Association of Gastric Myoelectric Activity with Dietary Intakes, Substrate Utilization, and Energy Expenditure in Adults with Obesity
Abstract
1. Introduction
2. Materials and Methods
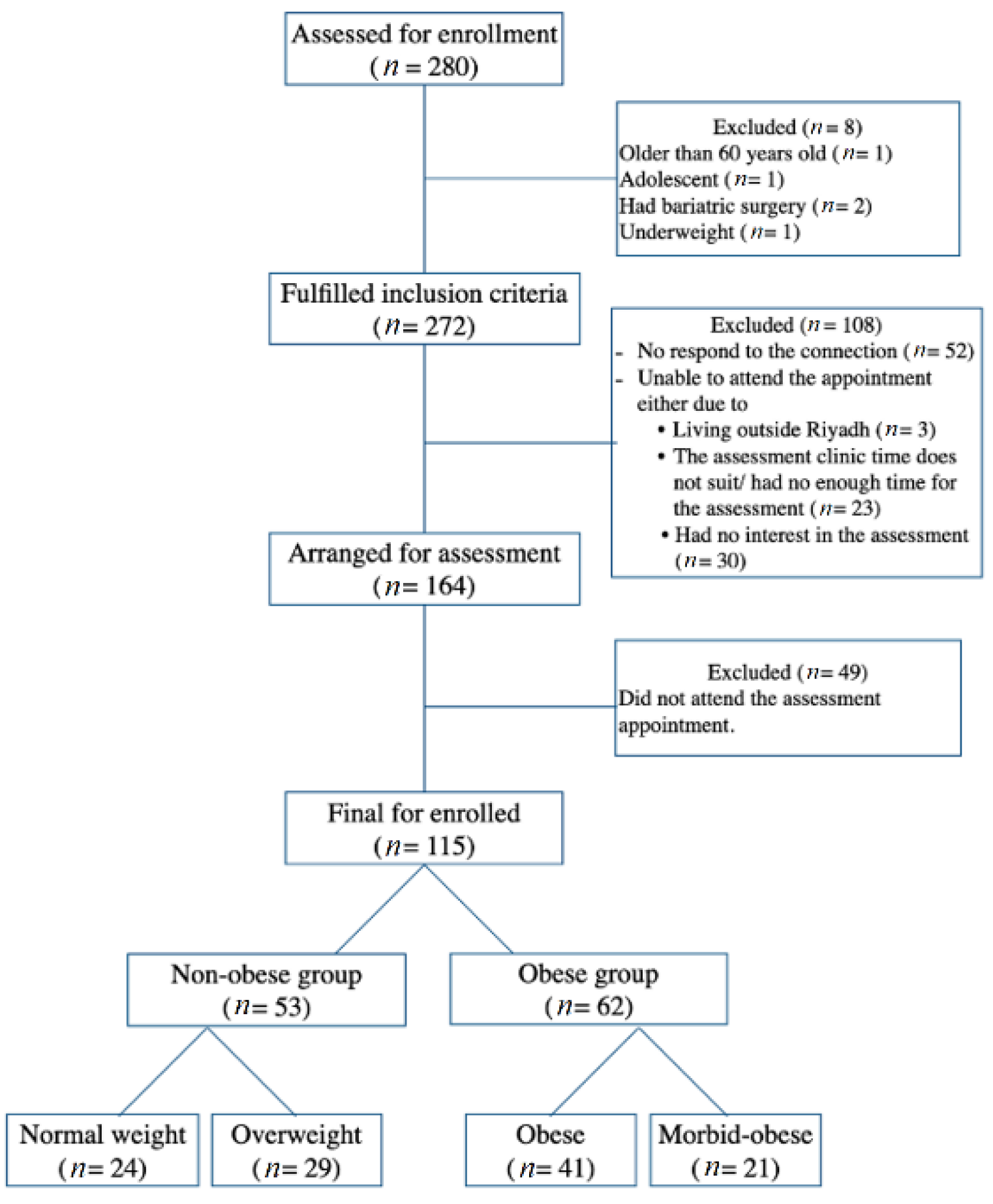
2.1. Study Design and Sample Size
2.2. Demographic Data
2.3. Anthropometric Measurement
2.4. Resting Energy Expenditure
2.5. Dietary Assessment
2.6. EGG for Measuring the GMA Activity
2.7. Statistical Analysis
3. Results
3.1. Demographic and Descriptive Results
3.2. Differences in Indirect Calorimetry and Other Data among Subgroups
3.3. Differences in Dietary Intake among Subgroups
3.4. EGG Recordings among Subgroups
3.5. Correlations of the Average Dominant Frequency of the EGG with Dietary Intakes and Energy Expenditure
3.6. Correlations in Study Participants Assigned into Two Groups Only (Non-Obese vs. Obese Groups)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Chapter 1—Worldwide epidemic of obesity. In Obesity and Obstetrics, 2nd ed.; Mahmood, T.A., Arulkumaran, S., Chervenak, F.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. [Google Scholar]
- Andrade, F.B.; Gualberto, A.; Rezende, C.; Percegoni, N.; Gameiro, J.; Hottz, E.D. The Weight of Obesity in Immunity from Influenza to COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 638852–638856. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, B.J.; Hill, J.O.; Gunn, A.J. Obesity: Scope, lifestyle interventions, and medical management. Tech. Vasc. Interv. Radiol. 2020, 23, 100653. [Google Scholar] [CrossRef]
- Weimer, K.; Sauer, H.; Horing, B.; Valitutti, F.; Mazurak, N.; Zipfel, S.; Stengel, A.; Enck, P.; Mack, I. Impaired gastric myoelectrical reactivity in children and adolescents with obesity compared to normal-weight controls. Nutrients 2018, 10, 699. [Google Scholar] [CrossRef]
- Stiegler, P.; Cunliffe, A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006, 36, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Molé, P.A. Impact of energy intake and exercise on resting metabolic rate. Sports Med. 1990, 10, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Koch, K.; Levine, M.; Muth, E.; Cacioppo, J.; Tassinary, L. Handbook of Psychophysiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Riezzo, G.; Russo, F.; Indrio, F. Electrogastrography in adults and children: The strength, pitfalls, and clinical significance of the cutaneous recording of the gastric electrical activity. BioMed Res. Int. 2013, 2013, 282757. [Google Scholar] [CrossRef] [PubMed]
- Abulmeaty, M.M.A.; Aldisi, D.; Aljuraiban, G.S.; Almajwal, A.; El Shorbagy, E.; Almuhtadi, Y.; Albaran, B.; Aldossari, Z.; Alsager, T.; Razak, S.; et al. Association of Gastric Myoelectrical Activity with Ghrelin, Gastrin, and Irisin in Adults with Metabolically Healthy and Unhealthy Obesity. Front. Physiol. 2022, 13, 815026. [Google Scholar] [CrossRef] [PubMed]
- Mcnearney, T.A.; Sallam, H.S.; Hunnicutt, S.E.; Doshi, D.; Wollaston, D.E.; Mayes, M.D.; Chen, J.D.Z. Gastric slow waves, gastrointestinal symptoms and peptides in systemic sclerosis patients. Neurogastroenterol. Motil. 2009, 21, 1269-e120. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Tassinary, L.G.; Berntson, G. Handbook of Psychophysiology, 4th ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Muth, E.R.; Koch, K.L.; Stern, R.M.; Thayer, J.F. Effect of autonomic nervous system manipulations on gastric myoelectrical activity and emotional responses in healthy human subjects. Psychosom. Med. 1999, 61, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Muth, E.R.; Thayer, J.F.; Stern, R.M.; Friedman, B.H.; Drake, C. The effect of autonomic nervous system activity on gastric myoelectrical activity: Does the spectral reserve hypothesis hold for the stomach? Biol. Psychol. 1998, 47, 265–278. [Google Scholar] [CrossRef]
- Riezzo, G.; Pezzolla, F.; Giorgio, I. Effects of age and obesity on fasting gastric electrical activity in man: A cutaneous electrogastrographic study. Digestion 1991, 50, 176–181. [Google Scholar] [CrossRef]
- Febo-Rodriguez, L.; Shulman, R.J. Chapter 22—Pediatric gastroparesis. In Gastroparesis; McCallum, R.W., Parkman, H.P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 291–307. [Google Scholar]
- Vasavid, P.; Chaiwatanarat, T.; Pusuwan, P.; Sritara, C.; Roysri, K.; Namwongprom, S.; Kuanrakcharoen, P.; Premprabha, T.; Chunlertrith, K.; Thongsawat, S.; et al. Normal Solid Gastric Emptying Values Measured by Scintigraphy Using Asian-style Meal:A Multicenter Study in Healthy Volunteers. J. Neurogastroenterol. Motil. 2014, 20, 371–378. [Google Scholar] [CrossRef]
- Wisén, O.; Hellström, P. Gastrointestinal motility in obesity. J. Intern. Med. 1995, 237, 411–418. [Google Scholar] [CrossRef]
- Xu, X.; Chen, D.D.; Yin, J.; Chen, J.D. Altered postprandial responses in gastric myoelectrical activity and cardiac autonomic functions in healthy obese subjects. Obes. Surg. 2014, 24, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 213–221. [Google Scholar] [CrossRef]
- Wan, X.; Yin, J.; Chen, J. Characteristics of Intestinal Myoelectrical and Motor Activities in Diet-Induced Obese Rats: Obesity and Motility. Dig. Dis. Sci. 2019, 64, 1478–1485. [Google Scholar] [CrossRef]
- Carvalho, N.S.; Baima, D.C.; Barbuti, R.C.; Carvalho, P.; Rezende Filho, J.; Navarro-Rodriguez, T. Transcutaneous multichannel electrogastrography: Normal parameters in a brazilian population. Arq. Gastroenterol. 2020, 57, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, G.; Chiloiro, M.; Guerra, V. Electrogastrography in Healthy Children Evaluation of Normal Values, Influence of Age, Gender, and Obesity. Dig. Dis. Sci. 1998, 43, 1646–1651. [Google Scholar] [CrossRef]
- Buchholz, V.; Berkenstadt, H.; Goitein, D.; Dickman, R.; Bernstine, H.; Rubin, M. Gastric emptying is not prolonged in obese patients. Surg. Obes. Relat. Dis. 2013, 9, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Verdich, C.; Lysgård Madsen, J.; Toubro, S.; Buemann, B.; Holst, J.J.; Astrup, A. Effect of obesity and major weight reduction on gastric emptying. Int. J. Obes. 2000, 24, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Simonian, H.P.; Panganamamula, K.; Parkman, H.P.; Xu, X.; Chen, J.Z.; Lindberg, G.; Xu, H.; Shao, C.; Ke, M.-Y.; Lykke, M.; et al. Multichannel electrogastrography (EGG) in normal subjects: A multicenter study. Dig. Dis. Sci. 2004, 49, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, F.; Zhang, S.; Li, S.; Chen, J. Characteristics of myoelectrical activities along the small intestine and their responses to test meals of different glycemic index in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R997–R1003. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandis, T.G.; Dissanayake, A.S.; De Silva, H.J. Effects of carbohydrate meals of varying consistency on gastric myoelectrical activity. Singap. Med. J. 2002, 43, 579–582. [Google Scholar]
- Brownlee, I. The impact of dietary fibre intake on the physiology and health of the stomach and upper gastrointestinal tract. Bioact. Carbohydr. Diet. Fibre 2014, 4, 155–169. [Google Scholar] [CrossRef]
- Wolpert, N.; Rebollo, I.; Tallon-Baudry, C. Electrogastrography for psychophysiological research: Practical considerations, analysis pipeline, and normative data in a large sample. Psychophysiology 2020, 57, e13599. [Google Scholar] [CrossRef] [PubMed]
| Variables | Non-Obese | Obese | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HW (n = 24) | OW (n = 29) | OB (n = 41) | MO (n = 21) | ||||||
| %within Gender | %within Subgroup | %within Gender | %within Subgroup | %within Gender | %within Subgroup | %within Gender | %within Subgroup | ||
| Gender | 0.01 * | ||||||||
| Female | 33.33 | 70.80 | 23.53 | 41.38 | 33.33 | 41.46 | 9.80 | 23.81 | |
| Male | 10.94 | 29.17 | 26.56 | 58.62 | 37.50 | 58.54 | 25 | 76.19 | |
| Smoking | 0.53 | ||||||||
| Not smoker | 19.28 | 84.21 | 27.71 | 82.14 | 36.15 | 93.75 | 16.87 | 82.35 | |
| Smoker | 23.08 | 15.79 | 38.46 | 17.85 | 15.39 | 6.25 | 23.08 | 17.65 | |
| Physical activity | 0.11 | ||||||||
| Active | 13.85 | 40.91 | 29.23 | 65.52 | 32.31 | 56.76 | 24.62 | 76.19 | |
| Inactive | 29.55 | 59.09 | 22.73 | 34.48 | 36.36 | 43.24 | 11.36 | 23.81 | |
| Variables | HW (n = 24) Mean ± SD | OW (n = 29) Mean ± SD | OB (n = 41) Mean ± SD | MO (n = 21) Mean ± SD | p-Value |
|---|---|---|---|---|---|
| Age | 24.46 ± 7.44 a | 29.07 ± 9.80 a,b | 30.98 ± 10.74 b,c | 34.90 ± 11.32 c | 0.006 * |
| BMI | 21.86 ± 1.91 a | 27.69 ± 1.16 a | 35.32 ± 3.36 a | 44.08 ± 4.11 a | <0.001 * |
| RMR_m (kcal/day) | 1412.92 ± 202.64 a | 1578.17 ± 268.81 b | 1835.85 ± 326.08 c | 2222.38 ± 334.25 d | <0.001 ** |
| RMR_pred (kcal/day) | 1497.96 ± 187.24 a | 1678.59 ± 238.01 b | 2004.63 ± 373.47 c | 2392.19 ± 338.37 d | <0.001 ** |
| Prediction % | 94.52 ± 8.82 a | 94.56 ± 13.80 a | 92.50 ± 11.16 a | 93.71 ± 12.77 a | 0.87 |
| Variability % | 6.96 ± 1.79 a | 8.88 ± 2.44 b | 8.15 ± 2.06 b,c | 7.71 ± 1.35 a,c | 0.007 * |
| RQ | 0.84 ± 0.07 a | 0.83 ± 0.07 a b | 0.83 ± 0.05 a,b | 0.81 ± 0.05 b | 0.28 |
| VO2 | 204.75 ± 29.61 a | 228.93 ± 38.62 b | 266.17 ± 47.07 c | 323.71 ± 49.08 d | <0.001 ** |
| VCO2 | 172.58 ± 25.19 a | 190.66 ± 35.0 a | 220.54 ± 40.27 b | 260.57 ± 38.66 c | <0.001 ** |
| Fat % | 40.05 ± 18.12 a | 44.90 ± 21.27 a | 47.24 ± 18.31 a b | 56.63 ± 16.73 b | 0.03 * |
| CHO % | 36.02 ± 17.96 a | 32.76 ± 21.04 a | 34.48 ± 17.90 a | 28.10 ± 16.08 a | 0.50 |
| Protein % | 23.91 ± 3.30 a | 22.34 ± 4.34 a | 18.29 ± 4.53 b | 15.26 ± 2.29 b | <0.001 ** |
| Variables | HW (n = 24) Mean ± SD | OW (n = 29) Mean ± SD | OB (n = 41) Mean ± SD | MO (n = 21) Mean ± SD | p-Value |
|---|---|---|---|---|---|
| Water-load | 509.17 ± 182.40 a | 514.48 ± 216.94 a | 512.88 ± 244.35 a | 774.29 ± 388.07 b | 0.001 ** |
| BL-BradayG | 48.10 ± 18.93 a | 50.22 ± 20.35 a | 51.05 ± 25.10 a | 66.86 ± 15.08 b | 0.014 * |
| BL-NromoG | 24.86 ± 18.85 a | 13.51 ± 8.62 b | 11.23 ± 7.42 b | 13.49 ± 7.28 b | <0.001 ** |
| BL-TachyG | 18.39 ± 7.81 a | 21.02 ± 13.15 a | 21.57 ± 16.69 a | 14.83 ± 9.25 a | 0.242 |
| BL-Duodenal | 8.62 ± 6.96 a,c | 15.25 ± 16.55 a,b | 16.15 ± 16.90 b | 5.30 ± 4.84 c | 0.01 * |
| Min10_BradyG | 58.48 ± 18.34 a,b | 57.97 ± 15.22 a,b | 49.80 ± 22.46 a | 62.08 ± 16.28 b | 0.069 |
| Min10_NormoG | 14.20 ± 8.35 a | 13.88 ± 6.74 a | 11.29 ± 6.62 a | 15.27 ± 11.97 a | 0.257 |
| Min10_TachyG | 19.83 ± 13.17 a | 20.37 ± 9.59 a | 25.29 ± 13.96 a | 17.88 ± 11.87 b | 0.106 |
| Min10_Duodenal | 7.48 ± 7.86 a | 7.78 ± 7.49 a | 13.61 ± 12.84 b | 4.77 ± 4.78 a | 0.003 * |
| Min20_BradyG | 48.98 ± 22.73 a | 50.92 ± 14.81 a | 46.65 ± 19.19 a | 58.33 ± 16.46 b | 0.135 |
| Min20_NormoG | 23.85 ± 18.34 a | 20.37 ± 12.64 a | 13.96 ± 6.39 b | 18.49 ± 15.26 a,b | 0.024 * |
| Min20_TachyG | 19.83 ± 10.92 a | 21.24 ± 9.30 a,b | 25.89 ± 13.73 b | 18.06 ± 8.23 a | 0.042 |
| Min20_Duodenal | 7.35 ± 8.79 a | 7.47 ± 8.95 a | 13.50 ± 16.50 b | 5.11 ± 5.15 a | 0.031 * |
| Min30_BradyG | 48.90 ± 20.50 a,b,c | 51.18 ± 16.17 a,b,c | 46.63 ± 19.24 b | 58.39 ± 16.19 c | 0.119 |
| Min30_NormoG | 21.91 ± 15.90 a | 19.53 ± 12.20 a | 20.16 ± 14.51 a | 21.13 ± 16.06 a | 0.937 |
| Min30_TachyG | 21.40 ± 11.25 a | 22.54 ± 9.51 a | 22.28 ± 12.78 a | 16.75 ± 8.33 a | 0.238 |
| Min30_Duodenal | 7.79 ± 7.90 a,b,c | 6.75 ± 5.54 a,b,c | 10.93 ± 14.65 b | 3.72 ± 2.78 c | 0.054 * |
| ADF-10 min | 1.74 ± 0.81 a,b | 2.29 ± 1.73 a,b | 2.70 ± 3.11 a | 1.29 ± 0.44 b | 0.069 |
| ADF-20 min | 1.90 ± 1.19 a,b | 1.62 ± 0.52 a | 2.80 ± 3.33 b | 1.49 ± 0.45 a | 0.047 * |
| ADF-30 min | 2.53 ± 2.23 a | 1.74 ± 1.02 a | 2.56 ± 2.62 a | 1.60 ± 0.70 a | 0.141 |
| ADF-40 min | 1.61 ± 0.96 a,b | 1.6 ± 0.76 a,b | 2.36 ± 3.02 a | 1.16 ± 0.68 b | 0.105 |
| HW (n = 24) | OW (n = 29) | OB (n = 41) | MO (n = 21) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kcal | Fat | CHO | Pro. | Kcal | Fat | CHO | Pro. | Kcal | Fat | CHO | Pro. | Kcal | Fat | CHO | Pro. | |
| Water load | 0.18 | −0.28 | 0.29 | 0.41 * | −0.04 | 0.05 | −0.25 | 0.04 | −0.07 | −0.11 | −0.03 | −0.07 | 0.35 | 0.17 | 0.49 * | 0.14 |
| The ADF at first 10 min. | −0.21 | −0.01 | −0.08 | −0.21 | 0.49 * | 0.43 * | 0.42 * | 0.36 | −0.19 | 0.01 | −0.17 | −0.26 | −0.13 | −0.27 | −0.09 | −0.06 |
| The ADF at 10–20 min. | 0.16 | 0.12 | −0.33 | −0.18 | −0.05 | −0.13 | 0.13 | −0.10 | 0.12 | 0.19 | 0.25 | −0.19 | −0.18 | −0.19 | −0.20 | −0.01 |
| The ADF at 20–30 min. | 0.15 | −0.34 | 0.15 | 0.56 * | −0.10 | −0.25 | 0.10 | −0.09 | 0.09 | 0.10 | 0.22 | −0.15 | 0.19 | 0.10 | 0.29 | 0.01 |
| The ADF at 30–40 min. | 0.16 | −0.13 | 0.34 | 0.18 | −0.28 | −0.29 | −0.12 | −0.26 | 0.11 | 0.12 | 0.19 | −0.15 | 0.24 | 0.31 | 0.26 | 0.29 |
| HW (n = 24) | OW (n = 29) | OB (n = 41) | MO (n = 21) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMR_m | RQ | Fat % | CHO % | Prot % | RMR_m | RQ | Fat % | CHO% | Prot % | RMR_m | RQ | Fat % | CHO% | Prot % | RMR_m | RQ | Fat % | CHO% | Prot % | |
| Water load Volume | −0.04 | 0.14 | −0.17 | 0.17 | 0.01 | 0.32 | −0.32 | 0.37 * | −0.29 | −0.40 * | 0.37 * | 0.14 | −0.12 | 0.16 | −0.16 | 0.13 | −0.22 | 0.25 | −0.24 | −0.16 |
| The ADF at first 10 min. | −0.18 | −0.23 | 0.20 | −0.23 | 0.16 | 013 | −0.18 | 0.25 | −0.22 | −0.17 | −0.06 | −0.11 | 0.11 | −0.09 | −0.08 | 0.06 | −0.06 | 0.02 | −0.01 | −0.07 |
| The ADF at 10–20 min. | 0.10 | 0.31 | −0.27 | 0.29 | −0.13 | −0.13 | 0.27 | −0.33 | 0.28 | 0.26 | 0.02 | −0.36 * | 0.37 * | −0.32 * | −0.23 | −0.34 | 0.02 | −0.06 | 0.02 | 0.33 |
| The ADF at 20–30 min. | −0.27 | 0.19 | −0.23 | 0.19 | 0.26 | −0.10 | 0.15 | −0.12 | 0.11 | 0.08 | 0.12 | −0.29 | 0.06 | −0.24 | −0.44 ** | 0.05 | −0.10 | 0.11 | −0.10 | −0.06 |
| The ADF at 30–40 min. | 0.01 | −0.04 | 0.03 | −0.04 | 0.04 | 0.01 | 0.68 * | −0.68 * | 0.66 * | 0.14 | 0.08 | 0.01 | 0.15 | 0.05 | −0.46 ** | −0.13 | 0.17 | −0.18 | 0.17 | 0.17 |
| Non-Obese (n = 53) | Obese (n = 62) | Non-Obese (n = 53) | Obese (n = 62) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kcal | Fat | CHO | Pro. | Fiber | Kcal | Fat | CHO | Pro. | Fiber | RMR_m | RQ | Fat % | CHO % | Prot % | RMR_m | RQ | Fat % | CHO % | Prot % | |
| Water load | 0.04 | −0.07 | −0.04 | 0.20 | 0.08 | 0.18 | 0.02 | 0.28 * | 0.01 | 0.10 | 0.19 | −0.14 | 0.17 | −0.12 | −0.26 | 0.39 * | −0.08 | 0.13 | −0.07 | −0.26 |
| The ADF at first 10 min. | 0.32 * | 0.30 * | 0.31 * | 0.16 | 0.29 * | −0.15 | −0.01 | −0.13 | −0.18 | 0.06 | 0.12 | −0.20 | 0.25 | −0.23 | −0.13 | −0.17 | −0.04 | 0.02 | −0.03 | 0.02 |
| The ADF at 10–20 min. | 0.06 | 0.03 | 0.19 | −0.14 | 0.03 | 0.05 | 0.13 | 0.13 | −0.13 | 0.33 * | −0.05 | 0.28 * | −0.27 * | 0.26 | 0.06 | −0.12 | −0.24 | 0.23 | −0.21 | −0.11 |
| The ADF at 20–30 min. | 0.03 | −0.24 | 0.07 | 0.33 * | 0.05 | 0.07 | 0.09 | 0.16 | −0.10 | 0.38 * | −0.26 | 0.18 | −0.19 | 0.16 | 0.20 | −0.02 | −0.20 | 0.23 | −0.16 | −0.31 * |
| The ADF at 30–40 min. | −0.08 | −0.21 | 0.10 | −0.02 | 0.06 | 0.08 | 0.12 | 0.13 | −0.07 | 0.29 * | −0.01 | 0.32 * | −0.34 * | 0.33 * | 0.09 | −0.07 | 0.05 | −0.02 | 0.10 | −0.30 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abulmeaty, M.M.A.; Aljuraiban, G.S.; Aldisi, D.; Albaran, B.; Aldossari, Z.; Alsager, T.; Razak, S.; Almuhtadi, Y.; El-Shorbagy, E.; Berika, M.; et al. Association of Gastric Myoelectric Activity with Dietary Intakes, Substrate Utilization, and Energy Expenditure in Adults with Obesity. Nutrients 2022, 14, 4021. https://doi.org/10.3390/nu14194021
Abulmeaty MMA, Aljuraiban GS, Aldisi D, Albaran B, Aldossari Z, Alsager T, Razak S, Almuhtadi Y, El-Shorbagy E, Berika M, et al. Association of Gastric Myoelectric Activity with Dietary Intakes, Substrate Utilization, and Energy Expenditure in Adults with Obesity. Nutrients. 2022; 14(19):4021. https://doi.org/10.3390/nu14194021
Chicago/Turabian StyleAbulmeaty, Mahmoud M. A., Ghadeer S. Aljuraiban, Dara Aldisi, Batool Albaran, Zaid Aldossari, Thamer Alsager, Suhail Razak, Yara Almuhtadi, Eman El-Shorbagy, Mohamed Berika, and et al. 2022. "Association of Gastric Myoelectric Activity with Dietary Intakes, Substrate Utilization, and Energy Expenditure in Adults with Obesity" Nutrients 14, no. 19: 4021. https://doi.org/10.3390/nu14194021
APA StyleAbulmeaty, M. M. A., Aljuraiban, G. S., Aldisi, D., Albaran, B., Aldossari, Z., Alsager, T., Razak, S., Almuhtadi, Y., El-Shorbagy, E., Berika, M., Al Zaben, M., & Almajwal, A. (2022). Association of Gastric Myoelectric Activity with Dietary Intakes, Substrate Utilization, and Energy Expenditure in Adults with Obesity. Nutrients, 14(19), 4021. https://doi.org/10.3390/nu14194021







