J. Effect of coffee consumption on non-alcoholic fatty liver disease incidence, prevalence and risk of significant liver fibrosis: Systematic review with meta-analysis of observational studies. Nutrients 2021, 13, 3042. [Google Scholar] [CrossRef]
- Ma, J.; Fox, C.S.; Jacques, P.F.; Speliotes, E.K.; Hoffmann, U.; Smith, C.E.; Saltzman, E.; McKeown, N.M. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J. Hepatol. 2015, 63, 462–469. [Google Scholar] [CrossRef]
- Pourreza, S.; Khademi, Z.; Mirzababaei, A.; Yekaninejad, M.S.; Sadeghniiat-Haghighi, K.; Naghshi, S.; Mirzaei, K. Association of plant-based diet index with inflammatory markers and sleep quality in overweight and obese female adults: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e14429. [Google Scholar] [CrossRef]
- Baden, M.Y.; Satija, A.; Hu, F.B.; Huang, T. Change in Plant-Based Diet Quality Is Associated with Changes in Plasma Adiposity-Associated Biomarker Concentrations in Women. J. Nutr. 2019, 149, 676–686. [Google Scholar] [CrossRef]
- Bolori, P.; Setaysh, L.; Rasaei, N.; Jarrahi, F.; Yekaninejad, M.S.; Mirzaei, K. Adherence to a healthy plant diet may reduce inflammatory factors in obese and overweight women-a cross-sectional study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2795–2802. [Google Scholar] [CrossRef]
- Cantero, I.; Abete, I.; Babio, N.; Arós, F.; Corella, D.; Estruch, R.; Fitó, M.; Hebert, J.R.; Martínez-González, M.; Pintó, X.; et al. Dietary Inflammatory Index and liver status in subjects with different adiposity levels within the PREDIMED trial. Clin. Nutr. 2018, 37, 1736–1743. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Moucari, R.; Asselah, T.; Cazals–Hatem, D.; Voitot, H.; Boyer, N.; Ripault, M.; Sobesky, R.; Martinot–Peignoux, M.; Maylin, S.; Nicolas–Chanoine, M.; et al. Insulin resistance in chronic hepatitis C: Association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 2008, 134, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Guo, J.; Moshfegh, A.J. Race/ethnicity and gender modify the association between diet and cognition in U.S. older adults: National Health and Nutrition Examination Survey 2011–2014. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12128. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.S.; Halbower, A.; Pan, Z.; Robbins, K.; Capocelli, K.E.; Klawitter, J.; Shearn, C.T.; Sokol, R.J. Nocturnal hypoxia-induced oxidative stress promotes progression of pediatric non-alcoholic fatty liver disease. J. Hepatol. 2016, 65, 560–569. [Google Scholar] [CrossRef]
- Dinwiddie, G.Y.; Zambrana, R.E.; Doamekpor, L.A.; Lopez, L. The impact of educational attainment on observed race/ethnic disparities in inflammatory risk in the 2001-2008 National Health and Nutrition Examination Survey. Int. J. Environ. Res. Public Health 2015, 13, 42. [Google Scholar] [CrossRef]
- Haffner, S.M.; Ralph, D.; Saad, M.F.; Rewers, M.; Mykkänen, L.; Selby, J.; Howard, G.; Savage, P.J.; Hamman, R.F.; Wegenknecht, L.E.; et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 1996, 45, 742–748. [Google Scholar] [CrossRef]
- Pool, L.R.; Ning, H.; Lloyd-Jones, D.M.; Allen, N.B. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999–2012. J. Am. Heart Assoc. 2017, 6, e006027. [Google Scholar] [CrossRef]
- Chung, H.-K.; Nam, J.S.; Lee, M.-Y.; Kim, Y.-B.; Won, Y.-S.; Song, W.-J.; Kim, Y.-H.; Ahn, C.W.; Sung, K.-C. The increased amount of coffee consumption lowers the incidence of fatty liver disease in Korean men. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1653–1661. [Google Scholar] [CrossRef]
- DiStefano, J.K. NAFLD and NASH in postmenopausal women: Implications for diagnosis and treatment. Endocrinology 2020, 161, bqaa134. [Google Scholar] [CrossRef]
- Sumien, N.; Cunningham, J.T.; Davis, D.L.; Engelland, R.; Fadeyibi, O.; Farmer, G.E.; Mabry, S.; Mensah-Kane, P.; Trinh, O.T.P.; Vann, P.H.; et al. Neurodegenerative disease: Roles for sex, hormones, and oxidative stress. Endocrinology 2021, 162, bqab185. [Google Scholar] [CrossRef] [PubMed]

| Variable | Overall PDI | hPDI | uPDI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | |
| N | 1195 | 1408 | 1297 | 1298 | 1263 | 1339 | 1406 | 1253 | 1241 |
| PDI score, median (IQR) | 48 (46–49) | 53 (52–54) | 58 (57–61) | 48 (45–49) | 53 (52–54) | 59 (57–62) | 48 (45–50) | 54 (53–55) | 60 (58–62) |
| Age, years | 45.6 (19.4) | 49.0 (18.2) | 52.6 (16.9) | 44.6 (18.5) | 50.0 (18.6) | 52.8 (17.2) | 54.0 (17.2) | 49.6 (18.4) | 43.2 (18.0) |
| Female, % | 50.5 | 51.6 | 54.5 | 42.1 | 52.9 | 60.3 | 52.1 | 53.4 | 50.9 |
| BMI, kg/m2 | 29.8 (7.3) | 29.7 (7.1) | 28.9 (6.8) | 30.4 (7.4) | 29.6 (7.2) | 28.4 (6.4) | 28.6 (6.4) | 29.6 (7.0) | 30.3 (7.7) |
| Total energy, kcal/d | 1809 (673) | 1980 (723) | 2133 (703) | 2293 (698) | 1906 (674) | 1734 (633) | 2013 (688) | 1947 (705) | 1960 (743) |
| Diabetes, % | 17.2 | 17.5 | 15.7 | 15.0 | 17.4 | 17.7 | 16.7 | 15.7 | 18.4 |
| Race/ethnicity, % | |||||||||
| Non-Hispanic white | 38.7 | 35.3 | 30.8 | 37.9 | 34.6 | 34.0 | 38.6 | 31.5 | 34.3 |
| Non-Hispanic black | 27.3 | 23.0 | 18.0 | 29.2 | 24.9 | 13.8 | 14.6 | 24.3 | 29.6 |
| Other races | 34.0 | 41.6 | 51.2 | 33.0 | 40.6 | 52.2 | 46.8 | 44.3 | 36.1 |
| Education, % | |||||||||
| ≤12th grade | 18.2 | 17.5 | 18.3 | 18.0 | 18.2 | 16.5 | 12.1 | 19.3 | 22.4 |
| High school graduate/GED or equivalent | 30.1 | 26.5 | 17.2 | 29.2 | 26.0 | 18.7 | 20.4 | 24.5 | 28.8 |
| More than high school | 51.4 | 56.0 | 64.3 | 52.7 | 55.5 | 64.6 | 67.3 | 56.0 | 48.7 |
| Marital status, % | |||||||||
| Married | 52.7 | 56.8 | 60.7 | 55.0 | 55.3 | 60.4 | 62.9 | 55.6 | 52.5 |
| Widowed/divorced/separated | 23.1 | 20.2 | 19.5 | 20.0 | 21.9 | 20.5 | 18.2 | 23.1 | 21.2 |
| Never married | 17.6 | 18.1 | 16.2 | 19.5 | 16.0 | 16.1 | 14.8 | 16.7 | 19.9 |
| Ratio of family income to poverty | |||||||||
| <1.30 | 25.9 | 25.5 | 22.7 | 26.9 | 24.5 | 21.8 | 19.1 | 24.4 | 30.2 |
| 1.30–3.49 | 37.1 | 36.1 | 35.1 | 39.5 | 33.9 | 34.2 | 33.7 | 36.0 | 38.8 |
| ≥3.50 | 24.2 | 27.0 | 32.3 | 21.3 | 28.5 | 34.5 | 36.3 | 27.0 | 19.7 |
| Physical activity, METS–h/week | |||||||||
| <8.3 | 34.7 | 35.0 | 34.3 | 34.7 | 36.8 | 32.4 | 28.3 | 36.9 | 39.3 |
| 8.3–16.7 | 9.6 | 7.9 | 11.3 | 8.0 | 8.7 | 11.8 | 11.2 | 8.8 | 8.2 |
| >16.7 | 54.6 | 56.1 | 54.0 | 56.4 | 53.5 | 55.3 | 60.1 | 53.2 | 51.7 |
| Smoking, % | |||||||||
| Never smokers | 56.9 | 60.2 | 66.1 | 55.9 | 60.8 | 66.1 | 64.7 | 61.4 | 57.8 |
| Former smokers | 23.5 | 23.3 | 23.5 | 23.9 | 23.7 | 23.3 | 25.0 | 23.0 | 21.9 |
| Current smokers | 19.6 | 16.5 | 10.4 | 20.2 | 15.5 | 10.6 | 10.3 | 15.7 | 20.2 |
| Alcohol drinking, % | |||||||||
| Never drinkers | 11.6 | 10.0 | 11.2 | 9.5 | 10.3 | 12.5 | 11.3 | 10.3 | 11.2 |
| Former drinkers | 19.6 | 19.4 | 19.4 | 21.5 | 19.2 | 17.9 | 15.4 | 20.8 | 24.1 |
| Current drinkers | 65.9 | 68.7 | 66.1 | 67.1 | 68.0 | 66.3 | 70.4 | 66.6 | 62.1 |
| OR (95% CI) | ptrendd | ||||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Per 10–Point Increase | ||
| Overall PDI | |||||
| Model 1 a | Reference | 1.02 (0.77, 1.35) | 0.99 (0.75, 1.30) | 0.96 (0.78, 1.18) | 0.673 |
| Model 2 b | Reference | 0.94 (0.70, 1.24) | 0.88 (0.67, 1.16) | 0.86 (0.69, 1.06) | 0.147 |
| Model 3 c | Reference | 0.92 (0.66, 1.28) | 1.03 (0.76, 1.38) | 1.06 (0.86, 1.30) | 0.609 |
| hPDI | |||||
| Model 1 a | Reference | 0.77 (0.62, 0.97) | 0.53 (0.39, 0.72) | 0.62 (0.49, 0.78) | <0.001 |
| Model 2 b | Reference | 0.74 (0.57, 0.97) | 0.50 (0.35, 0.72) | 0.59 (0.46, 0.77) | <0.001 |
| Model 3 c | Reference | 0.78 (0.58, 1.06) | 0.64 (0.46, 0.87) | 0.74 (0.59, 0.92) | 0.006 |
| uPDI | |||||
| Model 1 a | Reference | 1.20 (0.93, 1.54) | 1.32 (0.89, 1.95) | 1.29 (1.02, 1.62) | 0.034 |
| Model 2 b | Reference | 1.25 (0.98, 1.59) | 1.37 (0.93, 2.02) | 1.37 (1.08, 1.74) | 0.009 |
| Model 3 c | Reference | 1.18 (0.93, 1.51) | 1.14 (0.79, 1.66) | 1.16 (0.94, 1.45) | 0.173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Peng, Z.; Li, M.; Zeng, X.; Li, H.; Zhu, Y.; Chen, H.; Hu, A.; Zhao, Q.; Zhang, Z.; et al. A Healthful Plant-Based Diet Is Associated with Lower Odds of Nonalcoholic Fatty Liver Disease. Nutrients 2022, 14, 4099. https://doi.org/10.3390/nu14194099
Li X, Peng Z, Li M, Zeng X, Li H, Zhu Y, Chen H, Hu A, Zhao Q, Zhang Z, et al. A Healthful Plant-Based Diet Is Associated with Lower Odds of Nonalcoholic Fatty Liver Disease. Nutrients. 2022; 14(19):4099. https://doi.org/10.3390/nu14194099
Chicago/Turabian StyleLi, Xiude, Zhaohong Peng, Meiling Li, Xueke Zeng, Haowei Li, Yu Zhu, Hui Chen, Anla Hu, Qihong Zhao, Zhuang Zhang, and et al. 2022. "A Healthful Plant-Based Diet Is Associated with Lower Odds of Nonalcoholic Fatty Liver Disease" Nutrients 14, no. 19: 4099. https://doi.org/10.3390/nu14194099
APA StyleLi, X., Peng, Z., Li, M., Zeng, X., Li, H., Zhu, Y., Chen, H., Hu, A., Zhao, Q., Zhang, Z., Wang, H., Yuan, C., & Yang, W. (2022). A Healthful Plant-Based Diet Is Associated with Lower Odds of Nonalcoholic Fatty Liver Disease. Nutrients, 14(19), 4099. https://doi.org/10.3390/nu14194099








