Butyrate Glycerides Protect against Intestinal Inflammation and Barrier Dysfunction in Mice
Abstract
1. Introduction
2. Materials and Methods
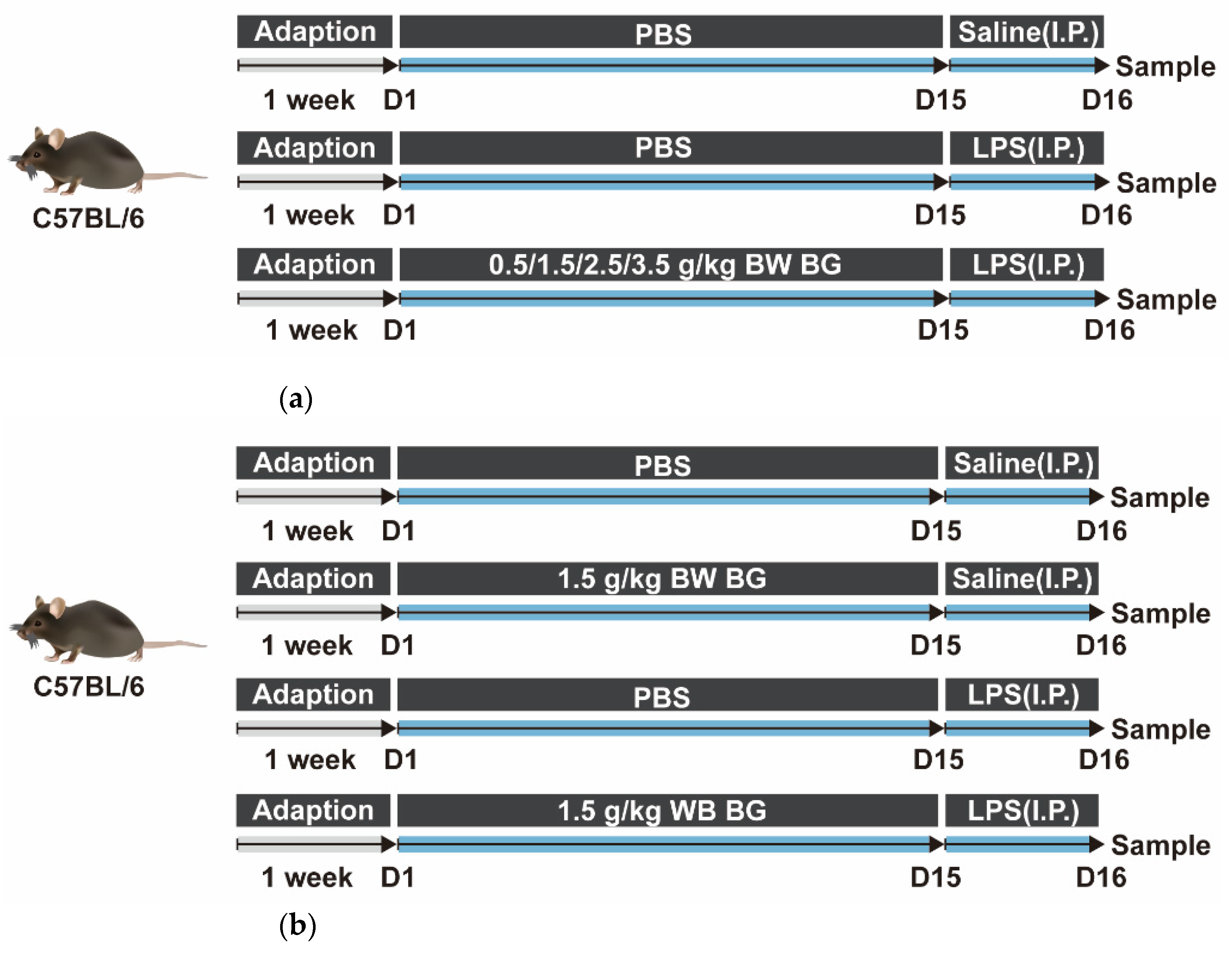
2.1. Animals and Experimental Treatment
2.1.1. Animal Experiment 1
2.1.2. Animal Experiment 2
2.2. Enzyme-Linked Immunosorbent (ELISA) Assay
2.3. Intestinal Morphology Analysis
2.4. Microvilli and Tight Junction Structure Observation by SEM and TEM
2.5. Quantitative Real-Time PCR
2.6. Immunofluorescence Analysis
2.7. Statistical Analysis
3. Results
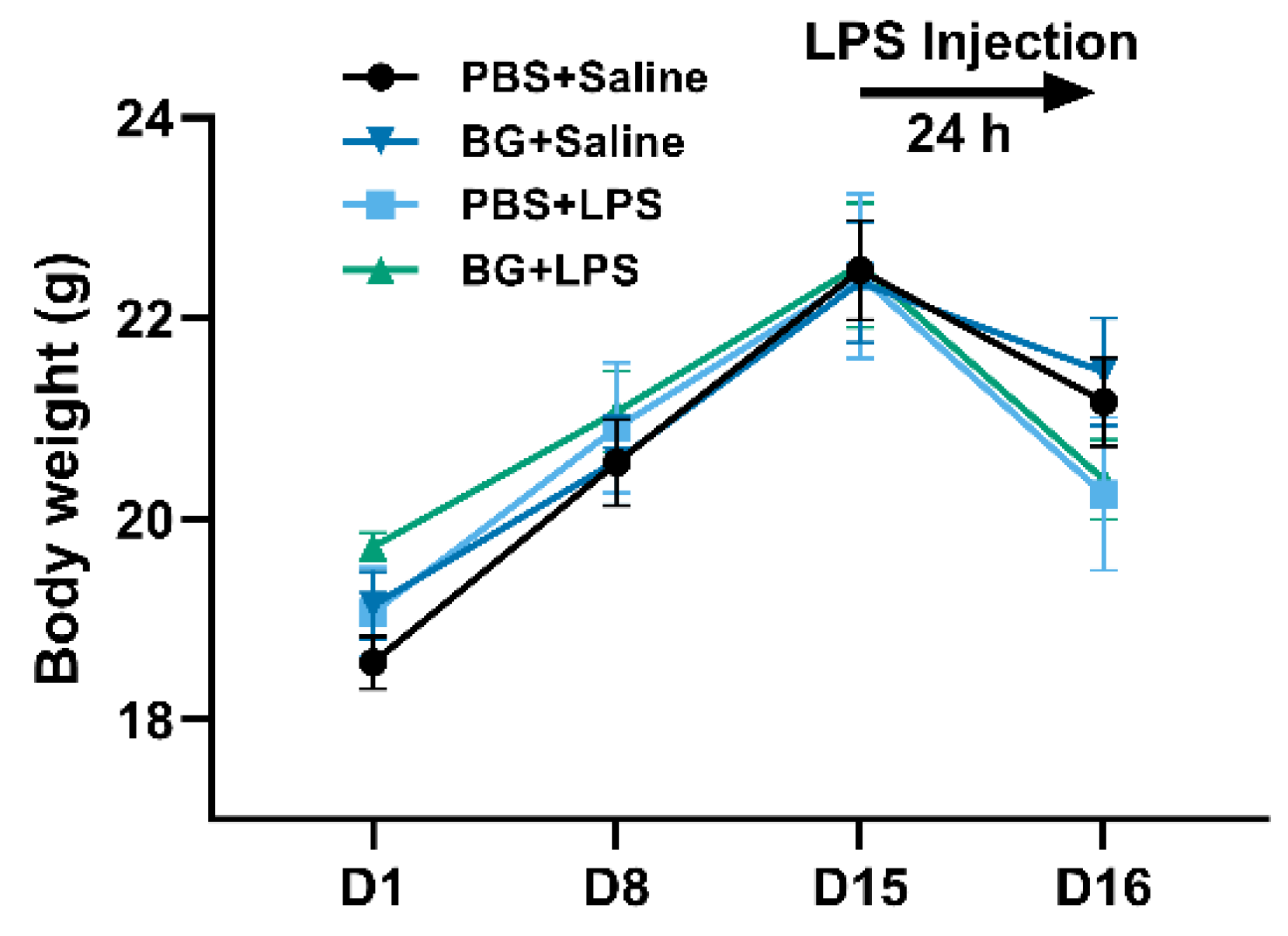
3.1. Growth Performance
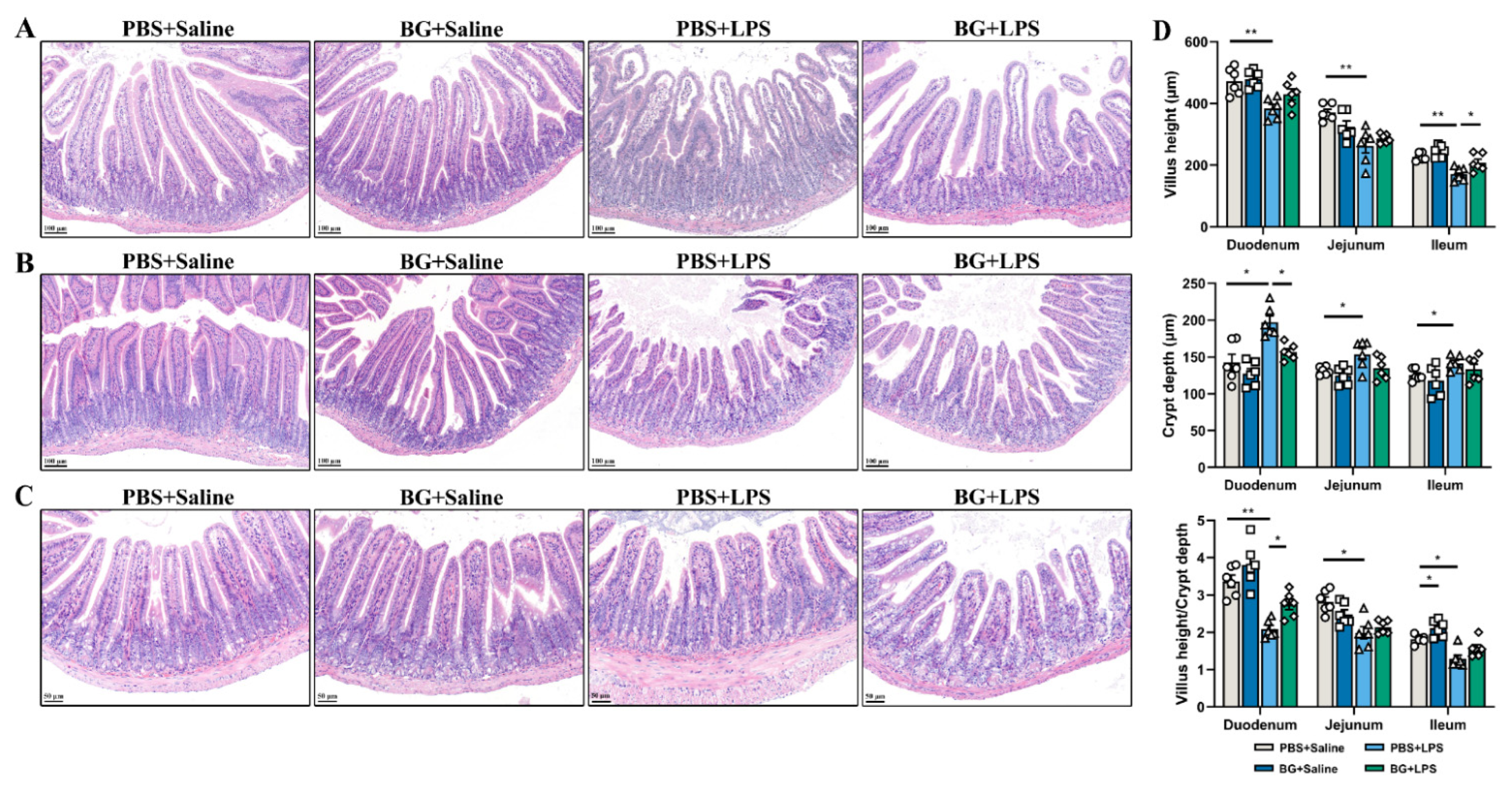
3.2. Intestinal Morphology of LPS-Stimulated Mice
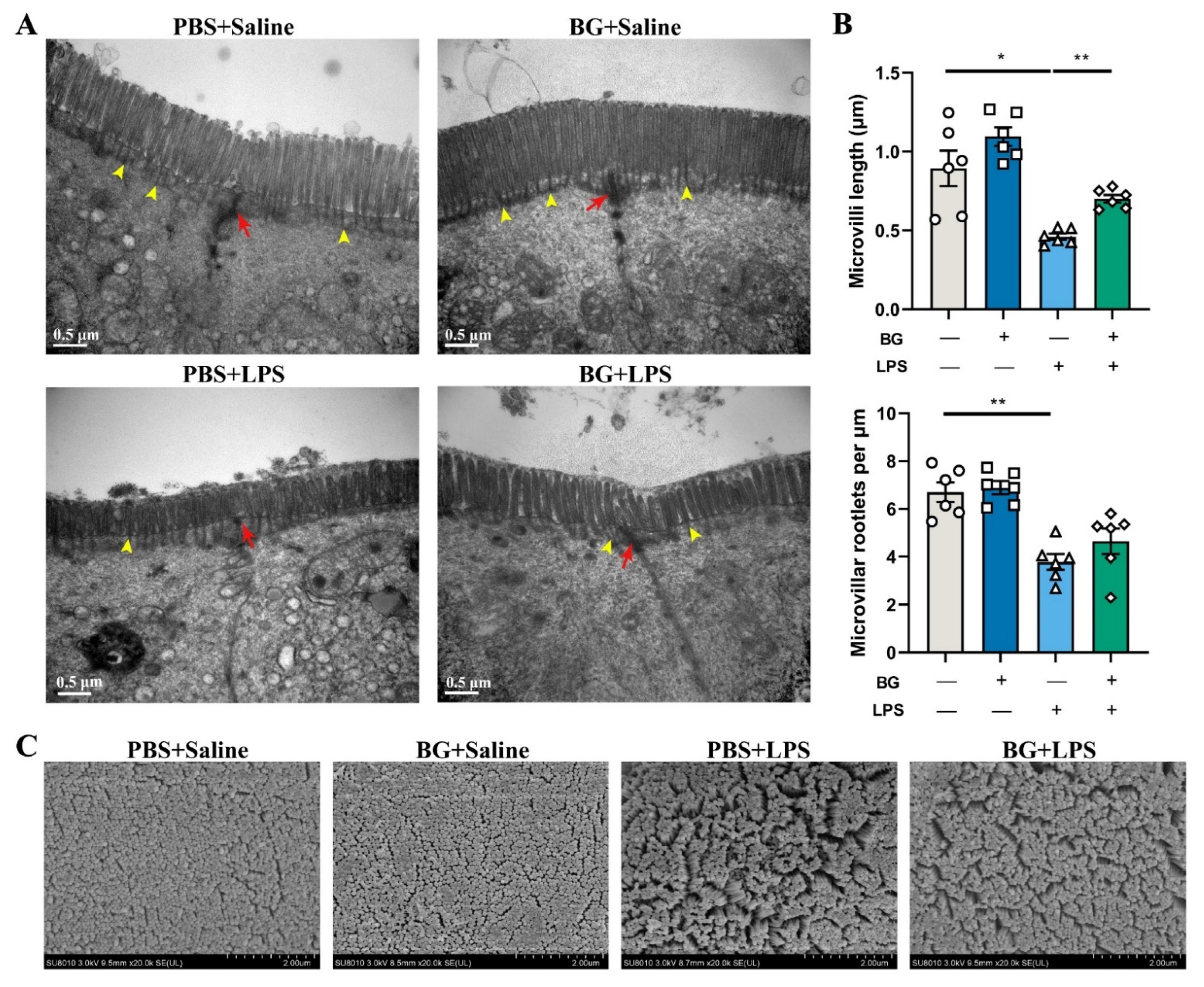
3.3. Ultrastructure of Jejunal and Ileum Epithelial Cells in Mice Induced by LPS Challenging
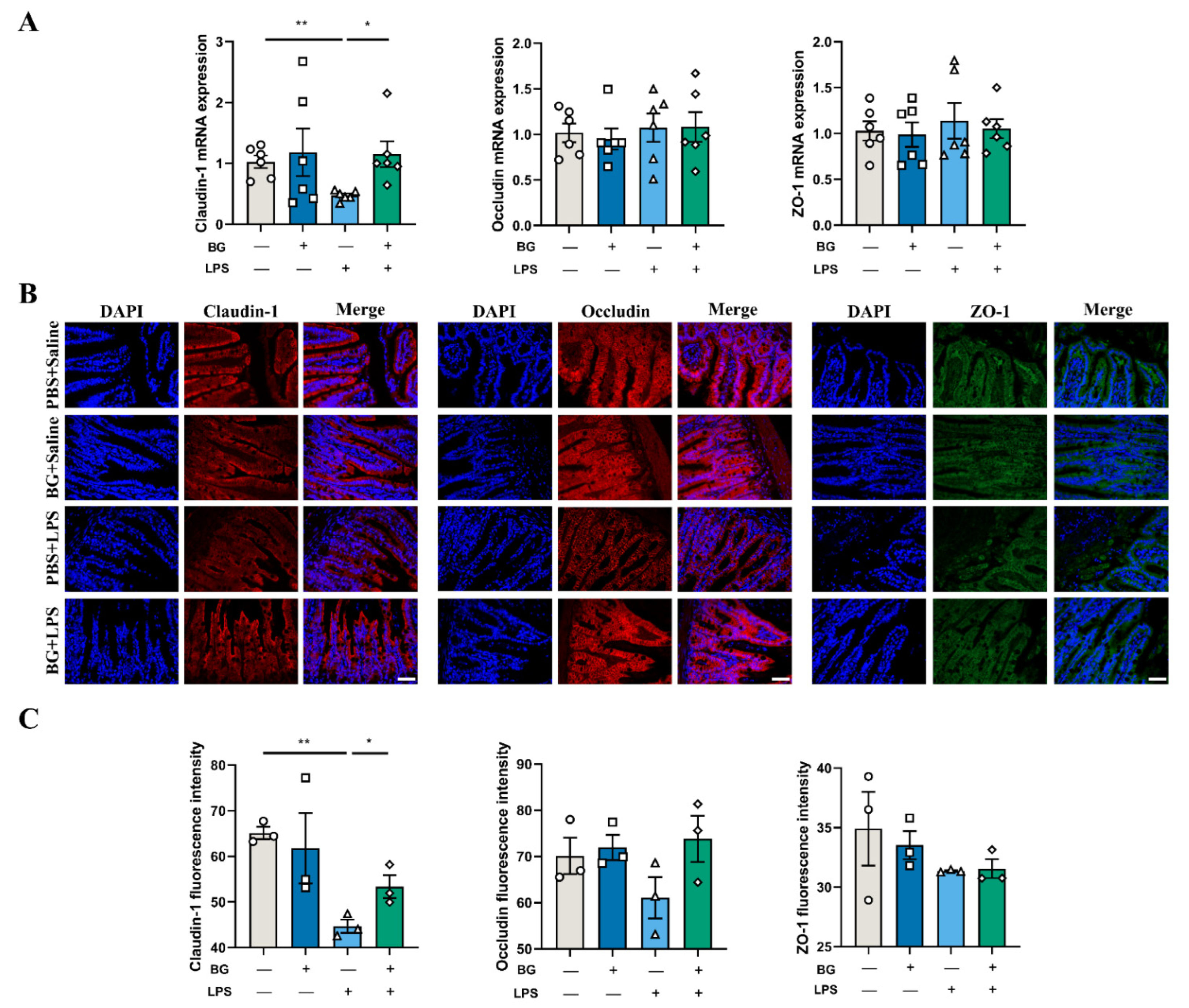
3.4. BG Alleviates LPS-Induced Ileal Intestinal Barrier Function Injuries
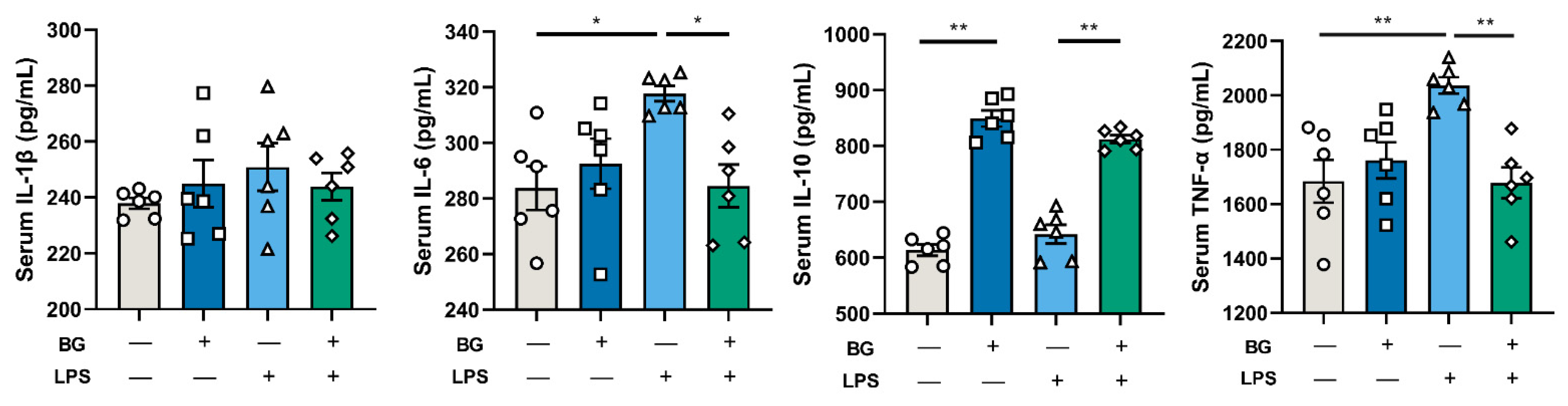
3.5. BG Reduce Ileal Inflammatory Response
3.6. Inflammatory Responses of LPS-Stimulated Mice
3.7. BG Alleviated Intestinal Inflammatory Response Induced by LPS-Stimulated via JNK Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.B.; Zhou, Y.L.; Fang, J.Y. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Delacour, D.; Salomon, J.; Robine, S.; Louvard, D. Plasticity of the brush border--the yin and yang of intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 161–174. [Google Scholar] [CrossRef]
- Yu, L.C.H. Commensal bacterial internalization by epithelial cells: An alternative portal for gut leakiness. Tissue Barriers 2015, 3, e1008895. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Guerrant, R.L.; Deboer, M.D.; Moore, S.R.; Scharf, R.J.; Lima, A.A.M. The impoverished gut—A triple burden of diarrhoea, stunting and chronic disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 220–229. [Google Scholar] [CrossRef]
- Ahmad, R.; Sorrell, M.F.; Batra, S.K.; Dhawan, P.; Singh, A.B. Gut permeability and mucosal inflammation: Bad, good or context dependent. Mucosal Immunol. 2017, 10, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, A.; Stein, J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur. J. Nutr. 2000, 39, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Galvez, J.; Rodríguez-Cabezas, M.E.; Zarzuelo, A. Effects of dietary fiber on inflammatory bowel disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef]
- Kovanda, L.L.; Hejna, M.; Liu, Y.H. Butyric acid and derivatives: In vitro effects on barrier integrity of porcine intestinal epithelial cells quantified by transepithelial electrical resistance. J. Anim. Sci. 2020, 98, 109. [Google Scholar] [CrossRef]
- Sampugna, J.; Quinn, J.G.; Pitas, R.E.; Carpenter, D.L.; Jensen, R.G. Digestion of butyrate glycerides by pancreatic lipase. Lipids 1967, 2, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Luo, X.L.; Wang, J.; Li, Y.; Feng, F.Q.; Zhao, M.J. Digestive behavior of unemulsified triglycerides with different chain lengths: In vitro dynamic and static simulated digestion models. LWT 2021, 149, 112006. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Prykhodko, O.; Hallenius, F.F.; Nyman, M. Monobutyrin reduces liver cholesterol and improves intestinal barrier function in rats fed high-fat diets. Nutrients 2019, 11, 308. [Google Scholar] [CrossRef]
- Dong, L.; Zhong, X.; He, J.T.; Zhang, L.L.; Bai, K.W.; Xu, W.; Wang, T.; Huang, X.X. Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin. Nutr. 2016, 35, 399–407. [Google Scholar] [CrossRef]
- Lee, S.; Knotts, T.A.; Goodson, M.L.; Barboza, M.; Wudeck, E.; England, G.; Raybould, H.E. Metabolic responses to butyrate supplementation in LF- and HF-fed mice are cohort-dependent and associated with changes in composition and function of the gut microbiota. Nutrients 2020, 12, 3524. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Prykhodko, O.; Hallenius, F.F.; Nyman, M. Effects of monobutyrin and tributyrin on liver lipid profile, caecal microbiota composition and SCFA in high-fat diet-fed rats. J. Nutr. Sci. 2017, 6, e51. [Google Scholar] [CrossRef]
- Namkung, H.; Yu, H.; Gong, J.; Leeson, S. Antimicrobial activity of butyrate glycerides toward salmonella typhimurium and clostridium perfringens. Poult. Sci. 2011, 90, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.; Lærke, H.; Hedemann, M.; Nielsen, T.; Ingerslev, A.; Gundelund Nielsen, D.; Theil, P.; Purup, S.; Hald, S.; Schioldan, A.; et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Snoeck, V.; Goddeeris, B.; Cox, E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 2005, 7, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.P.; Liu, L.J.; Jin, Y.Y.; Pei, X.; Sun, W.J.; Wang, M.Q. A potential prophylactic probiotic for inflammatory bowel disease: The overall investigation of clostridium tyrobutyricum ATCC25755 attenuates LPS-induced inflammation via regulating intestinal immune cells. Mol. Nutr. Food Res. 2021, 65, 2001213. [Google Scholar] [CrossRef]
- Hou, Y.Q.; Wang, L.; Yi, D.; Ding, B.Y.; Chen, X.; Wang, Q.J.; Zhu, H.L.; Liu, Y.L.; Yin, Y.L.; Gong, J.S.; et al. Dietary supplementation with tributyrin alleviates intestinal injury in piglets challenged with intrarectal administration of acetic acid. Br. J. Nutr. 2014, 111, 1748–1758. [Google Scholar] [CrossRef]
- Asadullah, K.; Sterry, W.; Volk, H.D. Interleukin-10 therapy—Review of a new approach. Pharmacol. Rev. 2003, 55, 241–269. [Google Scholar] [CrossRef]
- Fu, J.H.; Li, G.F.; Wu, X.M.; Zang, B. Sodium butyrate ameliorates intestinal injury and improves survival in a rat model of cecal ligation and puncture-induced sepsis. Inflammation 2019, 42, 1276–1286. [Google Scholar] [CrossRef]
- Chen, G.X.; Ran, X.; Li, B.; Li, Y.H.; He, D.W.; Huang, B.X.; Fu, S.P.; Liu, J.X.; Wang, W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a tnbs-induced inflammatory bowel disease mice model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, S.R.; Gan, P.Y. Cytokines: Names and numbers you should care about. Clin. J. Am. Soc. Nephrol. 2015, 10, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Wang, B.Y.; Wang, T.T.; Gao, L.; Yang, Z.J.; Wang, F.F.; Shang, H.W.; Hua, R.X.; Xu, J.D. Biological characteristics of IL-6 and related intestinal diseases. Int. J. Biol. Sci. 2021, 17, 204–219. [Google Scholar] [CrossRef]
- Paul, G.; Khare, V.; Gasche, C. Inflamed gut mucosa: Downstream of interleukin-10. Eur. J. Clin. Investig. 2011, 42, 95–109. [Google Scholar] [CrossRef]
- Bornhorst, G.M.; Paul Singh, R. Gastric digestion in vivo and in vitro: How the structural aspects of food influence the digestion process. Annu. Rev. Food Sci. Technol. 2014, 5, 111–132. [Google Scholar] [CrossRef]
- Schneeberger, K.; Roth, S.; Nieuwenhuis, E.; Middendorp, S. Intestinal epithelial cell polarity defects in disease: Lessons from microvillus inclusion disease. Dis. Models Mech. 2018, 11, dmm031088. [Google Scholar] [CrossRef]
- Crawley, S.W.; Mooseker, M.S.; Tyska, M.J. Shaping the intestinal brush border. J. Cell Biol. 2014, 207, 441–451. [Google Scholar] [CrossRef]
- Mooseker, M.S. Organization, Chemistry, and Assembly of the Cytoskeletal Apparatus of the Intestinal Brush Border. Annu. Rev. Cell Biol. 1985, 1, 209–241. [Google Scholar] [CrossRef]
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 59. [Google Scholar] [CrossRef]
- Vandussen, K.L.; Stojmirović, A.; Li, K.; Liu, T.C.; Kimes, P.K.; Muegge, B.D.; Simpson, K.F.; Ciorba, M.A.; Perrigoue, J.G.; Friedman, J.R. Abnormal small intestinal epithelial microvilli in patients with Crohn’s disease. Gastroenterology 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Le, S. Tight junctions on the move: Molecular mechanisms for epithelial barrier regulation. Ann. N. Y. Acad. Sci. 2012, 1258, 9–18. [Google Scholar] [CrossRef]
- Lechuga, S.; Ivanov, A.I. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim. Biophys. Acta 2017, 1864, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Talavera, M.M.; Kralik, N.; Jin, Y.; Chen, B.; Liu, Y.; Nelin, L.D. Mitogen-activated protein kinase phosphatase-1 prevents lipopolysaccharide-induced apoptosis in immature rat intestinal epithelial cells. Pediatr. Res. 2015, 78, 128–136. [Google Scholar] [CrossRef]
- Osaki, L.; Gama, P. Mapks and signal transduction in the control of gastrointestinal epithelial cell proliferation and differentiation. Int. J. Mol. Sci. 2013, 14, 10143–10161. [Google Scholar] [CrossRef]



| Gene | Sequences | Gen Bank Accession Number of mRNAs |
|---|---|---|
| IL-1β | F: TCGCAGCAGCACATCAACAGAG R: AGGTCCACGGGAAAGCACAGG | NM_008361 |
| IL-6 | F: CTGCAAGAGACTTCCATCCAG R: AGTGGTATAGCAGGTCTGTTGG | NM_031168 |
| IL-10 | F: TTCTTTCAAACAAAGGACCAGC R: GCAACCCAAGTAACCCTTAAAG | NM_010548 |
| TNF-α | F: ATGTCTCAGCCTCTTCTCATTC R: GCTTGTCACTCGAATTTTGAGA | NM_001278601 |
| TLR4 | F: GCCTTTCAGGGAATTAAGCTCC R: GATCAACCGATGGACGTGTAAA | NM_021297 |
| Myd88 | F: ATCGCTGTTCTTGAACCCTCG R: CTCACGGTCTAACAAGGCCAG | NM_010851 |
| ERK1 | F: ACCACATTCTAGGTATCTTGGGT R: AGTTTCGGGCCTTCATGTTAAT | NM_011952 |
| ERK2 | F: TTGCTTTCTCTCCCGCACAAA R: AGAGCCTGTTCAACTTCAATCC | NM_001038663 |
| P38 | F: GAGAAGATGCTCGTTTTGGACT R: GGACTGGTCATAAGGGTCAGC | NM_011951 |
| JNK | F: AGCAGAAGCAAACGTGACAAC R: GCTGCACACACTATTCCTTGAG | NM_016700 |
| Claudin-1 | F: GGGGACAACATCGTGACCG R: AGGAGTCGAAGACTTTGCACT | NM_016674 |
| Occludin | F: TTGAAAGTCCACCTCCTTACAGA R: CCGGATAAAAAGAGTACGCTGG | NM_008756 |
| ZO-1 | F: GCCGCTAAGAGCACAGCAA R: TCCCCACTCTGAAAATGAGGA | NM_009386 |
| β-actin | F: TATGCTCTCCCTCACGCCATCC R: GTCACGCACGATTTCCCTCTCAG | NM_007393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chen, H.; Lin, Y.; Wang, G.; Luo, Y.; Li, X.; Wang, M.; Huai, M.; Li, L.; Barri, A. Butyrate Glycerides Protect against Intestinal Inflammation and Barrier Dysfunction in Mice. Nutrients 2022, 14, 3991. https://doi.org/10.3390/nu14193991
Wang H, Chen H, Lin Y, Wang G, Luo Y, Li X, Wang M, Huai M, Li L, Barri A. Butyrate Glycerides Protect against Intestinal Inflammation and Barrier Dysfunction in Mice. Nutrients. 2022; 14(19):3991. https://doi.org/10.3390/nu14193991
Chicago/Turabian StyleWang, Haidong, Haohan Chen, Yueying Lin, Geng Wang, Yanqiu Luo, Xinyu Li, Minqi Wang, Mingyan Huai, Lily Li, and Adriana Barri. 2022. "Butyrate Glycerides Protect against Intestinal Inflammation and Barrier Dysfunction in Mice" Nutrients 14, no. 19: 3991. https://doi.org/10.3390/nu14193991
APA StyleWang, H., Chen, H., Lin, Y., Wang, G., Luo, Y., Li, X., Wang, M., Huai, M., Li, L., & Barri, A. (2022). Butyrate Glycerides Protect against Intestinal Inflammation and Barrier Dysfunction in Mice. Nutrients, 14(19), 3991. https://doi.org/10.3390/nu14193991





