Positive Effect of a Pea–Clam Two-Peptide Composite on Hypertension and Organ Protection in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Animal Experiment Scheme Design
2.4. Serum Biochemical Analysis
2.5. Pathological Section and Histopathological Analysis
2.6. Intestinal Microbial Diversity Analysis
2.7. Statistical Data Analysis
3. Results
3.1. Peptide Content, Molecular Weight, and ACE Inhibitory Activity of Pea and Clam Peptides
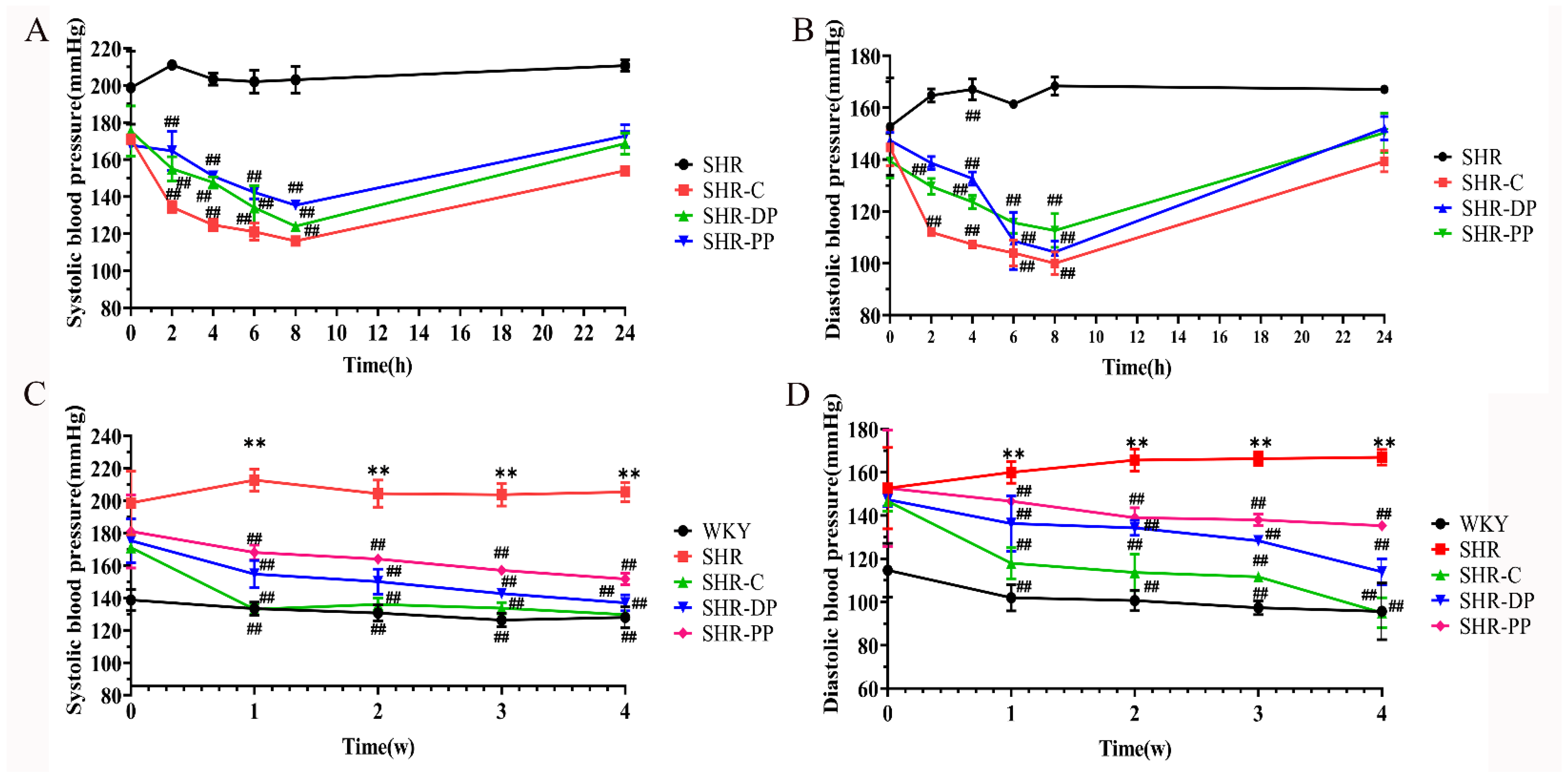
3.2. Effects of Pea Peptide and Two-Peptide Composite on Blood Pressure of SHRs and WKY Rats
3.3. Effect of Pea Peptides and Two-Peptide Composite on Kidney and the RAS in SHRs and WKY Rats
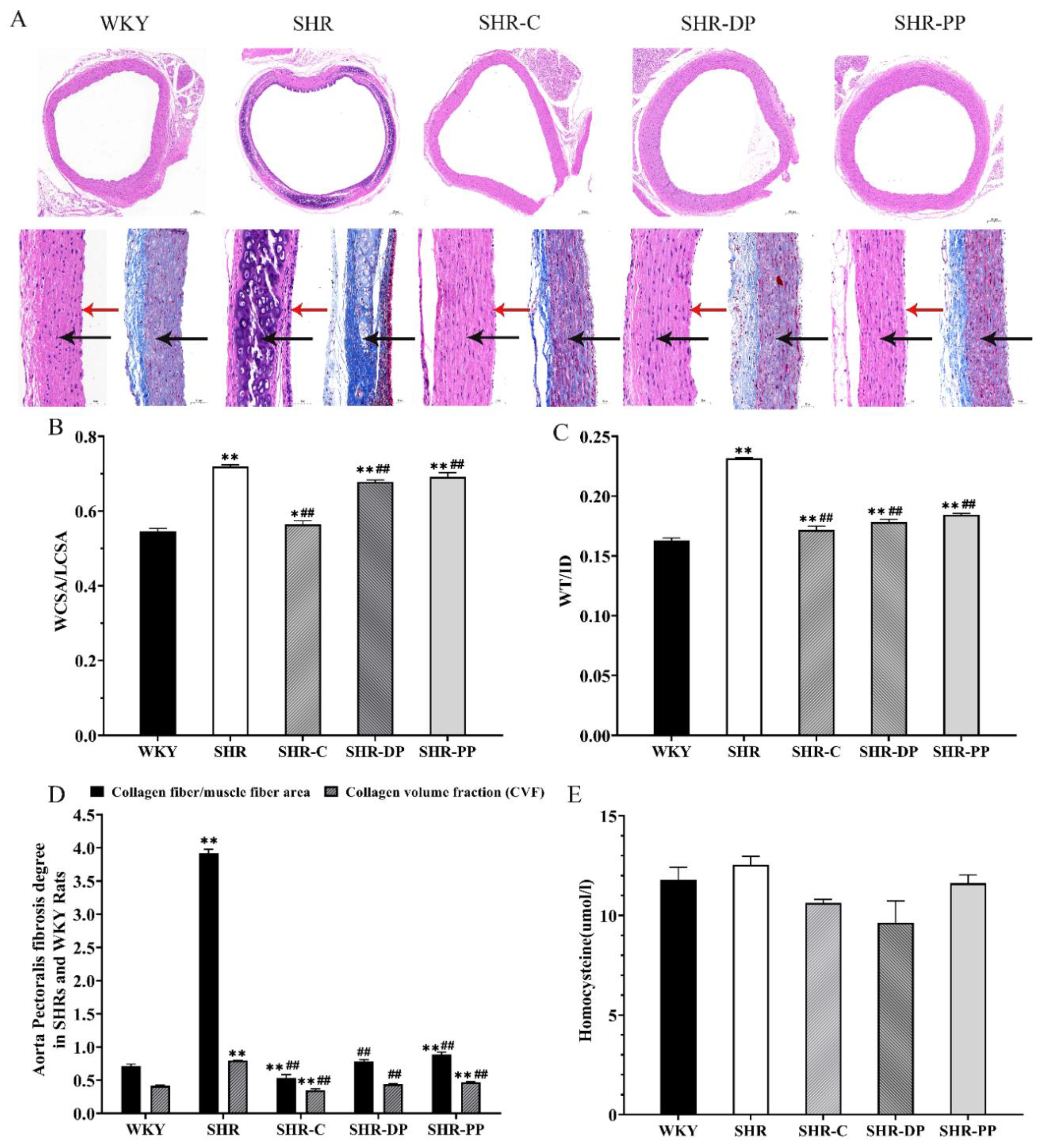
3.4. Effect of Pea Peptides and the Two-Peptide Composite on SHR and WKY Rat Vascular Remodeling
3.5. Effect of Pea Peptides and Two-Peptide Composite on Myocardial Fibrosis and Heart Function in SHRs and WKY Rats
3.6. Effect of Pea Peptides and the Two-Peptide Composite on the Brain of SHRs and WKY Rats
3.7. Effect of Pea Peptides and the Two-Peptide Composite on the Liver of SHRs and WKY Rats
3.8. Effect of Pea Peptides and the Two-Peptide Composite on the Gut Microbiota of SHRs and WKY Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hall, J.E.; Granger, J.P.; do Carmo, J.M.; da Silva, A.A.; Dubinion, J.; George, E.; Hamza, S.; Speed, J.; Hall, M.E. Hypertension: Physiology and Pathophysiology. Hypertens. Physiol. Pathophysiol. Compr. Physiol. 2012, 2, 2393–2442. [Google Scholar]
- Nowacki, D.; Martynowicz, H.; Skoczynska, A.; Wojakowska, A.; Turczyn, B.; Bobak, L.; Trziszka, T.; Szuba, A. Lecithin derived from omega-3 PUFA fortified eggs decreases blood pressure in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 12373. [Google Scholar] [CrossRef]
- Wang, M.; Han, W.Z.; Zhang, M.; Fang, W.Y.; Zhai, X.R.; Guan, S.F.; Qu, X.K. Long-term renal sympathetic denervation ameliorates renal fibrosis and delays the onset of hypertension in spontaneously hypertensive rats. Am. J. Transl. Res. 2018, 10, 4042–4053. [Google Scholar]
- Beaney, T.; Schutte, A.E.; Tomaszewski, M. May Measurement Month 2017: An analysis of blood pressure screening results worldwide. Lancet Glob. Health 2018, 6, e736–e743. [Google Scholar] [CrossRef]
- Sarzani, R.; Salvi, F.; Dessi-Fulgheri, P.; Rappelli, A. Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: An integrated view in humans. J. Hypertens. 2008, 26, 831–843. [Google Scholar] [CrossRef]
- Gomes, C.; Ferreira, D.; Carvalho, J.P.F.; Barreto, C.A.V.; Fernandes, J.; Gouveia, M.; Ribeiro, F.; Duque, A.S.; Vieira, S.I. Current genetic engineering strategies for the production of antihypertensive ACEI peptides. Biotechnol. Bioeng. 2020, 117, 2610–2628. [Google Scholar] [CrossRef]
- Berard, E.; Niel, O.; Rubio, A. Is the renin-angiotensin system actually hypertensive? Pediatr. Nephrol. 2014, 29, 951–960. [Google Scholar] [CrossRef]
- Van Thiel, B.S.; van der Pluijm, I.; Riet, L.T.; Essers, J.; Danser, A.H.J. The renin-angiotensin system and its involvement in vascular disease. Eur. J. Pharmacol. 2015, 763, 3–14. [Google Scholar] [CrossRef]
- Colafella, K.M.M.; Hilliard, L.M.; Denton, K.M. Epochs in the depressor/pressor balance of the renin-angiotensin system. Clin. Sci. 2016, 130, 761–771. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Zhao, L.; Yu, J.; Wang, S.L.; Cao, T.F.; Gao, X.; Wei, Y.X. Identification and Antihypertension Study of Novel Angiotensin I-Converting Enzyme Inhibitory Peptides from the Skirt of Chlamys farreri Fermented with Bacillus natto. J. Agric. Food Chem. 2021, 69, 146–158. [Google Scholar] [CrossRef]
- Bakhle, Y.S. How ACE inhibitors transformed the renin-angiotensin system. Br. J. Pharmacol. 2020, 177, 2657–2665. [Google Scholar] [CrossRef]
- Spence, J.D. Controlling resistant hypertension. Stroke Vasc. Neurol. 2018, 3, 69–75. [Google Scholar] [CrossRef]
- Hamrahian, S.M.; Falkner, B. Hypertension in Chronic Kidney Disease. In Hypertension: From Basic Research to Clinical Practice, Vol 2; Islam, M.S., Ed.; Springer International Publishing Ag: Cham, Switzerland, 2017; Volume 956, pp. 307–325. [Google Scholar]
- Messerli, F.H.; Bangalore, S.; Bavishi, C.; Rimoldi, S.F. Angiotensin-Converting Enzyme Inhibitors in Hypertension To Use or Not to Use? J. Am. Coll. Cardiol. 2018, 71, 1474–1482. [Google Scholar] [CrossRef]
- Jankovic, S.M.; Dajic, M.; Jacovic, S.; Markovic, S.; Papic, T.; Petrusic, T.; Radojkovic, M.; Rankovic, A.; Tanaskovic, M.; Vasic, M.; et al. Measuring Patients’ Knowledge About Adverse Effects of Angiotensin-Converting Enzyme Inhibitors. J. Patient Saf. 2019, 15, E28–E31. [Google Scholar] [CrossRef]
- Gorsane, I.; Ben Ayed, T.; Aoudia, R.; Kaaroud, H.; Ben Hamida, F.; Harzallah, A.; Ben Abdallah, T. Simultaneous Acute Pancreatitis and Angioedema Associated with Angiotensin-Converting Enzyme Inhibitor. Saudi J. Kidney Dis. Transplant. 2019, 30, 1479–1484. [Google Scholar] [CrossRef]
- Rai, A.K.; Sanjukta, S.; Jeyaram, K. Production of angiotensin I converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension. Crit. Rev. Food Sci. Nutr. 2017, 57, 2789–2800. [Google Scholar] [CrossRef]
- Wu, J.P.; Liao, W.; Udenigwe, C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Technol. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Liu, P.R.; Lan, X.D.; Yaseen, M.; Wu, S.G.; Feng, X.Z.; Zhou, L.Q.; Sun, J.H.; Liao, A.P.; Liao, D.K.; Sun, L.X. Purification, Characterization and Evaluation of Inhibitory Mechanism of ACE Inhibitory Peptides from Pearl Oyster (Pinctada fucata martensii) Meat Protein Hydrolysate. Mar. Drugs 2019, 17, 463. [Google Scholar] [CrossRef]
- Kodera, T.; Nio, N. Identification of an angiotensin I-converting enzyme inhibitory peptides from protein hydrolysates by a soybean protease and the antihypertensive effects of hydrolysates in spontaneously hypertensive model rats. J. Food Sci. 2006, 71, C164–C173. [Google Scholar] [CrossRef]
- Vallabha, V.S.; Tiku, P.K. Antihypertensive Peptides Derived from Soy Protein by Fermentation. Int. J. Pept. Res. Ther. 2014, 20, 161–168. [Google Scholar] [CrossRef]
- Li, H.; Prairie, N.; Udenigwe, C.C.; Adebiyi, A.P.; Tappia, P.S.; Aukema, H.M.; Jones PJ, H.; Aluko, R.E. Blood Pressure Lowering Effect of a Pea Protein Hydrolysate in Hypertensive Rats and Humans. J. Agric. Food Chem. 2011, 59, 9854–9860. [Google Scholar] [CrossRef]
- Nawaz, K.A.A.; David, S.M.; Murugesh, E.; Thandeeswaran, M.; Kiran, K.G.; Mahendran, R.; Palaniswamy, M.; Angayarkanni, J. Identification and in silico characterization of a novel peptide inhibitor of angiotensin converting enzyme from pigeon pea (Cajanus cajan). Phytomedicine 2017, 36, 1–7. [Google Scholar] [CrossRef]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino Acid Availability of a Dairy and Vegetable Protein Blend Compared to Single Casein, Whey, Soy, and Pea Proteins: A Double-Blind, Cross-Over Trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Gao, X.; Wei, Y.X.; Liu, Q.; Jiang, Y.H.; Zhao, L.; Ulaah, S. Isolation, purification and the anti-hypertensive effect of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from Ruditapes philippinarum fermented with Bacillus natto. Food Funct. 2018, 9, 5230–5237. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Cao, T.F.; Liu, J.L.; Fan, F.; Yin, D.C.; Zhou, S.C. Optimization of Enzymatic Hydrolysis of Hongdao Clam and Anti-hypertensive Activity of the Resulted Products. Sci. Technol. Food Ind. 2021, 42, 216–222. [Google Scholar]
- Zhang, F.F.; Dang, Y.; Li, Y.X.; Hao, Q.Q.; Li, R.; Qi, X.Y. Cardiac Contractility Modulation Attenuate Myocardial Fibrosis by Inhibiting TGF-beta 1/Smad3 Signaling Pathway in a Rabbit Model of Chronic Heart Failure. Cell. Physiol. Biochem. 2016, 39, 294–302. [Google Scholar] [CrossRef]
- Hua, M.; Liu, Z.B.; Sha, J.Y.; Li, S.S.; Dong, L.N.; Sun, Y.S. Effects of ginseng soluble dietary fiber on serum antioxidant status, immune factor levels and cecal health in healthy rats. Food Chem. 2021, 365, 130641. [Google Scholar] [CrossRef]
- Fogo, A.B.; Kashgarian, M. Chapter 3-Glomerular Diseases. In Diagnostic Atlas of Renal Pathology, 3rd ed.; Fogo, A.B., Kashgarian, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 19–294. [Google Scholar]
- Mancia, G.; Scopelliti, F.; Grassi, G. Hypertension and the heart. Semin. Cardiothorac. Vasc. Anesth. 2006, 10, 198–202. [Google Scholar] [CrossRef]
- Miller, J.B.; Suchdev, K.; Jayaprakash, N.; Hrabec, D.; Sood, A.; Sharma, S.; Levy, P.D. New Developments in Hypertensive Encephalopathy. Curr. Hypertens. Rep. 2018, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- McArt, S.H.; Cook-Patton, S.C.; Thaler, J.S. Relationships between arthropod richness, evenness, and diversity are altered by complementarity among plant genotypes. Oecologia 2012, 168, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Schmieder, R.E. New Approaches in the Treatment of Hypertension. Circ. Res. 2015, 116, 1074–1095. [Google Scholar] [CrossRef] [PubMed]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 2014, 28, 74–79. [Google Scholar] [CrossRef]
- Tsai, B.C.K.; Kuo, W.W.; Day, C.H.; Hsieh, D.J.Y.; Kuo, C.H.; Daddam, J.; Chen, R.J.; Padma, V.V.; Wang, G.Q.; Huang, C.Y. The soybean bioactive peptide VHVV alleviates hypertension-induced renal damage in hypertensive rats via the SIRT1-PGC1 alpha/Nrf2 pathway. J. Funct. Foods 2020, 75, 104255. [Google Scholar] [CrossRef]
- Jing, Y.; Hu, J.J.; Zhao, J.R.; Yang, J.; Huang, N.; Song, P.; Xu, J.; Zhang, M.X.; Li, P.; Yin, Y.L. Experimental study of blood pressure and its impact on spontaneous hypertension in rats with Xin Mai Jia. Biomed. Pharmacother. 2019, 112, 108689. [Google Scholar] [CrossRef]
- Ma, C.; Xin, H.; Jiang, X.Y.; Wang, Y.X.; Zhang, Y.S. Relationship between renal injury and the antagonistic roles of angiotensin-converting enzyme (ACE) and ACE2. Genet. Mol. Res. 2014, 13, 2333–2342. [Google Scholar] [CrossRef]
- Moon, J.-Y. ACE2 and Angiotensin-(1-7) in Hypertensive Renal Disease. Electrolytes Blood Press. E BP 2011, 9, 41–44. [Google Scholar] [CrossRef]
- Wang, X.X.; Ye, Y.; Gong, H.; Wu, J.; Yuan, J.; Wang, S.J.; Yin, P.P.; Ding, Z.W.; Kang, L.; Jiang, Q.; et al. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1-7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J. Mol. Cell. Cardiol. 2016, 97, 180–190. [Google Scholar] [CrossRef]
- Moon, J.-Y. Recent Update of Renin-angiotensin-aldosterone System in the Pathogenesis of Hypertension. Electrolyte Blood Press. E BP 2013, 11, 41–45. [Google Scholar] [CrossRef]
- Lazic, S.E.; Semenova, E.; Williams, D.P. Determining organ weight toxicity with Bayesian causal models: Improving on the analysis of relative organ weights. Sci. Rep. 2020, 10, 6625. [Google Scholar] [CrossRef]
- Renna, N.F.; de Las Heras, N.; Miatello, R.M. Pathophysiology of vascular remodeling in hypertension. Int. J. Hypertens. 2013, 2013, 808353. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Wo, D.; Huang, Y.; Yu, N.; Zeng, J.W.; Chen, H.W.; Wang, H.; Bao, L.Y.; Lin, S.; Chu, J.F.; et al. Alkaloids from Nelumbinis Plumula (AFNP) ameliorate aortic remodeling via RhoA/ROCK pathway. Biomed. Pharmacother. 2019, 112, 108651. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.A.M.; Diederich, L.; Good, M.E.; DeLalio, L.J.; Murphy, S.A.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, P.; Tatarkova, Z.; Sivonova, M.K.; Racay, P.; Lehotsky, J. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. Int. J. Mol. Sci. 2020, 21, 7698. [Google Scholar] [CrossRef]
- Rodrigo, R.; Passalacqua, W.; Araya, J.; Orellana, M.; Rivera, G. Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. J. Cardiovasc. Pharmacol. 2003, 42, 453–461. [Google Scholar] [CrossRef]
- Chen, L.Y.; Pan, C.S.; Wei, X.H.; Li, L.; Han, J.Y.; Huang, L. Sang-qi Granula Reduces Blood Pressure and Myocardial Fibrosis by Suppressing Inflammatory Responses Associated with the Peroxisome Proliferator-Activated Receptors and Nuclear Factor kappa B Protein in Spontaneously Hypertensive Rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 721729. [Google Scholar] [CrossRef]
- Firoz, C.K.; Jabir, N.R.; Kamal, M.A.; Alama, M.N.; Damanhouri, G.A.; Khan, W.; Alzahrani, A.S.; Almehdar, H.A.; Tabrez, S. Neopterin: An immune biomarker of coronary artery disease and its association with other CAD markers. IUBMB Life 2015, 67, 453–459. [Google Scholar] [CrossRef]
- Miao, Y.L.; Kang, X.I. Evaluation of left ventricular function in patients with heart failure after myocardial infarction by real-time three-dimensional transesophageal echocardiography. Am. J. Transl. Res. 2021, 13, 10380–10387. [Google Scholar]
- Miao, Y.L.; Liu, Y.; Liu, C.; Yao, L.; Kang, X.N.; Lv, M.T. Diagnostic Value of Echocardiography Combined with Serum h-FABP and cTnI in Myocardial Infarction and Its Evaluation Value in Left Ventricular Function. Evid.-Based Complement. Altern. Med. 2022, 2022, 8809708. [Google Scholar] [CrossRef]
- Gasparini, S.; Ferlazzo, E.; Sueri, C.; Cianci, V.; Ascoli, M.; Cavalli, S.M.; Beghi, E.; Belcastro, V.; Bianchi, A.; Benna, P.; et al. Hypertension, seizures, and epilepsy: A review on pathophysiology and management. Neurol. Sci. 2019, 40, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Douros, A.; Kauffmann, W.; Bronder, E.; Klimpel, A.; Garbe, E.; Kreutz, R. Ramipril-Induced Liver Injury: Case Report and Review of the Literature. Am. J. Hypertens. 2013, 26, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Lucena, M.I.; Kaplowitz, N.; Garcia-Munoz, B.; Borraz, Y.; Pachkoria, K.; Garcia-Cortes, M.; Fernandez, M.C.; Pelaez, G.; Rodrigo, L.; et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology 2006, 44, 1581–1588. [Google Scholar] [CrossRef]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef]
- Czuczejko, J.; Mila-Kierzenkowska, C.; Szewczyk-Golec, K. Plasma alpha-Glutathione S-Transferase Evaluation in Patients with Acute and Chronic Liver Injury. Can. J. Gastroenterol. Hepatol. 2019, 2019, 5850787. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.H.; Qi, Y.F.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef]
- Ahlawat, S.; Sharma, K.K. Gut-organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Mell, B.; Jala, V.R.; Mathew, A.V.; Byun, J.; Waghulde, H.; Zhang, Y.J.; Haribabu, B.; Vijay-Kumar, M.; Pennathur, S.; Joe, B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 2015, 47, 187–197. [Google Scholar] [CrossRef]
- Hu, R.K.; Guo, W.L.; Huang, Z.R.; Li, L.; Liu, B.; Lv, X.C. Extracts of Ganoderma lucidum attenuate lipid metabolism and modulate gut microbiota in high-fat diet fed rats. J. Funct. Foods 2018, 46, 403–412. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, W.B.; Tian, Z.; Wu, H.Y.; Ning, H.; Yan, G.C.; Zhang, Z.W.; Li, Z.X.; Dong, F.; Sun, Y.Z.; et al. Streptococcus and g. Eubacterium_coprostanoligenes_group combined with sphingosine to modulate the serum dyslipidemia in high-fat diet mice. Clin. Nutr. 2021, 40, 4234–4245. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Q.; Wang, J.; Zhan, R.H.; Zhang, L.; Wang, X.F. Fecal metabonomics combined with 16S rRNA gene sequencing to analyze the changes of gut microbiota in rats with kidney-yang deficiency syndrome and the intervention effect of You-gui pill. J. Ethnopharmacol. 2019, 244, 112139. [Google Scholar] [CrossRef] [PubMed]
- Verhaar BJ, H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Riezu-Boj, J.I.; Guruceaga, E.; Milagro, F.I.; Martinez, J.A. Sex-Specific Associations between Gut Prevotellaceae and Host Genetics on Adiposity. Microorganisms 2020, 8, 938. [Google Scholar] [CrossRef]
- Jia, Y.J.; Li, T.Y.; Han, P.; Chen, Y.; Pan, L.J.; Jia, C.S. Effects of different courses of moxibustion treatment on intestinal flora and inflammation of a rat model of knee osteoarthritis. J. Integr. Med. 2022, 20, 173–181. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Wang, M.; Xu, C.; Wang, S.; Li, L.; Zou, S.; Yu, J.; Wei, Y. Positive Effect of a Pea–Clam Two-Peptide Composite on Hypertension and Organ Protection in Spontaneously Hypertensive Rats. Nutrients 2022, 14, 4069. https://doi.org/10.3390/nu14194069
Sun X, Wang M, Xu C, Wang S, Li L, Zou S, Yu J, Wei Y. Positive Effect of a Pea–Clam Two-Peptide Composite on Hypertension and Organ Protection in Spontaneously Hypertensive Rats. Nutrients. 2022; 14(19):4069. https://doi.org/10.3390/nu14194069
Chicago/Turabian StyleSun, Xiaopeng, Min Wang, Chuanjin Xu, Shanglong Wang, Li Li, Shengcan Zou, Jia Yu, and Yuxi Wei. 2022. "Positive Effect of a Pea–Clam Two-Peptide Composite on Hypertension and Organ Protection in Spontaneously Hypertensive Rats" Nutrients 14, no. 19: 4069. https://doi.org/10.3390/nu14194069
APA StyleSun, X., Wang, M., Xu, C., Wang, S., Li, L., Zou, S., Yu, J., & Wei, Y. (2022). Positive Effect of a Pea–Clam Two-Peptide Composite on Hypertension and Organ Protection in Spontaneously Hypertensive Rats. Nutrients, 14(19), 4069. https://doi.org/10.3390/nu14194069






