Association of Body Mass Index with Risk of Household Catastrophic Health Expenditure in China: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
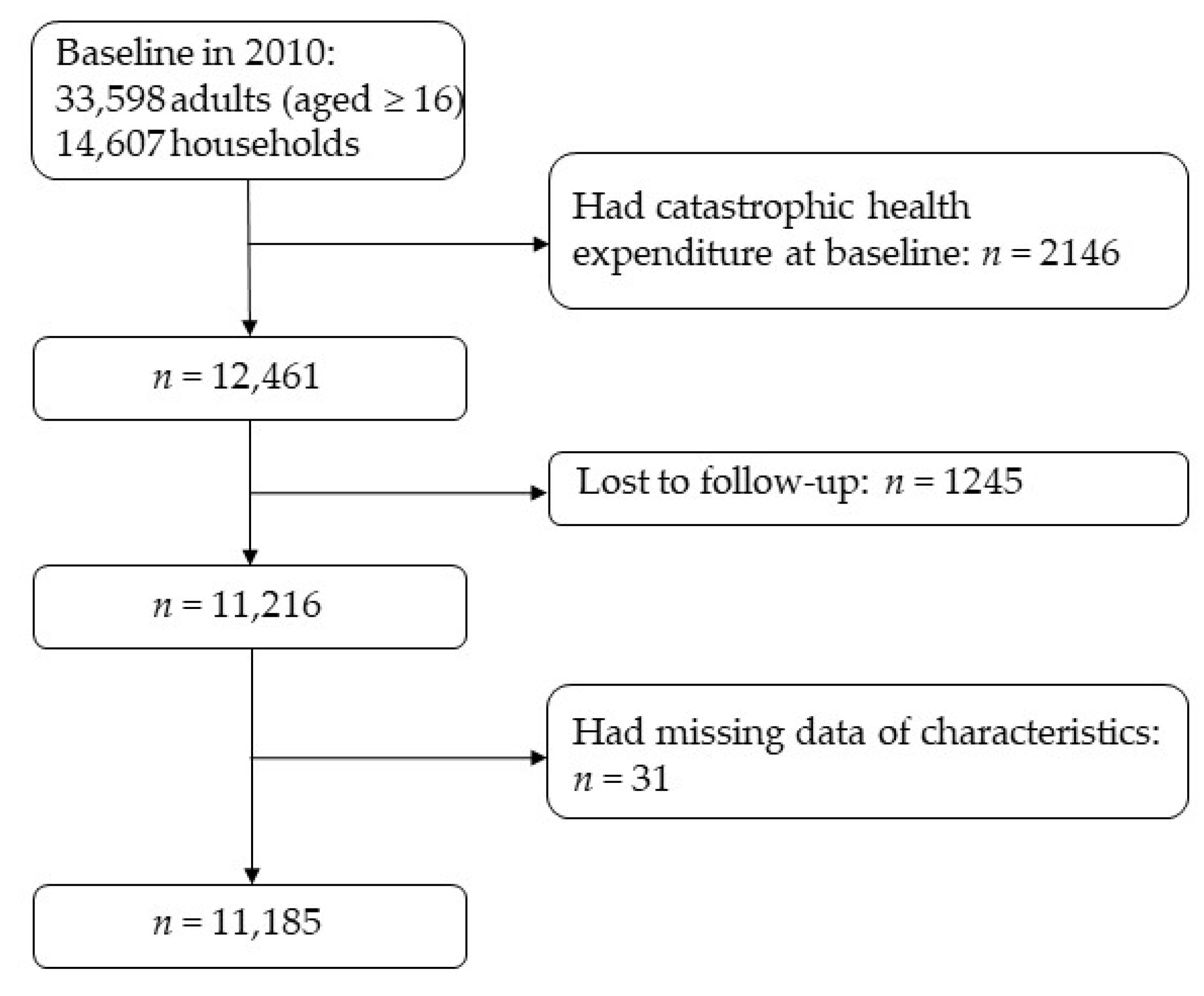
2.1. Study Design and Participants
2.2. Procedure
2.3. Outcome
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
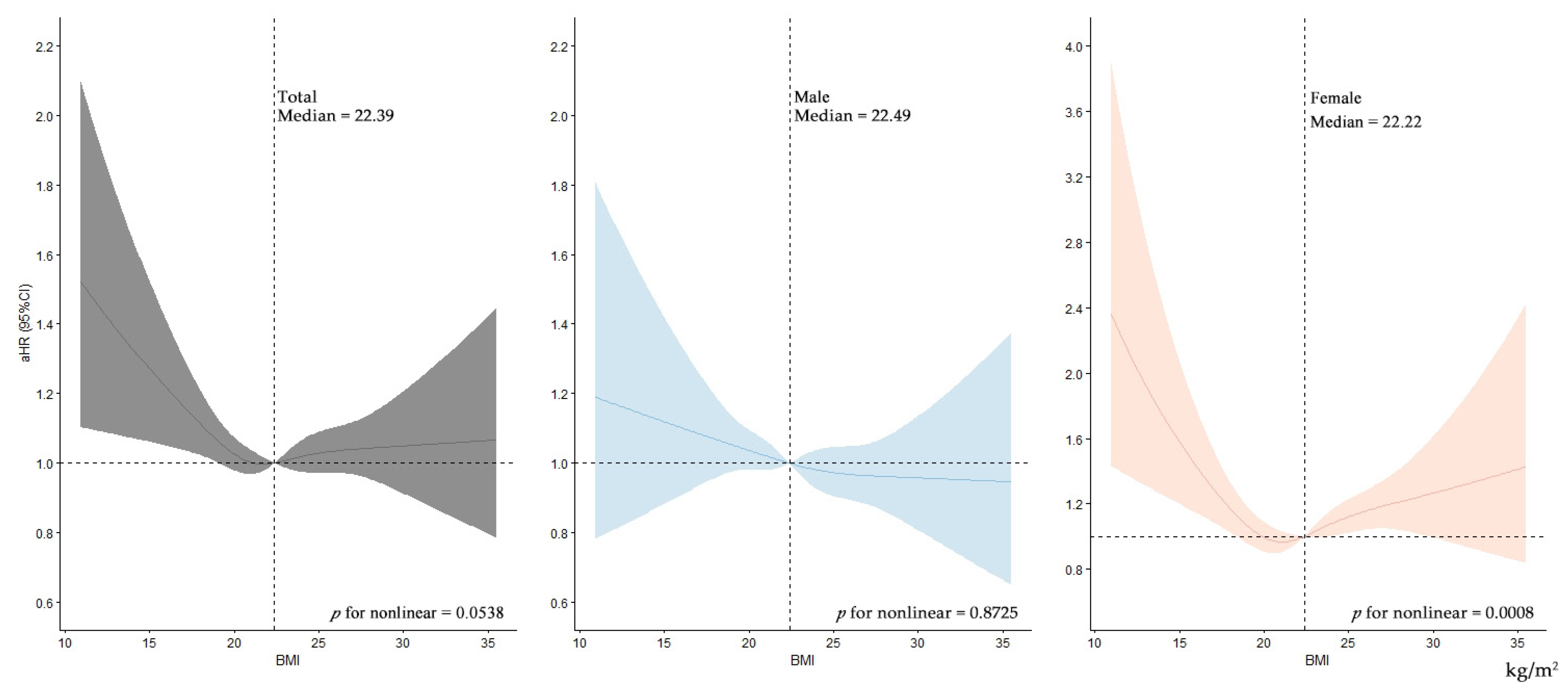
3.2. Risk of CHE
3.3. Sensitivity Analyses and Subgroup Analyses
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Universal Health Coverage. Available online: https://www.who.int/health-topics/universal-health-coverage#tab=tab_1 (accessed on 6 August 2022).
- Sustainable Development Goals—SDG Indicators Metadata Repository. Available online: https://unstats.un.org/sdgs/metadata/ (accessed on 28 August 2022).
- Tracking Universal Health Coverage: 2021 Global Monitoring Report. Available online: https://cdn.who.int/media/docs/default-source/world-health-data-platform/events/tracking-universal-health-coverage-2021-global-monitoring-report_uhc-day.pdf?sfvrsn=fd5c65c6_5&download=true (accessed on 6 August 2022).
- Doshmangir, L.; Hasanpoor, E.; Abou Jaoude, G.J.; Eshtiagh, B.; Haghparast-Bidgoli, H. Incidence of Catastrophic Health Expenditure and Its Determinants in Cancer Patients: A Systematic Review and Meta-analysis. Appl. Health Econ. Health Policy 2021, 19, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Atun, R.; Oldenburg, B.; McPake, B.; Tang, S.; Mercer, S.W.; Cowling, T.E.; Sum, G.; Qin, V.M.; Lee, J.T. Physical multimorbidity, health service use, and catastrophic health expenditure by socioeconomic groups in China: An analysis of population-based panel data. Lancet Global Health 2020, 8, e840–e849. [Google Scholar] [CrossRef]
- Mulaga, A.N.; Kamndaya, M.S.; Masangwi, S.J. Examining the incidence of catastrophic health expenditures and its determinants using multilevel logistic regression in Malawi. PLoS ONE 2021, 16, e0248752. [Google Scholar] [CrossRef]
- Njagi, P.; Arsenijevic, J.; Groot, W. Understanding variations in catastrophic health expenditure, its underlying determinants and impoverishment in Sub-Saharan African countries: A scoping review. Syst. Rev. 2018, 7, 136. [Google Scholar] [CrossRef]
- Sun, C.Y.; Shi, J.F.; Fu, W.Q.; Zhang, X.; Liu, G.X.; Chen, W.Q.; He, J. Catastrophic Health Expenditure and Its Determinants Among Households With Breast Cancer Patients in China: A Multicentre, Cross-Sectional Survey. Front. Public Health 2021, 9, 704700. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, M.; Si, L. Multimorbidity and catastrophic health expenditure among patients with diabetes in China: A nationwide population-based study. BMJ Global Health 2022, 7, e007714. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Blond, K.; Aarestrup, J.; Vistisen, D.; Bjerregaard, L.G.; Jensen, G.B.; Petersen, J.; Nordestgaard, B.G.; Jørgensen, M.E.; Jensen, B.W.; Baker, J.L. Associations between body mass index trajectories in childhood and cardiovascular risk factors in adulthood. Atherosclerosis 2020, 314, 10–17. [Google Scholar] [CrossRef]
- Qu, Y.; Hu, H.Y.; Ou, Y.N.; Shen, X.N.; Xu, W.; Wang, Z.T.; Dong, Q.; Tan, L.; Yu, J.T. Association of body mass index with risk of cognitive impairment and dementia: A systematic review and meta-analysis of prospective studies. Neurosci. Biobehav. Rev. 2020, 115, 189–198. [Google Scholar] [CrossRef]
- Anstey, K.J.; Cherbuin, N.; Budge, M.; Young, J. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes. Rev. 2011, 12, e426–e437. [Google Scholar] [CrossRef]
- Chiolero, A. Body mass index as socioeconomic indicator. BMJ 2021, 373, n1158. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.; Fusco, F.; Gray, A.; Jebb, S.A.; Cairns, B.J.; Mihaylova, B. Body mass index and healthcare costs: A systematic literature review of individual participant data studies. Obes. Rev. 2017, 18, 869–879. [Google Scholar] [CrossRef]
- Wang, Y.C.; McPherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011, 378, 815–825. [Google Scholar] [CrossRef]
- Guariglia, A.; Monahan, M.; Pickering, K.; Roberts, T. Financial health and obesity. Soc. Sci. Med. 2021, 276, 113665. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, P. The Sampling Design of the China Family Panel Studies (CFPS). Chin. J. Sociol. 2015, 1, 471–484. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, J. An Introduction to the China Family Panel Studies (CFPS). Chin. Sociol. Rev. 2014, 47, 3–29. [Google Scholar]
- Zhou, B.F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—Study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar]
- Body Mass Index (BMI). Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index?introPage=intro_3.html (accessed on 17 September 2022).
- Cylus, J.; Thomson, S.; Evetovits, T. Catastrophic health spending in Europe: Equity and policy implications of different calculation methods. Bull. World Health Organ. 2018, 96, 599–609. [Google Scholar] [CrossRef]
- National Bureau of Statistics of the People’s Republic of China. China Statistical Yearbook 2010; China Statistical Publishing House: Beijing, China, 2010. [Google Scholar]
- Chen, Z.; Yu, B.; Yang, C.; Zhou, Y.; Yao, S.; Qian, X.; Wang, C.; Wu, B.; Wu, J. An extended time-series (2000–2018) of global NPP-VIIRS-like nighttime light data from a cross-sensor calibration. Earth Syst. Sci. Data 2021, 13, 889–906. [Google Scholar] [CrossRef]
- GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef]
- Larsson, S.C.; Bäck, M.; Rees, J.M.B.; Mason, A.M.; Burgess, S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: A Mendelian randomization study. Eur. Heart J. 2020, 41, 221–226. [Google Scholar] [CrossRef]
- Teufel, F.; Seiglie, J.A.; Geldsetzer, P.; Theilmann, M.; Marcus, M.E.; Ebert, C.; Arboleda, W.A.L.; Agoudavi, K.; Andall-Brereton, G.; Aryal, K.K.; et al. Body-mass index and diabetes risk in 57 low-income and middle-income countries: A cross-sectional study of nationally representative, individual-level data in 685 616 adults. Lancet 2021, 398, 238–248. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Sasazuki, S.; Inoue, M.; Tsuji, I.; Sugawara, Y.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Nagata, C.; Tanaka, K.; Mizoue, T.; et al. Body mass index and mortality from all causes and major causes in Japanese: Results of a pooled analysis of 7 large-scale cohort studies. J. Epidemiol. 2011, 21, 417–430. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kawachi, I.; Coull, B.A.; Subramanian, S.V. Contribution of socioeconomic factors to the variation in body-mass index in 58 low-income and middle-income countries: An econometric analysis of multilevel data. Lancet Global Health 2018, 6, e777–e786. [Google Scholar] [CrossRef]
- Razak, F.; Corsi, D.J.; Slutsky, A.S.; Kurpad, A.; Berkman, L.; Laupacis, A.; Subramanian, S.V. Prevalence of Body Mass Index Lower Than 16 Among Women in Low- and Middle-Income Countries. JAMA 2015, 314, 2164–2171. [Google Scholar] [CrossRef]
- Chen, J.; Zha, S.; Hou, J.; Lu, K.; Qiu, Y.; Yang, R.; Li, L.; Yang, Y.; Xu, L. Dose-response relationship between body mass index and tuberculosis in China: A population-based cohort study. BMJ Open 2022, 12, e050928. [Google Scholar] [CrossRef]
- Katapa, R.S. A comparison of female- and male-headed households in Tanzania and poverty implications. J. Biosoc. Sci. 2006, 38, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Negesse, A.; Jara, D.; Habtamu, T.; Dessie, G.; Getaneh, T.; Mulugeta, H.; Abebaw, Z.; Taddege, T.; Wagnew, F.; Negesse, Y. The impact of being of the female gender for household head on the prevalence of food insecurity in Ethiopia: A systematic-review and meta-analysis. Public Health Rev. 2020, 41, 15. [Google Scholar] [CrossRef] [PubMed]
- Boneya, D.J.; Ahmed, A.A.; Yalew, A.W. The effect of gender on food insecurity among HIV-infected people receiving anti-retroviral therapy: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0209903. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.M.; de Bairros, F.S.; Pattussi, M.P.; Pauli, S.; Neutzling, M.B. Gender differences in the prevalence of household food insecurity: A systematic review and meta-analysis. Public Health Nutr. 2017, 20, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, K.; Briefel, R.R.; Frongillo, E.A., Jr.; Olson, C.M. Food insufficiency exists in the United States: Results from the third National Health and Nutrition Examination Survey (NHANES III). Am. J. Public Health 1998, 88, 419–426. [Google Scholar] [CrossRef]
- Sarlio-Lähteenkorva, S.; Lahelma, E. Food insecurity is associated with past and present economic disadvantage and body mass index. J. Nutr. 2001, 131, 2880–2884. [Google Scholar] [CrossRef]
- Drewnowski, A.; Specter, S.E. Poverty and obesity: The role of energy density and energy costs. Am. J. Clin. Nutr. 2004, 79, 6–16. [Google Scholar] [CrossRef]
- Elfassy, T.; Glymour, M.M.; Kershaw, K.N.; Carnethon, M.; Llabre, M.M.; Lewis, C.E.; Schneiderman, N.; Al Hazzouri, A.Z. Association Between Sustained Poverty and Changes in Body Mass Index, 1990-2015: The Coronary Artery Risk Development in Young Adults Study. Am. J. Epidemiol. 2018, 187, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, L. Catastrophic medical insurance in China. Lancet 2017, 390, 1724–1725. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 11,185, %) | BMI Group | χ2 | p-Value | |||
|---|---|---|---|---|---|---|---|
| Normal (n = 6833, %) | Underweight (n = 831, %) | Overweight (n = 2825, %) | Obesity (n = 696, %) | ||||
| Gender | 36.359 | <0.001 | |||||
| Male | 7864 (70.3) | 4859 (71.1) | 508 (61.1) | 2000 (70.8) | 497 (71.4) | ||
| Female | 3321 (29.7) | 1974 (28.9) | 323 (38.9) | 825 (29.2) | 199 (28.6) | ||
| Age group | 150.112 | <0.001 | |||||
| 16–39 | 2843 (25.4) | 1777 (26.0) | 223 (26.8) | 656 (23.2) | 187 (26.9) | ||
| 40–49 | 3452 (30.9) | 2134 (31.2) | 159 (19.1) | 937 (33.2) | 222 (31.9) | ||
| 50–59 | 2689 (24.0) | 1602 (23.4) | 173 (20.8) | 746 (26.4) | 168 (24.1) | ||
| ≥60 | 2201 (19.7) | 1320 (19.3) | 276 (33.2) | 486 (17.2) | 119 (17.1) | ||
| Marital status | 172.815 | <0.001 | |||||
| Married/partnered | 9836 (87.9) | 6013 (88.0) | 619 (74.5) | 2571 (91.0) | 633 (90.9) | ||
| Other | 1349 (12.1) | 820 (12.0) | 212 (25.5) | 254 (9.0) | 63 (9.1) | ||
| Education | 229.064 | <0.001 | |||||
| Illiterate/semiliterate | 2732 (24.4) | 1749 (25.6) | 316 (38.0) | 546 (19.3) | 121 (17.4) | ||
| Primary school | 2649 (23.7) | 1698 (24.9) | 204 (24.5) | 604 (21.4) | 143 (20.5) | ||
| Middle school | 3461 (30.9) | 2069 (30.3) | 207 (24.9) | 951 (33.7) | 234 (33.6) | ||
| High school and above | 2343 (20.9) | 1317 (19.3) | 104 (12.5) | 724 (25.6) | 198 (28.4) | ||
| Insurance | 241.867 | <0.001 | |||||
| None | 1394 (12.5) | 833 (12.2) | 121 (14.6) | 346 (12.2) | 94 (13.5) | ||
| UEBMI | 1354 (12.1) | 692 (10.1) | 43 (5.2) | 471 (16.7) | 148 (21.3) | ||
| URBMI | 757 (6.8) | 426 (6.2) | 39 (4.7) | 237 (8.4) | 55 (7.9) | ||
| NRCMS | 6685 (59.8) | 4318 (63.2) | 532 (64.0) | 1506 (53.3) | 329 (47.3) | ||
| Other | 995 (8.9) | 564 (8.3) | 96 (11.6) | 265 (9.4) | 70 (10.1) | ||
| Self-reported health | 102.954 | <0.001 | |||||
| Good | 5279 (47.2) | 3279 (48.0) | 296 (35.6) | 1395 (49.4) | 309 (44.4) | ||
| Medium | 4237 (37.9) | 2557 (37.4) | 324 (39.0) | 1077 (38.1) | 279 (40.1) | ||
| Poor | 1669 (14.9) | 997 (14.6) | 211 (25.4) | 353 (12.5) | 108 (15.5) | ||
| Outpatient services | 17.269 | 0.002 | |||||
| No | 9113 (81.5) | 5569 (81.5) | 636 (76.5) | 2342 (82.9) | 566 (81.3) | ||
| Yes | 2072 (18.5) | 1264 (18.5) | 195 (23.5) | 483 (17.1) | 130 (18.7) | ||
| Inpatient services | |||||||
| No | 10,512 (94.0) | 6432 (94.1) | 779 (93.7) | 2652 (93.9) | 649 (93.2) | 1.075 | 0.783 |
| Yes | 673 (6.0) | 401 (5.9) | 52 (6.3) | 173 (6.1) | 47 (6.8) | ||
| Chronic diseases | 38.440 | <0.001 | |||||
| No | 9550 (85.4) | 5940 (86.9) | 696 (83.8) | 2355 (83.4) | 559 (80.3) | ||
| Yes | 1635 (14.6) | 893 (13.1) | 135 (16.2) | 470 (16.6) | 137 (19.7) | ||
| Smoking | 38.664 | <0.001 | |||||
| No | 6053 (54.1) | 3543 (51.9) | 460 (55.4) | 1649 (58.4) | 401 (57.6) | ||
| Yes | 5132 (45.9) | 3290 (48.1) | 371 (44.6) | 1176 (41.6) | 295 (42.4) | ||
| Drinking | 18.624 | <0.001 | |||||
| No | 8364 (74.8) | 5091 (74.5) | 671 (80.7) | 2099 (74.3) | 503 (72.3) | ||
| Yes | 2821 (25.2) | 1742 (25.5) | 160 (19.3) | 726 (25.7) | 193 (27.7) | ||
| Residence | 238.227 | <0.001 | |||||
| urban | 5084 (45.5) | 2828 (41.4) | 292 (35.1) | 1548 (54.8) | 416 (59.8) | ||
| rural | 6101 (54.5) | 4005 (58.6) | 539 (64.9) | 1277 (45.2) | 280 (40.2) | ||
| Family size | 75.962 | <0.001 | |||||
| 1–2 | 2165 (19.4) | 1245 (18.2) | 191 (23.0) | 592 (21.0) | 137 (19.7) | ||
| 3–4 | 5431 (48.6) | 3249 (47.5) | 344 (41.4) | 1457 (51.6) | 381 (54.7) | ||
| ≥5 | 3589 (32.1) | 2339 (34.2) | 296 (35.6) | 776 (27.5) | 178 (25.6) | ||
| Family economic level | 185.968 | <0.001 | |||||
| Lowest | 2941 (26.3) | 1863 (27.3) | 325 (39.1) | 625 (22.1) | 128 (18.4) | ||
| Lower | 2567 (23.0) | 1642 (24.0) | 167 (20.1) | 616 (21.8) | 142 (20.4) | ||
| Higher | 3164 (28.3) | 1910 (28.0) | 222 (26.7) | 824 (29.2) | 208 (29.9) | ||
| Highest | 2513 (22.5) | 1418 (20.8) | 117 (14.1) | 760 (26.9) | 218 (31.3) | ||
| Socioeconomic development level | 238.177 | <0.001 | |||||
| Lowest | 2358 (21.1) | 1615 (23.6) | 268 (32.3) | 401 (14.2) | 74 (10.6) | ||
| Lower | 3234 (28.9) | 1930 (28.2) | 211 (25.4) | 907 (32.1) | 186 (26.7) | ||
| Higher | 2015 (18.0) | 1201 (17.6) | 111 (13.4) | 528 (18.7) | 175 (25.1) | ||
| Highest | 3578 (32.0) | 2087 (30.5) | 241 (29.0) | 989 (35.0) | 261 (37.5) | ||
| BMI Groups | Events/Incidence Rate * | Univariate Model | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| cHR (95%CI) | p-Value | aHR (95%CI) | p-Value | aHR (95%CI) | p-Value | aHR (95%CI) | p-Value | ||
| Total | 3275/3.85 | ||||||||
| Normal | 1968/3.77 | Ref. | — | Ref. | — | Ref. | — | Ref. | — |
| Underweight | 298/5.08 | 1.37 (1.22–1.55) | <0.001 | 1.18 (1.04–1.34) | 0.008 | 1.14 (1.01–1.29) | 0.036 | 1.15 (1.02–1.31) | 0.023 |
| Overweight | 822/3.82 | 1.01 (0.93–1.10) | 0.761 | 1.06 (0.98–1.15) | 0.162 | 1.06 (0.97–1.15) | 0.177 | 1.05 (0.97–1.15) | 0.210 |
| Obesity | 187/3.47 | 0.92 (0.79–1.07) | 0.293 | 0.98 (0.85–1.14) | 0.840 | 0.98 (0.84–1.13) | 0.750 | 0.98 (0.84–1.14) | 0.747 |
| Male | 2288/3.79 | ||||||||
| Normal | 1449/3.87 | Ref. | — | Ref. | — | Ref. | — | Ref. | — |
| Underweight | 180/4.94 | 1.29 (1.11–1.51) | 0.001 | 1.07 (0.92–1.26) | 0.368 | 1.03 (0.88–1.21) | 0.706 | 1.05 (0.90–1.23) | 0.547 |
| Overweight | 543/3.53 | 0.90 (0.82–1.00) | 0.045 | 0.98 (0.88–1.08) | 0.649 | 0.98 (0.89–1.08) | 0.702 | 0.97 (0.88–1.07) | 0.570 |
| Obesity | 116/2.99 | 0.77 (0.64–0.93) | 0.007 | 0.89 (0.73–1.07) | 0.210 | 0.88 (0.73–1.07) | 0.196 | 0.87 (0.72–1.05) | 0.152 |
| Female | 987/4.00 | ||||||||
| Normal | 519/3.49 | Ref. | — | Ref. | — | Ref. | — | Ref. | — |
| Underweight | 118/5.32 | 1.57 (1.28–1.91) | <0.001 | 1.43 (1.17–1.75) | 0.001 | 1.42 (1.16–1.74) | 0.001 | 1.42 (1.16–1.75) | 0.001 |
| Overweight | 279/4.57 | 1.32 (1.14–1.52) | <0.001 | 1.28 (1.10–1.48) | 0.001 | 1.26 (1.09–1.46) | 0.002 | 1.26 (1.09–1.47) | 0.002 |
| Obesity | 71/4.72 | 1.35 (1.06–1.73) | 0.017 | 1.23 (0.96–1.58) | 0.103 | 1.21 (0.94–1.56) | 0.132 | 1.22 (0.95–1.57) | 0.116 |
| Subgroup | Normal Weight (Events/Objects) | Underweight | Overweight | ||||
|---|---|---|---|---|---|---|---|
| Events/Objects | aHR (95%CI) | p-Value | Events/Objects | aHR (95%CI) | p-Value | ||
| All | 519/1974 | 118/323 | — | — | 279/825 | — | — |
| Age group | |||||||
| 16–39 | 109/662 | 25/120 | 1.33 (0.85–2.07) | 0.213 | 35/181 | 1.07 (0.73–1.59) | 0.724 |
| 40–49 | 127/575 | 24/70 | 1.60 (1.02–2.50) | 0.042 * | 70/262 | 1.25 (0.92–1.68) | 0.150 |
| 50–59 | 125/400 | 24/47 | 1.69 (1.07–2.67) | 0.025 * | 99/238 | 1.45 (1.10–1.91) | 0.008 * |
| ≥60 | 158/337 | 45/86 | 1.23 (0.87–1.73) | 0.240 | 75/144 | 1.30 (0.97–1.75) | 0.078 |
| Insurance | |||||||
| None | 74/291 | 17/51 | 1.41 (0.80–2.47) | 0.232 | 39/132 | 1.20 (0.79–1.81) | 0.402 |
| UEBMI | 48/254 | 3/18 | 0.90 (0.27–2.99) | 0.863 | 28/127 | 1.00 (0.61–1.64) | 0.994 |
| URBMI | 48/187 | 4/20 | 1.11 (0.38–3.29) | 0.845 | 32/88 | 1.33 (0.79–2.26) | 0.283 |
| NRCMS | 301/1029 | 81/189 | 1.52 (1.18–1.96) | 0.001 * | 151/407 | 1.24 (1.02–1.52) | 0.033 * |
| Other | 48/213 | 13/45 | 1.71 (0.87–3.33) | 0.117 | 29/71 | 1.66 (0.99–2.79) | 0.052 |
| Self-reported health | |||||||
| Good | 175/823 | 34/122 | 1.16 (0.79–1.70) | 0.443 | 104/327 | 1.44 (1.12–1.86) | 0.005 * |
| Medium | 199/791 | 43/126 | 1.53 (1.09–2.15) | 0.014 * | 108/335 | 1.23 (0.96–1.56) | 0.095 |
| Poor | 145/360 | 41/75 | 1.53 (1.06–2.20) | 0.022 * | 67/163 | 1.09 (0.80–1.48) | 0.573 |
| Current smoking | |||||||
| No | 491/1876 | 113/307 | 1.47 (1.19–1.81) | <0.001 * | 269/810 | 1.24 (1.07–1.45) | 0.005 * |
| Yes | 28/98 | 5/16 | 0.74 (0.21–2.60) | 0.639 | 10/15 | 2.60 (0.96–7.10) | 0.061 |
| Current drinking | |||||||
| No | 504/1910 | 115/314 | 1.44 (1.17–1.77) | 0.001 * | 262/791 | 1.23 (1.06–1.44) | 0.007 * |
| Yes | 15/64 | 3/9 | 2.07 (0.40–10.80) | 0.386 | 17/34 | 3.64 (1.40–9.47) | 0.008 * |
| Chronic diseases | |||||||
| No | 416/1678 | 89/266 | 1.41 (1.11–1.78) | 0.004 * | 205/667 | 1.26 (1.06–1.50) | 0.008 * |
| Yes | 103/296 | 29/57 | 1.58 (1.03–2.43) | 0.036 * | 74/158 | 1.35 (0.99–1.85) | 0.061 |
| Outpatient services | |||||||
| No | 381/1511 | 71/239 | 1.17 (0.90–1.51) | 0.239 | 202/641 | 1.19 (1.00–1.42) | 0.053 |
| Yes | 138/463 | 47/84 | 2.17 (1.53–3.07) | <0.001 * | 77/184 | 1.36 (1.02–1.82) | 0.038 * |
| Inpatient services | |||||||
| No | 476/1830 | 110/295 | 1.45 (1.18–1.80) | 0.001 * | 261/768 | 1.30 (1.12–1.52) | 0.001 * |
| Yes | 43/144 | 8/28 | 0.98 (0.43–2.25) | 0.969 | 18/57 | 0.63 (0.32–1.25) | 0.189 |
| Residence | |||||||
| Urban | 240/1048 | 35/138 | 1.17 (0.81–1.68) | 0.404 | 162/508 | 1.24 (1.01–1.52) | 0.044 * |
| Rural | 279/926 | 83/185 | 1.60 (1.25–2.06) | <0.001 * | 117/317 | 1.27 (1.02–1.58) | 0.036 * |
| Socioeconomic development level | |||||||
| Lowest | 89/351 | 33/75 | 1.86 (1.23–2.82) | 0.003 * | 33/93 | 1.32 (0.87–2.00) | 0.192 |
| Lower | 162/603 | 26/89 | 0.99 (0.65–1.52) | 0.974 | 99/291 | 1.23 (0.95–1.60) | 0.121 |
| Higher | 91/320 | 14/41 | 1.64 (0.92–2.94) | 0.096 | 58/140 | 1.47 (1.04–2.07) | 0.029 * |
| Highest | 177/700 | 45/118 | 1.33 (0.94–1.9) | 0.108 | 89/301 | 1.08 (0.84–1.41) | 0.541 |
| Family economic level | |||||||
| Lowest | 183/519 | 57/123 | 1.52 (1.12–2.07) | 0.008 * | 93/230 | 1.21 (0.93–1.58) | 0.148 |
| Lower | 113/437 | 23/68 | 1.29 (0.80–2.08) | 0.291 | 55/186 | 1.12 (0.80–1.56) | 0.528 |
| Higher | 138/585 | 26/86 | 1.24 (0.81–1.91) | 0.321 | 72/225 | 1.41 (1.05–1.90) | 0.024 * |
| Highest | 85/433 | 12/46 | 1.79 (0.95–3.37) | 0.074 | 59/184 | 1.56 (1.10–2.22) | 0.012 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, M.; Liu, J. Association of Body Mass Index with Risk of Household Catastrophic Health Expenditure in China: A Population-Based Cohort Study. Nutrients 2022, 14, 4014. https://doi.org/10.3390/nu14194014
Wang Y, Liu M, Liu J. Association of Body Mass Index with Risk of Household Catastrophic Health Expenditure in China: A Population-Based Cohort Study. Nutrients. 2022; 14(19):4014. https://doi.org/10.3390/nu14194014
Chicago/Turabian StyleWang, Yaping, Min Liu, and Jue Liu. 2022. "Association of Body Mass Index with Risk of Household Catastrophic Health Expenditure in China: A Population-Based Cohort Study" Nutrients 14, no. 19: 4014. https://doi.org/10.3390/nu14194014
APA StyleWang, Y., Liu, M., & Liu, J. (2022). Association of Body Mass Index with Risk of Household Catastrophic Health Expenditure in China: A Population-Based Cohort Study. Nutrients, 14(19), 4014. https://doi.org/10.3390/nu14194014







