The Link between Food Environment and Colorectal Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question Formulation
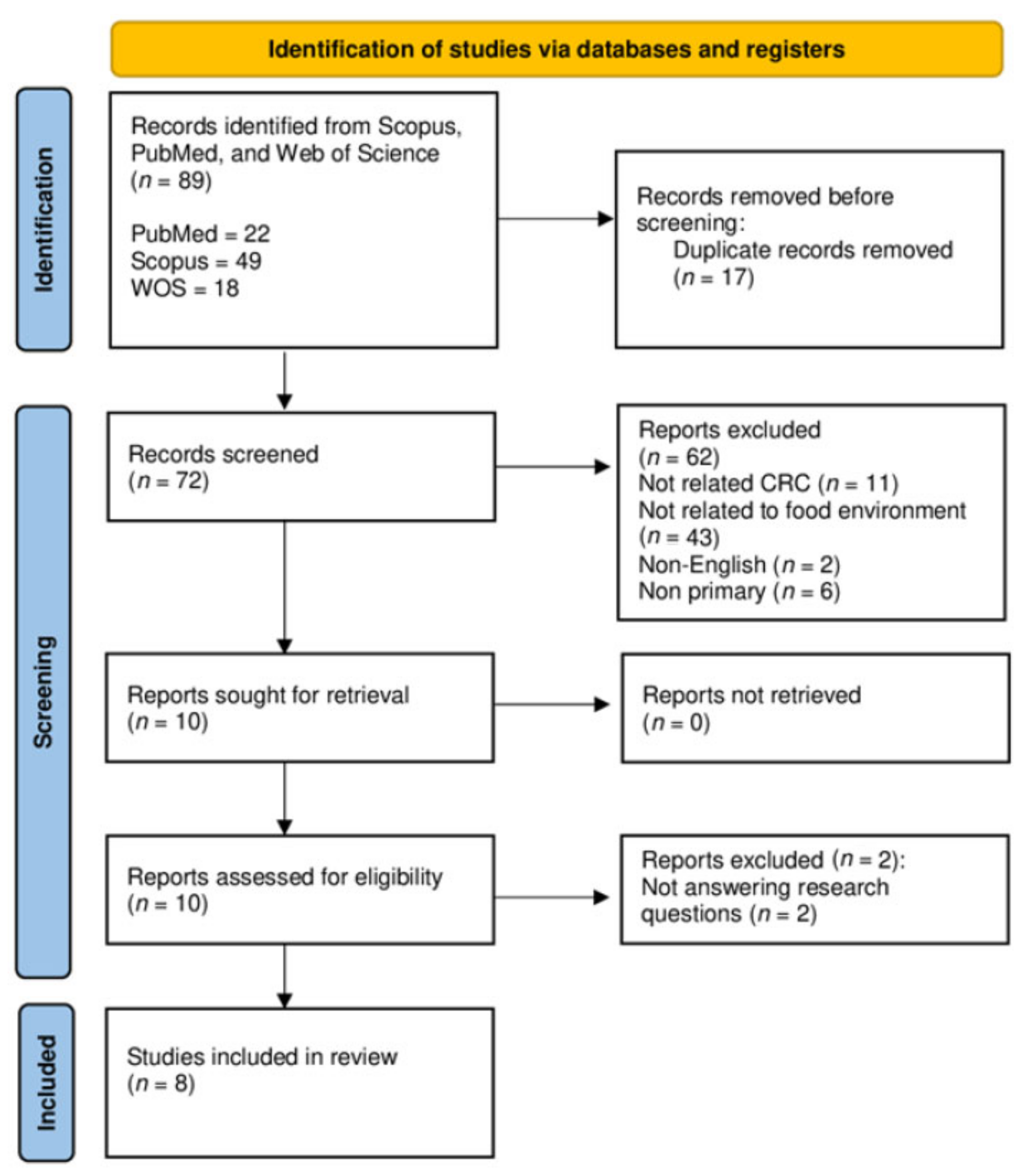
2.2. Identification
2.3. Screening
2.4. Eligibility
2.5. Data Extraction
2.6. Data Analysis
2.7. Quality Appraisal
3. Results
3.1. Characteristics of the Included Studies
3.2. FE Attributes
3.2.1. FE and CRC Incidence
3.2.2. FE and CRC Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC WHO Colorectal Cancer Factsheet. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 4 January 2021).
- Herforth, A.; Ahmed, S. The Food Environment, Its Effects on Dietary Consumption, and Potential for Measurement within Agriculture-Nutrition Interventions. Food Secur. 2015, 7, 505–520. [Google Scholar] [CrossRef]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global Trends in Colorectal Cancer Mortality: Projections to the Year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2021, 20, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-Analyses of Colorectal Cancer Risk Factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef]
- Hoang, T.; Kim, H.; Kim, J. Dietary Intake in Association with All-Cause Mortality and Colorectal Cancer Mortality among Colorectal Cancer Survivors: A Systematic Review and Meta-Analysis of Prospective Studies. Cancers 2020, 12, 3391. [Google Scholar] [CrossRef]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349. [Google Scholar] [CrossRef]
- HLPE. Nutrition and Food Systems. A Report by the High Panel of Experts on Food Security and Nutrition of the Committee on World Food Security; HLPE: Rome, Italy, 2017; Volume 44. [Google Scholar]
- Schüle, S.A.; Bolte, G. Interactive and Independent Associations between the Socioeconomic and Objective Built Environment on the Neighbourhood Level and Individual Health: A Systematic Review of Multilevel Studies. PLoS ONE 2015, 10, e0123456. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical Activity, Sedentary Behaviour, Diet, and Cancer: An Update and Emerging New Evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Nam, S.; Choi, Y.J.; Kim, D.W.; Park, E.C.; Kang, J.G. Risk Factors for Colorectal Cancer in Korea: A Population-Based Retrospective Cohort Study. Ann. Coloproctol. 2019, 35, 347–356. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk ofColorectal Cancer. Gastroenterology 2020, 158, 322. [Google Scholar] [CrossRef]
- Nawawi, K.N.M.; Mokhtar, N.M.; Wong, Z.; Azman, Z.A.M.; Chew, D.C.H.; Rehir, R.; Leong, J.; Ismail, F.; Rose, I.M.; Yaacob, Y.; et al. Incidence and Clinicopathological Features of Colorectal Cancer among Multi-Ethnic Patients in Kuala Lumpur, Malaysia: A Hospital-Based Retrospective Analysis over Two Decades. PeerJ 2021, 9, e12425. [Google Scholar] [CrossRef]
- Nawi, A.M.; Chin, S.F.; Mazlan, L.; Jamal, R. Delineating Colorectal Cancer Distribution, Interaction, and Risk Prediction by Environmental Risk Factors and Serum Trace Elements. Sci. Rep. 2020, 10, 18670. [Google Scholar] [CrossRef]
- Kearney, J. Food Consumption Trends and Drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793. [Google Scholar] [CrossRef]
- Munn, Z.; MClinSc, S.M.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological Guidance for Systematic Reviews of Observational Epidemiological Studies Reporting Prevalence and Cumulative Incidence Data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Dufault, B.; Klar, N. The Quality of Modern Cross-Sectional Ecologic Studies: A Bibliometric Review. Am. J. Epidemiol. 2011, 174, 1101–1107. [Google Scholar] [CrossRef]
- Betran, A.P.; Torloni, M.R.; Zhang, J.; Ye, J.; Mikolajczyk, R.; Deneux-Tharaux, C.; Oladapo, O.T.; Souza, J.P.; Tunçalp, Ö.; Vogel, J.P.; et al. What Is the Optimal Rate of Caesarean Section at Population Level? A Systematic Review of Ecologic Studies. Reprod Health 2015, 12, 57. [Google Scholar] [CrossRef]
- Aglago, E.K.; Bray, F.; Zotor, F.; Slimani, N.; Chajès, V.; Huybrechts, I.; Ferrari, P.; Gunter, M.J. Temporal Trends in Food Group Availability and Cancer Incidence in Africa: An Ecological Analysis. Public Health Nutr. 2019, 22, 2569–2580. [Google Scholar] [CrossRef]
- Shvetsov, Y.B.; Shariff-Marco, S.; Yang, J.; Conroy, S.M.; Canchola, A.J.; Albright, C.L.; Park, S.Y.; Monroe, K.R.; Le Marchand, L.; Gomez, S.L.; et al. Association of Change in the Neighborhood Obesogenic Environment with Colorectal Cancer Risk: The Multiethnic Cohort Study. SSM Popul. Health 2020, 10, 100532. [Google Scholar] [CrossRef]
- Besson, H.; Paccaud, F.; Marques-Vidal, P. Ecologic Correlations of Selected Food Groups with Disease Incidence and Mortality in Switzerland. J. Epidemiol. 2013, 23, 466–473. [Google Scholar] [CrossRef]
- Buamden, S. Association between Food Availability and Mortality Due to Colorectal Cancer in the Americas. Salud Colect 2018, 14, 579–595. [Google Scholar] [CrossRef]
- Fong, A.J.; Lafaro, K.; Ituarte, P.H.G.; Fong, Y. Association of Living in Urban Food Deserts with Mortality from Breast and Colorectal Cancer. Ann. Surg. Oncol. 2021, 28, 1311–1319. [Google Scholar] [CrossRef]
- Mo, J.; Luo, J.; Hendryx, M. Food Environment and Colorectal Cancer Incidence and Mortality Rates. J. Hunger Environ. Nutr. 2022, 17, 397–408. [Google Scholar] [CrossRef]
- Canchola, A.J.; Shariff-Marco, S.; Yang, J.; Albright, C.; Hertz, A.; Park, S.Y.; Shvetsov, Y.B.; Monroe, K.R.; Le Marchand, L.; Gomez, S.L.; et al. Association between the Neighborhood Obesogenic Environment and Colorectal Cancer Risk in the Multiethnic Cohort. Cancer Epidemiol. 2017, 50, 99–106. [Google Scholar] [CrossRef]
- Gibson, D.C.; Prochaska, J.D.; Yu, X.; Kaul, S. An Examination Between Census Tract Unhealthy Food Availability and Colorectal Cancer Incidence. Cancer Epidemiol. 2020, 67, 101761. [Google Scholar] [CrossRef]
- Downs, S.M.; Ahmed, S.; Fanzo, J.; Herforth, A. Food Environment Typology: Advancing an Expanded Definition, Framework, and Methodological Approach for Improved Characterization of Wild, Cultivated, and Built Food Environments toward Sustainable Diets. Foods 2020, 9, 532. [Google Scholar] [CrossRef]
- McKinnon, R.A.; Reedy, J.; Morrissette, M.A.; Lytle, L.A.; Yaroch, A.L. Measures of the Food Environment. A Compilation of the Literature, 1990–2007. Am. J. Prev. Med. 2009, 36, S124–S133. [Google Scholar] [CrossRef]
- Burgoine, T.; Sarkar, C.; Webster, C.J.; Monsivais, P. Examining the Interaction of Fast-Food Outlet Exposure and Income on Diet and Obesity: Evidence from 51,361 UK Biobank Participants. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 71. [Google Scholar] [CrossRef]
- Haynes-Maslow, L.; Leone, L.A. Examining the Relationship between the Food Environment and Adult Diabetes Prevalence by County Economic and Racial Composition: An Ecological Study. BMC Public Health 2017, 17, 648. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; Jackson, D.B. Food Insecurity, Food Deserts, and Waist-to-Height Ratio: Variation by Sex and Race/Ethnicity. J. Community Health 2019, 44, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Pineda, E.; Brunner, E.J.; Llewellyn, C.H.; Mindell, J.S. The Retail Food Environment and Its Association with Body Mass Index in Mexico. Int. J. Obes. 2021, 45, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Kelli, H.M.; Kim, J.H.; Tahhan, A.S.; Liu, C.; Ko, Y.A.; Hammadah, M.; Sullivan, S.; Sandesara, P.; Alkhoder, A.A.; Choudhary, F.K.; et al. Living in Food Deserts and Adverse Cardiovascular Outcomes in Patients With Cardiovascular Disease. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2019, 8, e010694. [Google Scholar] [CrossRef]
- Adams, A.T.; Ulrich, M.J.; Coleman, A. Food Deserts. J. Appl. Soc. Sci. 2010, 4, 58–62. [Google Scholar] [CrossRef]
- Nadadur, M.; Chen, C.; Duan, L.; Lee, M.; Chen, W.; Permanente, K. Food desert and its impact on mortality and hospitalization in heart failure patients. J. Am. Coll. Cardiol. 2019, 73, 861. [Google Scholar] [CrossRef]
- Sommer, I.; Griebler, U.; Mahlknecht, P.; Thaler, K.; Bouskill, K.; Gartlehner, G.; Mendis, S. Socioeconomic Inequalities in Non-Communicable Diseases and Their Risk Factors: An Overview of Systematic Reviews. BMC Public Health 2015, 15, 914. [Google Scholar] [CrossRef]
- Jin, H.; Lu, Y. Evaluating Consumer Nutrition Environment in Food Deserts and Food Swamps. Int. J. Environ. Res. Public Health 2021, 18, 2675. [Google Scholar] [CrossRef]
- Monsivais, P.; Thompson, C.; Astbury, C.C.; Penney, T.L. Environmental Approaches to Promote Healthy Eating: Is Ensuring Affordability and Availability Enough? BMJ 2021, 372, n549. [Google Scholar] [CrossRef]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean Diet and Colorectal Cancer: A Systematic Review. Nutrition 2017, 43–44, 83–88. [Google Scholar] [CrossRef]
- Romaguera, D.; Fernández-Barrés, S.; Gracia-Lavedán, E.; Vendrell, E.; Azpiri, M.; Ruiz-Moreno, E.; Martín, V.; Gómez-Acebo, I.; Obón, M.; Molinuevo, A.; et al. Consumption of Ultra-Processed Foods and Drinks and Colorectal, Breast, and Prostate Cancer. Clin. Nutr. 2021, 40, 1537–1545. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Bawadi, H.A.; Shehadah, I.; Bani-Hani, K.E.; Takruri, H.; Al-Jaberi, T.; Heath, D.D. Fast Foods, Sweets and Beverage Consumption and Risk of Colorectal Cancer: A Case-Control Study in Jordan. Asian Pac. J. Cancer Prev. 2018, 19, 261–269. [Google Scholar] [CrossRef]
- Chang, V.C.; Cotterchio, M.; De, P.; Tinmouth, J. Risk Factors for Early-Onset Colorectal Cancer: A Population-Based Case–Control Study in Ontario, Canada. Cancer Causes Control 2021, 32, 1063–1083. [Google Scholar] [CrossRef]
- Sasso, A.; Latella, G. Role of Heme Iron in the Association Between Red Meat Consumption and Colorectal Cancer. Nutr. Cancer 2018, 70, 1173–1183. [Google Scholar] [CrossRef]
- Singh, S.; Arcaroli, J.; Thompson, D.C.; Messersmith, W.; Vasiliou, V. Acetaldehyde and Retinaldehyde-Metabolizing Enzymes in Colon and Pancreatic Cancers. Adv. Exp. Med. Biol. 2015, 815, 281–294. [Google Scholar] [CrossRef]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-Analysis of 16 Studies of the Association of Alcohol with Colorectal Cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef]
- Rossi, M.; Anwar, M.J.; Usman, A.; Keshavarzian, A.; Bishehsari, F. Colorectal Cancer and Alcohol Consumption—Populations to Molecules. Cancers 2018, 10, 38. [Google Scholar] [CrossRef]
- Marley, A.R.; Nan, H. Epidemiology of Colorectal Cancer. Int. J. Mol. Epidemiol. Genet. 2016, 7, 105. [Google Scholar] [CrossRef]
- Kim, Y.I. Role of Folate in Colon Cancer Development and Progression. J. Nutr. 2003, 133, 3731S–3739S. [Google Scholar] [CrossRef]
- Ni Mhurchu, C.; Vandevijvere, S.; Waterlander, W.; Thornton, L.E.; Kelly, B.; Cameron, A.J.; Snowdon, W.; Swinburn, B. Monitoring the Availability of Healthy and Unhealthy Foods and Non-Alcoholic Beverages in Community and Consumer Retail Food Environments Globally. Obes. Rev. 2013, 14, 108–119. [Google Scholar] [CrossRef]
- Carethers, J.M. Racial and Ethnic Disparities in Colorectal Cancer Incidence and Mortality. Adv. Cancer Res. 2021, 151, 197. [Google Scholar] [CrossRef]
- Chang, W.Y.; Chiu, H.M. Beyond Colonoscopy: Physical Activity as a Viable Adjunct to Prevent Colorectal Cancer. Dig. Endosc. 2022. [Google Scholar] [CrossRef]
- Cho, M.Y.; Siegel, D.A.; Demb, J.; Richardson, L.C.; Gupta, S. Increasing Colorectal Cancer Incidence Before and After Age 50: Implications for Screening Initiation and Promotion of “On-Time” Screening. Dig. Dis. Sci. 2022, 67, 4086–4091. [Google Scholar] [CrossRef]
- Knudsen, M.D.; De Lange, T.; Botteri, E.; Nguyen, D.H.; Evensen, H.; Steen, C.B.; Hoff, G.; Bernklev, T.; Hjartåker, A.; Berstad, P. Favorable Lifestyle before Diagnosis Associated with Lower Risk of Screen-Detected Advanced Colorectal Neoplasia. World J. Gastroenterol. 2016, 22, 6276–6286. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.N.; Harris, R.B.; Oren, E.; Roe, D.J.; Lance, P.; Jacobs, E.T. Adherence to Nutrition and Physical Activity Cancer Prevention Guidelines and Development of Colorectal Adenoma. Nutrients 2018, 10, 1098. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Clarke, C.A.; Press, D.J.; Mannalithara, A.; Myer, P.A.; Cheng, I.; Gomez, S.L. Colorectal Cancer Incidence in Asian Populations in California: Effect of Nativity and Neighborhood-Level Factors. Am. J. Gastroenterol. 2014, 109, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Illari, P.; Russo, F. Causality in Cancer Research: A Journey through Models in Molecular Epidemiology and Their Philosophical Interpretation. Emerg. Themes Epidemiol. 2017, 14, 7. [Google Scholar] [CrossRef]
- Murphy, N.; Moreno, V.; Hughes, D.J.; Vodicka, L.; Vodicka, P.; Aglago, E.K.; Gunter, M.J.; Jenab, M. Lifestyle and Dietary Environmental Factors in Colorectal Cancer Susceptibility. Mol. Aspects Med. 2019, 69, 2–9. [Google Scholar] [CrossRef]
- Loke, Y.L.; Chew, M.T.; Ngeow, Y.F.; Lim, W.W.D.; Peh, S.C. Colon Carcinogenesis: The Interplay Between Diet and Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 603086. [Google Scholar] [CrossRef]
- Syed Soffian, S.S.; Mohammed Nawi, A.; Hod, R.; Ja’afar, M.H.; Isa, Z.M.; Chan, H.K.; Hassan, M.R.A. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutrients 2022, 14, 1555. [Google Scholar] [CrossRef] [PubMed]
- Puzzono, M.; Mannucci, A.; Grannò, S.; Zuppardo, R.A.; Galli, A.; Danese, S.; Cavestro, G.M. The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review. Cancers 2021, 13, 5933. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Jakubowski, C.D.; Fedewa, S.A.; Davis, A.; Azad, N.S. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology (ASCO): Alexandria, VA, USA, 2020; pp. e75–e88. [Google Scholar]
- You, Y.N.; Lee, L.D.; Deschner, B.W.; Shibata, D. Colorectal Cancer in the Adolescent and Young Adult Population. J. Oncol. Pract. 2020, 16, 19–27. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-Onset Colorectal Cancer: Initial Clues and Current Views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef]
- Zaurito, A.E.; Tschurtschenthaler, M. Microenvironmental Metabolites in the Intestine: Messengers between Health and Disease. Metabolites 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Gongora, V.M.; Matthes, K.L.; Castaño, P.R.; Linseisen, J.; Rohrmann, S. Dietary Heterocyclic Amine Intake and Colorectal Adenoma Risk: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2019, 28, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A.O. Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Cooked Meat Products: A Review. Polycycl. Aromat. Compd. 2020, 40, 1557–1567. [Google Scholar] [CrossRef]
- Hur, S.J.; Yoon, Y.; Jo, C.; Jeong, J.Y.; Lee, K.T. Effect of Dietary Red Meat on Colorectal Cancer Risk—A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1812–1824. [Google Scholar] [CrossRef]
- Le Marchand, L.; Kolonel, L.N. Cancer in Japanese migrants to Hawaii: Interaction between genes and environment. Rev. Epidemiol. Sante Publique 1992, 40, 425–430. [Google Scholar]
- Le Marchand, L.; Wilkens, L.R.; Hankin, J.H.; Kolonel, L.N.; Lyu, L.C. A Case-Control Study of Diet and Colorectal Cancer in a Multiethnic Population in Hawaii (United States): Lipids and Foods of Animal Origin. Cancer Causes Control 1997, 8, 637–648. [Google Scholar] [CrossRef]
- Maskarinec, G.; Noh, J. The Effect of Migration on Cancer Incidence among Japanese in Hawaii. Ethn. Dis. 2004, 14, 431–439. [Google Scholar]
- Stemmermann, G.N.; Nomura, A.M.Y.; Chyou, P.-H.; Kato, I.; Tetsuo, T. Cancer Incidence in Hawaiian Japanese: Migrants from Okinawa Compared with Those from Other Prefectures. Jpn. J. Cancer Res. 1991, 82, 1366–1370. [Google Scholar] [CrossRef]
- Ton, M.; Widener, M.J.; James, P.; Vopham, T. Food Environments and Hepatocellular Carcinoma Incidence. Int. J. Environ. Res. Public Health 2021, 18, 5740. [Google Scholar] [CrossRef]
- Ly, C.; Essman, M.; Zimmer, C.; Ng, S.W. Developing an Index to Estimate the Association between the Food Environment and CVD Mortality Rates. Health Place 2020, 66, 102469. [Google Scholar] [CrossRef]
- Remington, P.L.; Catlin, B.B.; Gennuso, K.P. The County Health Rankings: Rationale and Methods. Popul. Health Metr. 2015, 13, 11. [Google Scholar] [CrossRef]
- Richardson, A.S.; Ghosh-Dastidar, M.; Beckman, R.; Flórez, K.R.; DeSantis, A.; Collins, R.L.; Dubowitz, T. Can the Introduction of a Full-Service Supermarket in a Food Desert Improve Residents’ Economic Status and Health? Ann. Epidemiol. 2017, 27, 771. [Google Scholar] [CrossRef]
- Løvhaug, A.L.; Granheim, S.I.; Djojosoeparto, S.K.; Harrington, J.M.; Kamphuis, C.B.M.; Poelman, M.P.; Roos, G.; Sawyer, A.; Stronks, K.; Torheim, L.E.; et al. The Potential of Food Environment Policies to Reduce Socioeconomic Inequalities in Diets and to Improve Healthy Diets among Lower Socioeconomic Groups: An Umbrella Review. BMC Public Health 2022, 22, 433. [Google Scholar] [CrossRef]
- Custodio, M.C.; Ynion, J.; Samaddar, A.; Cuevas, R.P.; Mohanty, S.K.; Ray (Chakravarti), A.; Demont, M. Unraveling Heterogeneity of Consumers’ Food Choice: Implications for Nutrition Interventions in Eastern India. Glob. Food Secur. 2021, 28, 100497. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Pietrzyk, Ł. Food Properties and Dietary Habits in Colorectal Cancer Prevention and Development. Int. J. Food Prop. 2017, 20, 2323–2343. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Sedgwick, P. Ecological Studies: Advantages and Disadvantages. BMJ Clin. Res. 2014, 348, g2979. [Google Scholar] [CrossRef]
| Search Engine | Search Area | Search Date | Format | Search |
|---|---|---|---|---|
| WOS | Topic | 19 April 2022 | P | Colorectal cancer OR colorectal neoplasms OR colorectal carcinoma OR colorectal tumo* OR cancer colorectal OR bowel cancer OR large intestine cancer AND |
| E | Food environment OR eat environment OR food dessert OR food swamp OR café OR canteen OR restaurant OR takeaway OR food entry point OR food access OR food production OR food availab* OR food access* OR food obtain OR food purchase OR food prepare* OR food handy OR food afford OR Food convenience OR food retailer AND | |||
| Relation* OR Link OR connection OR association OR correlate OR tie AND | ||||
| O | Risk OR possibility OR probability OR frequency OR predictor OR Incidence OR occurrence rate OR frequency OR mortality OR death | |||
| SCOPUS | TITLE-ABS-KEY | 19 April 2022 | P | TITLE-ABS-KEY (“colorectal cancer” OR “colorectal neoplasms” OR “colorectal carcinoma” OR “colorectal tumo*” OR “cancer off colorectal” OR “bowel cancer” OR “large intestine cancer”) AND |
| E | TITLE-ABS-KEY (“food environment” OR “eat environment” OR “food dessert” OR “food swamp” OR cafe OR canteen OR restaurant OR takeaway OR “food entry point” OR “food point” OR “food access*” OR “food production” OR “food availab*” OR “food access*” OR “food obtain” OR “food purchase” OR “food prepare*” OR “food handy” OR “food afford” OR “food convenience” OR “food retailer”) AND (TITLE-ABS-KEY (relation* OR link OR connection OR association OR correlate OR tie) AND | |||
| O | TITLE-ABS-KEY (risk OR possibility OR probability OR frequency OR predictor OR incidence OR occurrence OR rate OR prevalence OR mortality or death) | |||
| Pubmed | Title/abstract | 19 April 2022 | P | Colorectal cancer OR colorectal neoplasms OR colorectal carcinoma OR colorectal tumo* OR cancer colorectal OR bowel cancer OR large intestine cancer AND |
| E | Food environment OR eat environment OR food dessert OR food swamp OR café OR canteen OR restaurant OR takeaway OR food entry point OR food access OR food production OR food availab* OR food access* OR food obtain OR food purchase OR food prepare* OR food handy OR food afford OR Food convenience OR food retailer AND | |||
| Relation* OR Link OR connection OR association OR correlate OR tie AND | ||||
| O | risk OR possibility OR probability OR frequency OR predictor OR incidence OR occurrence OR rate OR prevalence OR mortality OR death |
| Authors | Selection | Comparability | Outcome | Total Quality Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representative Eness of Exposed Cohort | Selection of Nonexposed cohort | Ascertainment of Exposure | Demonstration that Outcome of Interest Was Not Present at Start of Study | Adjust for the Most Important Risk Factors | Adjust for other Risk Factors | Assessment of Outcome | Follow-up Length Enough? | Loss to Follow-Up Rate | ||
| Shvetsov et al. [20] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Canchola et al. [21] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Evaluation Criterion | Categories | Points (Max = 21) | Study | |||||
|---|---|---|---|---|---|---|---|---|
| 1 1 | 2 2 | 3 3 | 4 4 | 5 5 | 6 6 | |||
| Study Design | ||||||||
| Design | Cross-sectional Longitudinal | 1 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sample size | <80% units ≥80% units | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Unbiased inclusion of units | No Yes | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Level of data aggregation | Other than below Regional, State National | 1 2 3 | 2 | 2 | 3 | 3 | 3 | 3 |
| Level of inference | Individual or unclear Ecologic | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Pre-specification of ecologic units | No Yes | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Outcomes of interest included | Some All | 1 2 | 1 | 1 | 1 | 2 | 2 | 2 |
| Source of data | Inadequate Adequate | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Statistical Methodology | ||||||||
| Analytic methodology | Spearman’s rank correlation, Linear regression model, Quadratic model, Exponential model, LOWESS, Fractional polynomial regression, Piecewise regression | 1 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Validity of regression | No Yes | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Use of covariates | None Socio-economic Socio-economic + clinical | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Proper adjustment for covariates | No Yes | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Quality Of Reporting | ||||||||
| Statement of study design | No Yes | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Justification of study design | No Yes | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Discussion of cross-level bias and limitations | No Yes | 0 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| TOTAL POINTS | 17 | 17 | 17 | 18 | 18 | 18 | ||
| Authors | Country | Study Design | Food Availability Data | Study Sample Population |
|---|---|---|---|---|
| Gibson et al. [22] | US | Ecological | 2005 Business Patterns Survey based on matching zip codes with the US Department of Housing and Urban Development zip code | Texas Cancer Registry (5215 census tracts) Individuals aged 40, residing in Texas, diagnosed with CRC (primary/malignant and/or invasive) |
| Canchola et al. [21] | US | Cohort | the Restaurant Environment Index (REI) and the Retail Food Environment Index (RFEI) from California Neighbourhoods Data System | Multi-ethnic Cohort Hawaii and California |
| Shvetsov et al. [20] | US | Cohort | California Neighbourhoods Data System (40,870 male and 54,602 female) | Multi-ethnic Cohort Lived in California |
| Fong et al. [23] | US | Ecological | USDA food desert data set with zip code level measures | Stage II/III CRC patients California Cancer Registry (CCR). |
| Besson et al. [24] | Switzerland | Ecological | Food availability data from food balance sheets produced by the FAO | Incidence rates from the Vaud Cancer Registry. |
| Aglago et al. [26] | Africa (sub Saharan countries) | Ecological | Food availability data from food balance sheets produced by the FAO | African Cancer Registries Network |
| Buamden [25] | US | Ecological | Food availability data from food balance sheets produced by the FAO | International Agency for Research on Cancer (IARC) and codes C18, C19, C20, and C21 of the International Classification of Diseases (ICD-10). |
| Mo et al. [15] | US | Ecological | US FEI from the 2020 County Health Rankings | Incidence: The State Cancer Profiles by CDC and NCI 2013–2017 Mortality: CDC Underlying Cause of Mortality data 2014–2018 |
| Authors (Year) | Attributes | Description | Interpretation |
|---|---|---|---|
| Gibson et al. [22] | Unhealthy food environment density (UFAD) | the number of all limited-service restaurants, businesses, and employment within each zip code | UFAD was divided into Quartiles1 to 4 Quartile 1 indicates the lowest unhealthy food availability Quartile 4 indicates the highest unhealthy food availability. |
| Canchola et al. [21] | Restaurant Environment Index | the ratio of the number of fast-food restaurants to other restaurants | - |
| Retail Food Environment Index | the ratio of the number of convenience stores, liquor stores, and fast-food restaurants to supermarkets and farmers’ markets | - | |
| Shvetsov et al. [20] | Fast food availability dynamic | Number of fast-food restaurants within blocks group | Up = increased number Down = decreased Same = similar |
| Supermarket availability dynamic | Number of fast-food restaurants within blocks group | Up = increased number, Down = decreased Same = similar | |
| Fong et al. [23] | Food desert | Areas that lack access to affordable that make up a full and healthy diet (fruits, vegetables, whole grains and low-fat milk) | Low access means: at least 500 people AND/OR at least 1/3rd of the census tract lives >1 mile in urban communities OR >10 miles in rural communities from a grocery store |
| Besson et al. [24] | Individual daily food availability | Estimation of the individual daily food availability of each food commodity (the total energy of animal products, vegetable products, cereals, sugars, vegetable oils, alcohol, meat, milk, fish, fruits, vegetable, fats) was made by integrating the yearly supply of domestic production+ imports + exports + stocks + non-food use, then divided by the average population and the number of days in the year to get daily availability. The values then converted to the corresponding calorie of each food commodity (kcal/person/day) | Increased total calorie of food means increased food availability |
| Aglago et al. [26] | Food and energy availability | Estimations of major foods and food groups available for human consumption, Total energy, proteins, fats, and carbohydrates values drawn from these food groups Data available in the food balance sheets were presented either as kilograms per capita per year or converted to kilocalories per capita per day to recover the energy contribution of the food considered. | A higher value of food and energy (in kilograms or kilocalories) means higher food availability |
| Buamden [25] | Food availability | Food availability represents the amount of food available per capita and provides a general picture of the populations’ diets. It does not account for food access or actual consumption | - |
| Mo et al. [15] | Food access | The percentage of the population that is low-income 1 and has low access 2 to a grocery store | A higher index means better food accessThe index ranges from 0 (worst) to 10 (best) |
| Food security | The percentage of people without a reliable food source in the past year. | A higher index means better food security The index ranges from 0 (worst) to 10 (best) |
| Authors | FE | Comparator | CRC Outcome | Conclusion | |||
|---|---|---|---|---|---|---|---|
| Incidence | 95% CI, p-Value | Mortality | 95% CI, p-Value | ||||
| Gibson et al. [22] | Quartile 2 UFA | Quartile 1 | Quartile 2 1.03 1 | 1.00–1.05 | - | - | No significant differences in colorectal cancer incidence between the lowest unhealthy food availability and quartile 2,3,4 |
| Quartile 3 UFA | Quartile 1 | Quartile 3 1.02 1 | 1.00, 1.05 | - | - | ||
| Quartile 4 UFA (Highest) | Quartile 1 | Quartile 4 1.02 1 | 0.99, 1.05 | - | - | ||
| Canchola et al. [21] | High REI Male Female | Low REI | 0.85 2 1.29 2 | 0.54, 1.33 0.84, 1.99 | - | - | No significant associations between neighbourhood obesogenic attributes and colorectal cancer risk. |
| High RFEI Male Female | Low RFEI | 1.11 2 0.90 2 | 0.91, 1.36 0.74–1.10 | - | - | ||
| Shvetsov et al. [20] | Upward change Fast food restaurants | No change | Men = 1.19 2 Women = 0.96 2 | 0.97, 1.45 (0.79–1.17 | - | - | Upward change in fast food and supermarket was not statistically significantly associated with CRC risk among the male and female. |
| Upward change in supermarket | No change | Men = 0.95 2 Women = 1.12 2 | 0.80, 1.13 0.96–1.30 | - | - | ||
| Fong et al. [23] | Living in Food desert Yes | No | - | - | UV HR = 1.12, MV HR: 1.18 | 1.05, 1.19 p = 0.001 1.05, 1.19 p = 0.001 | Food desert residence was associated with higher 5-year mortality. |
| Besson et al. [24] | Food availability Coefficients exceeding the cut-off of ± 0.70 are considered meaningful | All types of foods results are below than 0.7 | - | - | - | Colorectal cancer incidence was not associated with any food availability. Associations were found only for polyps with fish availability and decreased availability of animal fats | |
| Aglago et al. [26] | Food availability coefficients exceeding the cut-off of ± 0.50 are correlated
| Coefficient | - | - | - | Colorectal cancer incidence in men and women significantly positively correlated with red meat, meat, animal fats availability, and energy from animal sources | |
| Meat | |||||||
| Men | T0 = 0.72 T5 = 0.60 T20 = 0.64 | - | - | - | |||
| Women | T5 = 0.54 T20 = 0.54 | - | - | - | |||
| Red meat | |||||||
| Men | T20 = 0.53 | - | - | - | |||
| Women | T0 = 0.63 T5 = 0.58 T20 = 0.58 | - | - | - | |||
| Animal fats | |||||||
| Women | T10 = 0.67 T15 = 0.70 T20 = 0.66 | - | - | - | |||
| Energy from animal sources | T20 = 0.52 | - | - | - | |||
| Buamden [25] | Food availability coefficients from 0.50 to 0.75 show moderate correlation and greater than 0.75 show a very good or excellent correlation. | Coefficient | Strong relationships were found between colorectal cancer mortality rate and the availability of animal fat, red meat, alcoholic beverages, and calories. The availability of fruits and vegetables have no protective effect on the colorectal cancer mortality | ||||
| Red meat | - | - | 0.59 | - | |||
| Ethanol | - | - | 0.61 | - | |||
| Total fat | - | - | 0.47 | - | |||
| Animal fat | - | - | 0.60 | - | |||
| Calorie | - | - | 0.56 | - | |||
| Mo et al. 2022 [15] | Healthy FEI | 41.3 per 100,000 | -, p <0.004 | 14.9 per 100,000 | -, p < 0.01 | Healthy FEI scores (less food insecurity and better healthy food access were associated with lower colorectal cancer incidence and mortality A poorer food environment was significantly associated with higher colorectal cancer incidence and mortality | |
| Unhealthy FEI | 44.5 per 100,000 | 17.1 per 100,000 | |||||
| Food availability coefficients | Coefficient | p value | Coefficient | p value | |||
| FEI | −0.681 | 0.004 | −0.826 | <0.01 | |||
| Food insecure | −0.12 | 0.10 | 0.108 | 0.004 | |||
| Limited access to healthy food | 0.191 | 0.0001 | 0.096 | <0.01 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masdor, N.A.; Mohammed Nawi, A.; Hod, R.; Wong, Z.; Makpol, S.; Chin, S.-F. The Link between Food Environment and Colorectal Cancer: A Systematic Review. Nutrients 2022, 14, 3954. https://doi.org/10.3390/nu14193954
Masdor NA, Mohammed Nawi A, Hod R, Wong Z, Makpol S, Chin S-F. The Link between Food Environment and Colorectal Cancer: A Systematic Review. Nutrients. 2022; 14(19):3954. https://doi.org/10.3390/nu14193954
Chicago/Turabian StyleMasdor, Noor Azreen, Azmawati Mohammed Nawi, Rozita Hod, Zhiqin Wong, Suzana Makpol, and Siok-Fong Chin. 2022. "The Link between Food Environment and Colorectal Cancer: A Systematic Review" Nutrients 14, no. 19: 3954. https://doi.org/10.3390/nu14193954
APA StyleMasdor, N. A., Mohammed Nawi, A., Hod, R., Wong, Z., Makpol, S., & Chin, S.-F. (2022). The Link between Food Environment and Colorectal Cancer: A Systematic Review. Nutrients, 14(19), 3954. https://doi.org/10.3390/nu14193954







