Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet–Waxy Corn from Three Different Regions of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Materials and Sample Preparation
2.3. Quantification of Protein
2.4. Quantification of Amino Acid Composition
2.5. Amino Acid Nutritional Evaluation
2.5.1. Amino Acid Composition Analysis
2.5.2. Chemical Evaluation
2.5.3. Amino Acid Ratio Coefficient Method
2.5.4. Matching Degree
2.6. Factor Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Amino Acid Profiles of Fresh Corn from the Three Provinces of China
3.2. Chemical Evaluation
3.3. Evaluation of Amino Acid Pattern Spectrum
3.3.1. FAO/WHO Standard Pattern Spectrum
3.3.2. IOM Model
3.3.3. Egg Pattern Spectrum
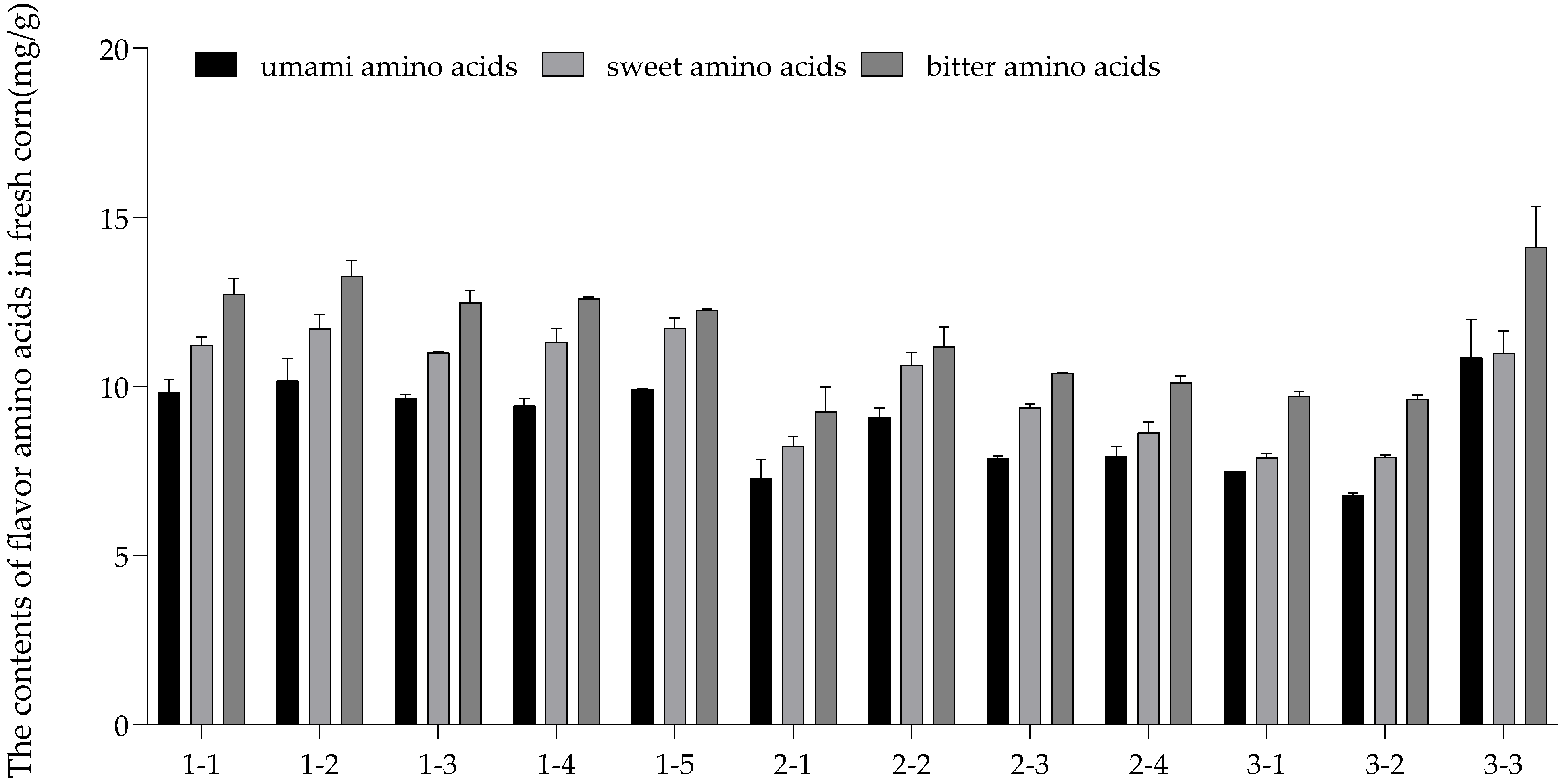
3.4. Flavor Evaluation
3.5. Evaluation of Amino Acid Matching Degree
3.6. Factor Analysis
3.6.1. Correlation Analysis
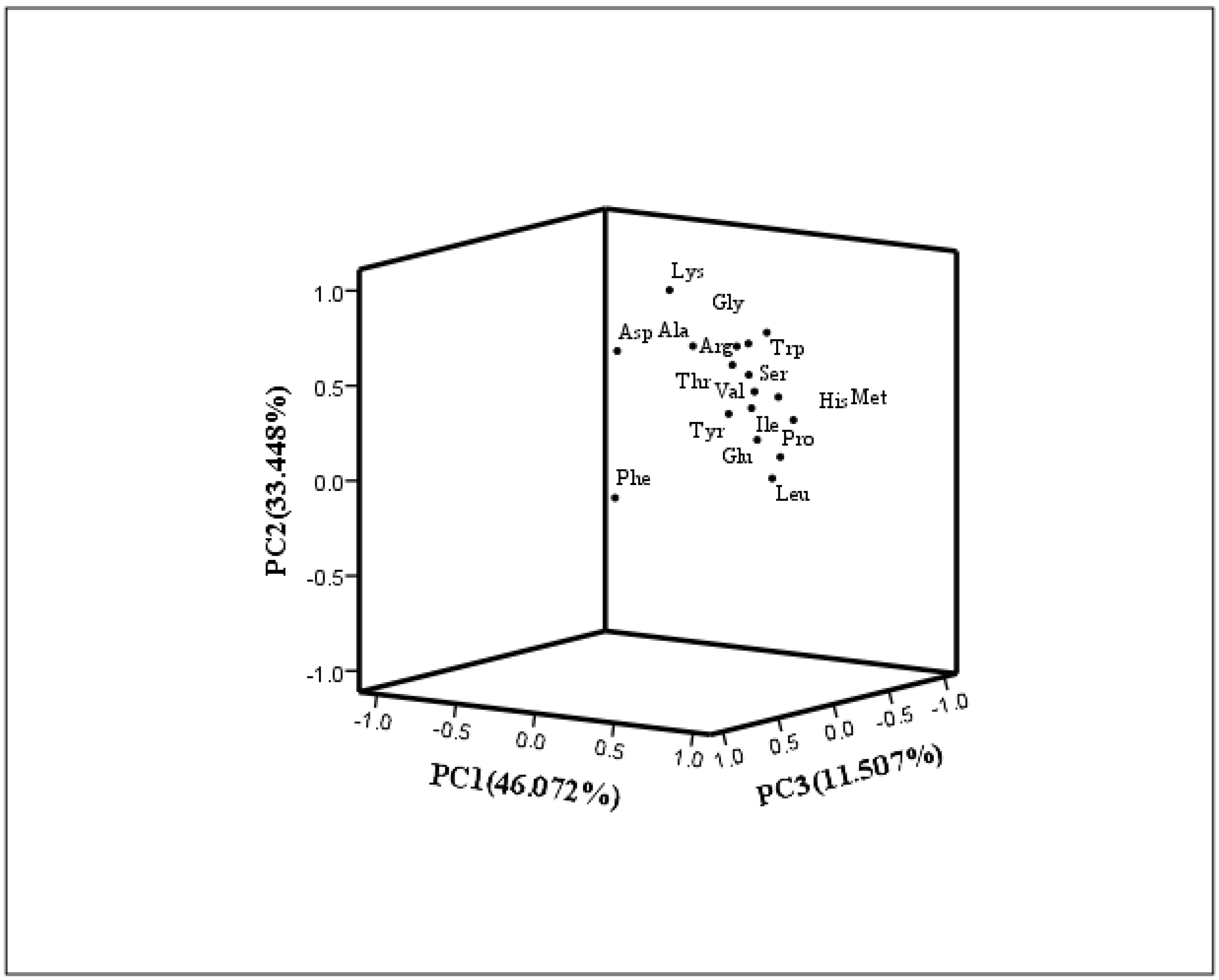
3.6.2. Principal Component Analysis (PCA)
3.6.3. Comprehensive Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, T.M. Waxy corn and WX gene. J. Maize Sci. 1993, 2, 1–2. [Google Scholar]
- Xu, L.; Zhao, J.; Lu, B.; Shi, Y.; Fan, Y. Current situation and development trend of fresh corn seed industry in China. Chin. Seed Ind. 2020, 10, 14–18. [Google Scholar]
- Li, Z.; Liu, J.; Chen, Y.; Li, M. Amino Acid Composition and Nutritional Value Evaluation of Lentinus edodes from Different Habitats. Storage Process 2020, 20, 167–172. [Google Scholar]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef]
- Oladipupo, Q.A.; Olasunkanmi, S.G.; Sumbo, H.A. Functional Properties and Protein Digestibility of Protein Concentrates and Isolates Produced from Kariya (Hildergadia bateri) Seed. J. Food Processing Preserv. 2016, 40, 979–989. [Google Scholar]
- Liu, H.; Wang, Z.H.; Li, C.F.; Li, K.Y.; Yang, N.; Yang, Y.E. Contents of Protein and Amino Acids of Wheat Grain in Different Wheat Production Regions and Their Evaluation. Acta Agron. Sin. 2016, 42, 768–777. [Google Scholar] [CrossRef]
- Mattila, P.; Suonpaa, K.; Piironen, V. Functional properties of edible mushrooms. Nutrition 2000, 16, 694–696. [Google Scholar] [CrossRef]
- Scientific Research Report on Dietary Guidelines for Chinese Residents (2021) has been released. Food Health 2021, 04, 4.
- Xiao, F.; Guo, F. Impacts of essential amino acids on energy balance. Mol. Metab. 2022, 57, 101393. [Google Scholar] [CrossRef]
- Shi, L.; Hao, G.; Chen, J.; Ma, S.; Weng, W. Nutritional evaluation of Japanese abalone (Haliotis discus hannai Ino) muscle: Mineral content, amino acid profile and protein digestibility. Food Res. Int. 2020, 129, 108876. [Google Scholar] [CrossRef]
- Feng, N.; Yue, Z.; Hou, D.; Li, J.; Chen, L.; Yang, C. Changes in Nutrient Quality and Amino Acid Composition during Germination of Foxtail Millet. Food Res. Dev. 2020, 41, 14–21. [Google Scholar]
- Long, R.; Su, L.; Wang, Q. Nutrient Composition Comparative Analysis of Pleurotus ostreatus from Different Main Cultivation Areas. Edible Fungi China 2020, 39, 43–49. [Google Scholar]
- Luo, X.; Zhang, S.; Yan, M.; Zhang, W.; Sun, D. Protein and Amino Acid Analysis and Nutritional Value Evaluation of Eight Cultivation Edible Fungi in Yunnan Province. Food Ind. 2021, 42, 328–332. [Google Scholar]
- Jiang, Y.G.; Xu, Q.S. Advances of Study on Effect and Mechanism of Conditionally Essential Amino Acid on Wound Healing. Biot. Resour. 2002, 3, 59–62. [Google Scholar]
- Jiang, J.P.; Sun, Y.L.; Pei, Z.C.; Wang, Y.; Zhang, L.J.; Zhou, J.H.; Meng, F.Y.; Tong, G.X.; Wang, Z.S.; Zhang, X.; et al. Analysis and Evaluation on Nutritional Components of Fresh Sweet Corn in Beijing City. Food Nutr. China 2020, 26, 55–59. [Google Scholar]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, L.; Wu, P.; Feng, L.; Jiang, W.; Liu, Y.; Kuang, S.; Li, S.; Mi, H.; Tang, L.; et al. Dietary protein levels changed the hardness of muscle by acting on muscle fiber growth and the metabolism of collagen in sub-adult grass carp (Ctenopharyngodon idella). J. Anim. Sci. Biotechnol. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Yang, T.; Yang, C.; Yang, W.; Li, G. Analysis of nutritional components of artificially cultivated Dendrobium officinale in Fanjing mountain. Cereals Oils 2021, 34, 146–149. [Google Scholar]
- Ren, A.J.; Yang, Z.Y.; Dai, Y.J.; Wang, F.F. Research on aquatic product demand forecasting in China. J. Shanghai Ocean Univ. 2012, 21, 145–150. [Google Scholar]
- Yan, Z.X.; Jin, Y.C. Quality evaluation of tibetan mutton based on amino acid and fatty acid. Sci. Technol. Food Ind. 2016, 37, 351–354. [Google Scholar]
- Wang, C.L.; Guo, X.H.; Yuan, D.S.; Teng, Y. Analysis on Scophthatmus maximus Amino Acids at Different Developing Stages. Mod. Food Sci. Technol. 2012, 28, 104–107. [Google Scholar]
- Yan, S.; Lin, X.; Li, W.; Lin, Q.; Yao, Q. Amino acid composition characteristics and nutritional evaluation of edible fungi produced in Fujian. J. Food Saf. Qual. 2021, 12, 7723–7731. [Google Scholar]
- Fanelli, N.S.; Bailey, H.M.; Guardiola, L.V.; Stein, H.H. Values for Digestible Indispensable Amino Acid Score (DIAAS) Determined in Pigs Are Greater for Milk Than for Breakfast Cereals, but DIAAS Values for Individual Ingredients Are Additive in Combined Meals. J. Nutr. 2021, 151, 540–547. [Google Scholar] [CrossRef]
- Energy and protein requirements. In Proceedings of the Report of a Joint FAO/WHO Ad Hoc Expert Committee, Rome, Italy, 22 March–2 April 1971.
- Fang, C.; Zhao, X.; Yang, F.; Zou, F.; Yang, M.; Zhao, Y.; Zhao, S. Evaluation of Muscle Amino Acids Composition of Chinese Indigenous Pig Breeds Based on FAO Pattern. China Anim. Husb. Vet. Med. 2019, 46, 2890–2901. [Google Scholar]
- UNU; FAO; WHO. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Oser, B.L. Method for integrating essential amino acid content in the nutritional evaluation of protein. J. Am. Diet. Assoc. 1951, 27, 396–402. [Google Scholar] [CrossRef]
- Shen, C.; Wang, X.; Huang, S. The analysis and evaluation of nutrients of sansui’s characteristic sheldrake duck eggs. Food Mach. 2017, 33, 55–60. [Google Scholar]
- Astermark, J.; Hogg, P.J.; Björk, I.; Stenflo, J. Effects of gamma–carboxyglutamic acid and epidermal growth factor–like modules of factor IX on factor X activation. Studies using proteolytic fragments of bovine factor IX. J. Biol. Chem. 1992, 267, 3249–3256. [Google Scholar] [CrossRef]
- Bano, Z.; Rajarathnam, S. Pleurotus mushroom as a nutritious food. In Tropical Mushrooms–Biological Nature and Cultivated Methods; The Chinese University of Hong Kong Press: Hong Kong, China, 1982. [Google Scholar]
- Oser, B.L. An Integrated Essential Amino Acid Index for Predicting the Biological Value of Proteins–ScienceDirect. Protein Amino Acid Nutr. 1959, Chapter 10, 281–295. [Google Scholar]
- Yan, S.; Yao, Q.; Lin, X.; Qian, A. Evaluation method of amino acid nutrition. Food Sci. Technol. 2012, 37, 286–290. [Google Scholar]
- Yang, X.; Xi, B.; Li, W.; Guo, T.; Du, T.; Gao, Y. Nutritional Evaluation and Composition Analysis of Amino Acid in Different Parts of Muscle of Bamei Pig. Sci. Technol. Food Ind. 2020, 41, 232–236. [Google Scholar]
- Huang, W.; Zhang, Z.H.; Shi, Y.H.; Zhang, G.Y.; Zhang, H.M.; Lu, G.H.; Xie, Y.D.; Luo, Z.Q. Analysis and Evaluation of Nutritional Components in Muscle of Cultured Synechogobius ommaturus. Chin. J. Anim. Nutr. 2014, 26, 2866–2873. [Google Scholar]
- Huang, B.Q.; Huang, C.; Wu, J.X.; Zou, Q.; Liu, J.F. Comparison of Amino Acids and Taste Characteristics in Four Kinds of Emydidae Meat Enzymatic Hydrolysate. Food Res. Dev. 2021, 42, 12–17. [Google Scholar]
- Anusha, M.B.; Shivanna, N.; Kumar, G.P.; Anilakumar, K.R. Efficiency of selected food ingredients on protein efficiency ratio, glycemic index and in vitro digestive properties. J. Food Sci. Technol. 2018, 55, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Alsmeyer, R.H.; Cunningham, A.E.; Happich, M.L. Equation to Predict PER from Amino Acid Analysis. Food Technol. 1974, 28, 34–36. [Google Scholar]
- Hou, D.; Huang, W.; Chen, H.; Wu, Y.; Bao, D.; Wang, W.; Tao, X.; Yang, R. Amino acid profile and protein quality of Lentinula edodes with ball–shaped fruiting bodies. Mycosystema 2021, 40, 2412–2422. [Google Scholar]
- Wu, Y.; Bao, D.; Wang, R.; Chen, H.; Wang, H. Amino Acid Composition and Nutritional Evaluation of Proteins in Six Samples of Cultivated Flammulina velutipes. Food Sci. 2018, 39, 263–268. [Google Scholar]
- Joyce, B.; Ramani, W.; Barbara, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar]
- Seligson, F.H.; Mackey, L.N. Variable predictions of protein quality by chemical score due to amino acid analysis and reference pattern. J. Nutr. 1984, 114, 682–691. [Google Scholar] [CrossRef]
- Zhu, S.T.; Wu, K. Nutritional evaluation of protein. Acta Nutr. Sin. 1988, 187–190. [Google Scholar]
- Zhao, F.M.; Li, S.J.; Zhang, X.Y.; Yang, B.N.; Liu, W.; Su, D.; Yang, Y.C. Nutritional Evaluation of Amino Acids in Different Potato Gultivars. J. Chin. Cereals Oils 2014, 29, 13–18. [Google Scholar]
- Sun, W.J.; Ma, C.Z.; Yang, L.; Chi, F.M.; Luo, Z.; Gu, X.D. Analysis of Nutritional Components in Tibetan Pig Heart. Food Ind. 2020, 41, 340–343. [Google Scholar]
- Huahua, Y.; Rongfeng, L.; Song, L.; Rong–e, X.; Xiaolin, C.; Pengcheng, L. Amino acid composition and nutritional quality of gonad from jellyfish Rhopilema esculentum. Biomed. Prev. Nutr. 2014, 4, 399–402. [Google Scholar]
- Xue, P.; Zhao, L.; Jing, J.; Wang, X.; Zhang, F. Amino Acid Analysis and Nutritional Evaluation of Quinoa Bran Protein. Food Res. Dev. 2019, 40, 65–70. [Google Scholar]
- He, S.S.; Zhou, Z.Q. Evaluation of amino acid composition and nutritional quality in different species of Citrus fruit. South China Fruits 2018, 47, 18–22. [Google Scholar]
- Yan, S.; Shi, M.; Lin, X.; Li, W.; Yao, Q. Principal Component Analysis and Cluster Analysis for Evaluating Amino Acid of Different Table Grapes (Vitis vinifera L.) Varieties. Sci. Technol. Food Ind. 2022, 43, 372–379. [Google Scholar]
- XWang, Y.; Wang, R.R.; Wang, T.; Yang, L.Z.; Liu, J.; Li, H.; Zhang, Q.; Shan, Y.; Ding, S.H. Principal Component Analysis and Cluster Analysis for Evaluating the Free Amino Acid Composition of Inner and Outer Lily Bulb Scales from Different Cultivars. Food Sci. 2020, 41, 211–220. [Google Scholar]
- Cai, L.; Zeng, X.M.; Wang, X.; Li, M.X.; Tang, G.L.; Chen, C. Analysis and Assessment of Amino Acid Component in Flowers of Hibiscus mutabilis L. among Different Cultivars. Sci. Technol. Food Ind. 2021, 42, 279–285. [Google Scholar]
- Shen, F.; Ying, Y.; Li, B.; Zheng, Y.; Zhuge, Q. Multivariate classification of rice wines according to ageing time and brand based on amino acid profiles. Food Chem. 2011, 129, 565–569. [Google Scholar] [CrossRef]
- Elzbieta, S.; Aleksandra, S. Metabotropic glutamate receptors (mGluRs) are involved in early phase of memory formation: Possible role of modulation of glutamate release. Neurochem. Int. 2003, 43, 469–474. [Google Scholar]
- Marquezi, M.L.; Roschel, H.A.; Dos, S.C.A.; Sawada, L.A.; Lancha, A.H. Effect of aspartate and asparagine supplementation on fatigue determinants in intense exercise. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 65–75. [Google Scholar] [CrossRef]
- Yano, S.; Tokumitsu, H.; Soderling, T.R. Calcium promotes cell survival through CaM–K kinase activation of the protein–kinase–B pathway. Nature 1998, 396, 584–587. [Google Scholar] [CrossRef]
- Beth, K.; Erika, L.P. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar]
- De Windt, L.J.; Lim, H.W.; Bueno, O.F.; Liang, Q.; Delling, U.; Braz, J.C.; Glascock, B.J.; Kimball, T.F.; Del, M.F.; Hajjar, R.J.; et al. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 3322–3327. [Google Scholar] [CrossRef]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef]
- Meguro, T.; Hong, C.; Asai, K.; Takagi, G.; McKinsey, T.A.; Olson, E.N.; Vatner, S.F. Cyclosporine Attenuates Pressure–Overload Hypertrophy in Mice While Enhancing Susceptibility to Decompensation and Heart Failure. Circ. Res. 1999, 84, 735–740. [Google Scholar] [CrossRef]
- Calder, P.C. Branched–chain amino acids and immunity. J. Nutr. 2006, 136, 288S–293S. [Google Scholar] [CrossRef]
- Adrian, H.; Sophie, E.J.; Marije, A.H.R. Behavioral and cognitive effects of tyrosine intake in healthy human adults. Pharmacol. Biochem. Behav. 2015, 133, 1–6. [Google Scholar]
- Peng, L.; Yu–Long, Y.; Defa, L.; Sung, W.K.; Guoyao, W. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar]
- Xu, J.; Liao, J.; Lin, Z.A.; Tang, Z.D.; Fan, X.P.; Sun, Q.X.; Liu, S.C.; Su, W.M. Amino acid composition analysis and nutrition value evaluation of peptide powder from grouper muscle. Food Ferment. Ind. 2021, 47, 221–226. [Google Scholar]
- Di, H. Analysis of the Adolescents Physical Growth Development Differences among the Provinces and the Change of Health Equity in China from 1985 to 2010. Master’s Thesis, Soochow University, Suzhou, China, 2014. [Google Scholar]
- Lin, Y.; Dengu, J.M.; Miao, L.; Simon, B.; Pan, W.; Liu, B.; Ge, X. Effects of dietary supplementary leucine in a wheat meal–rich diet on the growth performance and immunity of juvenile gibel carp (Carassius auratus gibelio var. CAS III). Aquac. Res. 2020, 52, 1501–1512. [Google Scholar] [CrossRef]
- Witham, M.D.; Adamson, S.; Avenell, A.; Band, M.M.; Donnan, P.T.; George, J.; Hapca, A.; Hume, C.; Kemp, P.; Mckenzie, E.; et al. 667 effect of leucine supplementation on physical performance, muscle mass and quality of life in older people with sarcopenia. Age Ageing 2022, 51, afac036-667. [Google Scholar] [CrossRef]
- Guoyao, W.; Fuller, W.B.; Robert, C.B.; Gregory, A.J.; Sung, W.K.; Darrell, A.K.; Peng, L.; Xilong, L.; Jason, R.M.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar]
- Satyajit, D.; Supratim, R.; Nagarajan, K. Glutamic acid as anticancer agent: An overview. Saudi Pharm. J. 2013, 21, 337–343. [Google Scholar]
- Vera–Aviles, M.; Vantana, E.; Kardinasari, E.; Koh, N.L.; Latunde–Dada, G.O. Protective Role of Histidine Supplementation Against Oxidative Stress Damage in the Management of Anemia of Chronic Kidney Disease. Pharmaceuticals 2018, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Ray, S.; Nagarajan, K. Glutamic acid analogues used as potent anticancer: A review. Pharma Chem. 2011, 3, 263–272. [Google Scholar]
- Liu, S.; Sun, Y.; Zhao, R.; Wang, Y.; Zhang, W.; Pang, W. Isoleucine increases muscle mass through promoting myogenesis and intramyocellular fat deposition. Food Funct. 2020, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Jontofsohn, R.; Heinze, V.; Katz, N.; Stuber, U.; Wilke, H.; Kluthe, R. Histidine and iron supplementation in dialysis and pre–dialysis patients. Proc. Eur. Dial. Transpl. Assoc. Eur. Dial. Transpl. Assoc. 1975, 11, 391–397. [Google Scholar]
- Zhang, Z.; TeSlaa, T.; Xu, X.; Zeng, X.; Yang, L.; Xing, G.; Tesz, G.J.; Clasquin, M.F.; Rabinowitz, J.D. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nat. Metab. 2021, 3, 1608–1620. [Google Scholar] [CrossRef]
- Marius, A.Ø.; Per, B.; Wenjun, J.; Leila, R.Z.; Knut, A.L.; Kumar, S.; Ian, H.D.B.; Stein, I.H. Gene expression studies and targeted metabolomics reveal disturbed serine, methionine, and tyrosine metabolism in early hypertensive nephrosclerosis. Kidney Int. Rep. 2018, 4, 321–333. [Google Scholar]
- Liao, X.; Liu, B.; Qu, H.; Zhang, L.; Lu, Y.; Xu, Y.; Lyu, Z.; Zheng, H. A High Level of Circulating Valine Is a Biomarker for Type 2 Diabetes and Associated with the Hypoglycemic Effect of Sitagliptin. Mediat. Inflamm. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Mcbride, K.L.; Pluciniczak, J.; Rhyand, T.; Bartholomew, D. Phenylalanine and tyrosine measurements across gestation by tandem mass spectrometer on dried blood spot cards from normal pregnant women. Genet. Med. 2019, 21, 1821–1826. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, R.H.; Men, J. Research Progress in the Analysis and Detection of Tyrosine. Fine Chem. Intermed. 2022, 52, 1–4. [Google Scholar]
- Amir, S.; Isaac, R.K.; George, C.T. The serine/threonine protein phosphatase 2A controls autoimmunity. Clin. Immunol. 2018, 186, 38–42. [Google Scholar]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Prog. Heritable Soft Connect. Tissue Dis. 2013, 802, 31–47. [Google Scholar]
- Han, M.; Xie, M.; Han, J.; Yuan, D.; Yang, T.; Xie, Y. Development and validation of a rapid, selective, and sensitive LC–MS/MS method for simultaneous determination of D- and L-amino acids in human serum: Application to the study of hepatocellular carcinoma. Anal. Bioanal. Chem. 2018, 410, 2517–2531. [Google Scholar] [CrossRef]
- Erens, C.; Van Broeckhoven, J.; Hoeks, C.; Schabbauer, G.; Cheng, P.N.; Chen, L.; Hellings, N.; Broux, B.; Lemmens, S.; Hendrix, S. L–Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction. Biomedicines 2022, 10, 205. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H. Tryptophan and Its Metabolites in Lung Cancer: Basic Functions and Clinical Significance. Front. Oncol. 2021, 11, 3019. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, C.; Yang, X.; Zhang, C. L–phenylalanine attenuates high salt–induced hypertension in Dahl SS rats through activation of GCH1–BH4. PLoS ONE 2021, 16, e0250126. [Google Scholar] [CrossRef]
- Wang, C.; Mao, Y.; Liu, J.; Dai, S.; Yan, B. Analysis and evaluation of amino acid components in Rosa rugosa Thunb. from different regions. J. Food Saf. Qual. 2022, 13, 544–553. [Google Scholar]
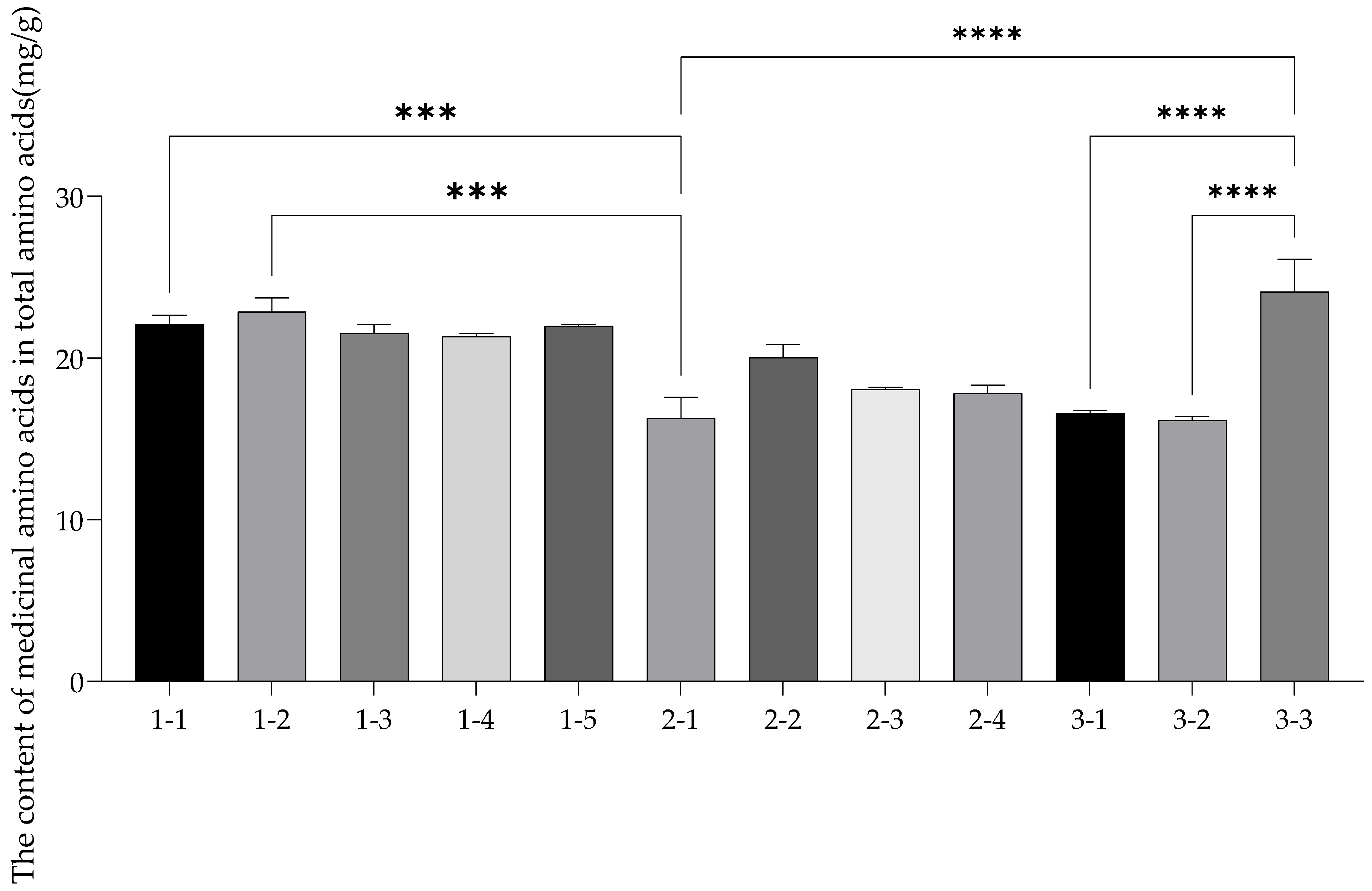
| Pattern Spectrum Amino Acid | His | Thr | Lys | Leu | Ile | Met + Cys | Phe + Tyr | Val | Trp |
|---|---|---|---|---|---|---|---|---|---|
| FAO/WHO/UNU Adults | 15 | 23 | 45 | 59 | 30 | 22 | 38 | 39 | 6 |
| IOM | 18 | 27 | 51 | 55 | 25 | 25 | 47 | 32 | 7 |
| Whole Egg | – | 47 | 70 | 86 | 54 | 57 | 93 | 66 | 17 |
| Amino Acids | Inner Mongolia | Jilin | Heilongjiang | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 2–1 | 2–2 | 2–3 | 2–4 | 3–1 | 3–2 | 3–3 | |
| Asp | 2.45 ± 0.02 abc | 2.33 ± 0.07 bcd | 2.24 ± 0.12 d | 2.18 ± 0.04 d | 2.62 ± 0.02 a | 2.25 ± 0.12 d | 2.58 ± 0.11 a | 2.29 ± 0.05 acd | 2.22 ± 0.06 d | 1.82 ± 0.02 e | 1.88 ± 0.02 e | 2.50 ± 0.17 b |
| Thr | 1.37 ± 0.03 abc | 1.41 ± 0.01 ab | 1.32 ± 0.04 bc | 1.31 ± 0.01 cd | 1.41 ± 0.02 a | 1.01 ± 0.05 f | 1.23 ± 0.03 de | 1.17 ± 0.02 e | 1.15 ± 0.02 e | 0.94 ± 0.04 f | 1.01 ± 0.01 f | 1.37 ± 0.08 abc |
| Ser | 1.82 ± 0.01 ab | 1.87 ± 0.09 a | 1.79 ± 0.07 ab | 1.72 ± 0.06 b | 1.83 ± 0.01 a | 1.22 ± 0.05 e | 1.56 ± 0.01 c | 1.46 ± 0.01 d | 1.51 ± 0.05 cd | 1.30 ± 0.00 e | 1.26 ± 0.01 e | 1.82 ± 0.05 ab |
| Glu | 7.37 ± 0.42 bc | 7.83 ± 0.73 ab | 7.40 ± 0.01 bc | 7.25 ± 0.18 bc | 7.29 ± 0.01 bc | 5.03 ± 0.45 e | 6.49 ± 0.19 cd | 5.58 ± 0.01 de | 5.70 ± 0.23 de | 5.64 ± 0.02 de | 4.90 ± 0.05 e | 8.33 ± 0.98 a |
| Gly | 1.36 ± 0.03 ab | 1.39 ± 0.02 a | 1.27 ± 0.07 cde | 1.21 ± 0.02 def | 1.31 ± 0.03 abc | 1.02 ± 0.04 i | 1.18 ± 0.05 efg | 1.14 ± 0.02 fgh | 1.07 ± 0.05 hi | 0.84 ± 0.05 j | 1.10 ± 0.01 ghi | 1.28 ± 0.01 bcd |
| Ala | 3.57 ± 0.07 de | 3.93 ± 0.00 bc | 3.64 ± 0.10 cd | 4.02 ± 0.04 b | 4.48 ± 0.01 a | 2.90 ± 0.19 g | 3.96 ± 0.18 bc | 3.28 ± 0.09 ef | 2.60 ± 0.08 gh | 2.44 ± 0.06 hi | 2.27 ± 0.02 i | 3.23 ± 0.36 f |
| Val | 2.02 ± 0.08 a | 1.94 ± 0.00 a | 1.89 ± 0.09 a | 1.86 ± 0.01 ab | 1.91 ± 0.02 a | 1.41 ± 0.11 def | 1.71 ± 0.07 bc | 1.57 ± 0.01 cd | 1.50 ± 0.05 de | 1.29 ± 0.04 f | 1.39 ± 0.01 ef | 1.92 ± 0.18 a |
| Met | 0.78 ± 0.07 a | 0.77 ± 0.02 a | 0.71 ± 0.00 ab | 0.73 ± 0.04 a | 0.72 ± 0.01 ab | 0.61 ± 0.07 c | 0.58 ± 0.02 c | 0.59 ± 0.02 c | 0.59 ± 0.01 c | 0.47 ± 0.01 d | 0.65 ± 0.01 bc | 0.78 ± 0.05 a |
| Ile | 1.33 ± 0.05 a | 1.32 ± 0.05 ab | 1.31 ± 0.09 ab | 1.29 ± 0.01 ab | 1.32 ± 0.04 ab | 0.94 ± 0.10 d | 1.16 ± 0.06 bc | 1.08 ± 0.02 cd | 1.03 ± 0.03 cd | 0.95 ± 0.05 d | 0.98 ± 0.01 d | 1.41 ± 0.16 a |
| Leu | 4.46 ± 0.25 bc | 4.92 ± 0.55 b | 4.61 ± 0.24 b | 4.74 ± 0.04 b | 4.33 ± 0.00 bc | 3.02 ± 0.33 e | 3.86 ± 0.07 cd | 3.33 ± 0.02 de | 3.25 ± 0.23 de | 3.79 ± 0.01 cd | 3.27 ± 0.03 de | 5.56 ± 0.65 a |
| Tyr | 1.24 ± 0.05 a | 1.24 ± 0.00 a | 1.33 ± 0.06 a | 1.24 ± 0.04 a | 1.30 ± 0.01 a | 0.93 ± 0.09 c | 1.24 ± 0.03 a | 1.12 ± 0.02 b | 1.08 ± 0.00 b | 1.03 ± 0.04 bc | 1.02 ± 0.01 bc | 1.34 ± 0.09 a |
| Phe | 1.50 ± 0.07 bc | 1.47 ± 0.08 bcd | 1.28 ± 0.08 cd | 1.47 ± 0.05 bcd | 1.47 ± 0.06 bcd | 1.26 ± 0.03 cd | 1.53 ± 0.16 b | 1.49 ± 0.15 bc | 1.53 ± 0.12 b | 1.41 ± 0.02 cd | 1.24 ± 0.05 d | 1.81 ± 0.14 a |
| Lys | 1.41 ± 0.10 ab | 1.27 ± 0.13 bc | 1.19 ± 0.11 c | 1.11 ± 0.02 cd | 1.46 ± 0.05 a | 1.00 ± 0.04 de | 1.23 ± 0.07 c | 1.22 ± 0.05 c | 1.12 ± 0.01 cd | 0.67 ± 0.08 f | 0.95 ± 0.01 e | 0.96 ± 0.05 de |
| His | 1.10 ± 0.01 ab | 1.18 ± 0.04 a | 1.19 ± 0.01 a | 1.12 ± 0.01 ab | 1.02 ± 0.04 bc | 0.84 ± 0.01 e | 0.98 ± 0.10 cd | 1.01 ± 0.05 bc | 0.95 ± 0.02 cd | 0.88 ± 0.08 de | 0.93 ± 0.01 cde | 1.12 ± 0.05 ab |
| Arg | 1.54 ± 0.05 ab | 1.65 ± 0.14 a | 1.49 ± 0.04 abc | 1.39 ± 0.09 bcd | 1.49 ± 0.04 abc | 1.16 ± 0.10 e | 1.36 ± 0.09 cd | 1.30 ± 0.01 de | 1.26 ± 0.03 de | 0.92 ± 0.05 f | 1.15 ± 0.01 e | 1.50 ± 0.02 abc |
| Pro | 3.10 ± 0.22 ab | 3.12 ± 0.35 ab | 2.97 ± 0.31 ab | 3.07 ± 0.29 ab | 2.68 ± 0.23 bc | 2.08 ± 0.06 d | 2.71 ± 0.12 bc | 2.33 ± 0.04 cd | 2.30 ± 0.13 cd | 2.36 ± 0.03 cd | 2.26 ± 0.02 cd | 3.27 ± 0.17 a |
| Trp | 0.25 ± 0.01 | – | 0.24 ± 0.00 | – | 0.19 ± 0.01 | – | 0.13 ± 0.02 | – | – | – | – | – |
| Total acid | 36.41 ± 0.93 a | 37.64 ± 1.38 a | 35.63 ± 0.62 ab | 35.70 ± 0.55 ab | 36.64 ± 0.35 a | 26.69 ± 1.72 d | 33.35 ± 1.35 b | 29.96 ± 0.12 c | 28.84 ± 0.85 cd | 26.75 ± 0.38 d | 26.25 ± 0.29 d | 38.23 ± 3.07 a |
| Protein | 40.26 ± 0.35 a | 40.09 ± 1.79 a | 34.99 ± 0.61 b | 38.78 ± 0.86 a | 39.14 ± 0.86 a | 32.38 ± 0.45 c | 35.16 ± 0.26 b | 30.49 ± 0.26 cd | 30.74 ± 0.24 cd | 35.98 ± 1.17 b | 29.18 ± 0.83 d | 35.41 ± 1.46 b |
| Amino Acids | Inner Mongolia | Jilin | Heilongjiang | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 2–1 | 2–2 | 2–3 | 2–4 | 3–1 | 3–2 | 3–3 | |
| Asp | 6.73 ± 0.24 | 6.21 ± 0.42 | 6.27 ± 0.22 | 6.11 ± 0.01 | 7.15 ± 0.02 | 8.41 ± 0.08 | 7.74 ± 0.01 | 7.64 ± 0.13 | 7.70 ± 0.01 | 6.82 ± 0.04 | 7.16 ± 0.01 | 6.55 ± 0.07 |
| Thr | 3.75 ± 0.17 | 3.74 ± 0.17 | 3.71 ± 0.04 | 3.66 ± 0.09 | 3.85 ± 0.01 | 3.78 ± 0.04 | 3.70 ± 0.06 | 3.90 ± 0.05 | 3.99 ± 0.03 | 3.50 ± 0.12 | 3.83 ± 0.01 | 3.59 ± 0.07 |
| Ser | 4.99 ± 0.10 | 4.97 ± 0.06 | 5.03 ± 0.11 | 4.80 ± 0.08 | 5.00 ± 0.01 | 4.58 ± 0.11 | 4.68 ± 0.22 | 4.86 ± 0.02 | 5.22 ± 0.01 | 4.87 ± 0.08 | 4.79 ± 0.01 | 4.77 ± 0.26 |
| Glu | 20.22 ± 0.64 | 20.79 ± 1.17 | 20.77 ± 0.34 | 20.31 ± 0.20 | 19.89 ± 0.21 | 18.84 ± 0.46 | 19.46 ± 0.21 | 18.62 ± 0.04 | 19.76 ± 0.23 | 21.1 ± 0.38 | 18.66 ± 0.03 | 21.77 ± 0.80 |
| Gly | 3.74 ± 0.18 | 3.68 ± 0.18 | 3.55 ± 0.12 | 3.39 ± 0.01 | 3.59 ± 0.06 | 3.83 ± 0.09 | 3.54 ± 0.01 | 3.79 ± 0.06 | 3.70 ± 0.06 | 3.14 ± 0.16 | 4.20 ± 0.01 | 3.36 ± 0.25 |
| Ala | 9.82 ± 0.07 | 10.45 ± 0.38 | 10.22 ± 0.12 | 11.25 ± 0.06 | 12.24 ± 0.10 | 10.86 ± 0.01 | 11.86 ± 0.06 | 10.93 ± 0.27 | 9.02 ± 0.03 | 9.11 ± 0.08 | 8.66 ± 0.02 | 8.45 ± 0.26 |
| Val | 5.55 ± 0.07 | 5.16 ± 0.19 | 5.30 ± 0.15 | 5.20 ± 0.10 | 5.20 ± 0.10 | 5.30 ± 0.09 | 5.13 ± 0.02 | 5.25 ± 0.05 | 5.20 ± 0.02 | 4.83 ± 0.07 | 5.28 ± 0.01 | 5.02 ± 0.06 |
| Met | 2.15 ± 0.13 | 2.06 ± 0.01 | 1.99 ± 0.04 | 2.05 ± 0.08 | 1.96 ± 0.02 | 2.27 ± 0.10 | 1.75 ± 0.02 | 1.98 ± 0.08 | 2.05 ± 0.02 | 1.75 ± 0.03 | 2.46 ± 0.01 | 2.05 ± 0.02 |
| Ile | 3.66 ± 0.03 | 3.51 ± 0.00 | 3.67 ± 0.19 | 3.60 ± 0.02 | 3.61 ± 0.07 | 3.52 ± 0.14 | 3.49 ± 0.04 | 3.61 ± 0.09 | 3.56 ± 0.01 | 3.56 ± 0.14 | 3.73 ± 0.01 | 3.69 ± 0.12 |
| Leu | 12.24 ± 0.38 | 13.07 ± 0.98 | 12.93 ± 0.44 | 13.27 ± 0.09 | 11.81 ± 0.11 | 11.31 ± 0.52 | 11.58 ± 0.27 | 11.1 ± 0.10 | 11.25 ± 0.47 | 14.18 ± 0.24 | 12.45 ± 0.01 | 14.53 ± 0.54 |
| Tyr | 3.40 ± 0.24 | 3.29 ± 0.12 | 3.74 ± 0.10 | 3.49 ± 0.16 | 3.54 ± 0.00 | 3.48 ± 0.13 | 3.71 ± 0.05 | 3.73 ± 0.08 | 3.74 ± 0.11 | 3.85 ± 0.11 | 3.89 ± 0.01 | 3.52 ± 0.04 |
| Phe | 4.10 ± 0.09 | 3.91 ± 0.36 | 3.61 ± 0.29 | 4.12 ± 0.20 | 4.02 ± 0.21 | 4.74 ± 0.19 | 4.58 ± 0.29 | 4.98 ± 0.48 | 5.31 ± 0.57 | 5.26 ± 0.00 | 4.74 ± 0.14 | 4.73 ± 0.01 |
| Lys | 3.89 ± 0.38 | 3.38 ± 0.48 | 3.34 ± 0.24 | 3.12 ± 0.01 | 3.98 ± 0.09 | 3.75 ± 0.08 | 3.67 ± 0.07 | 4.09 ± 0.19 | 3.89 ± 0.09 | 2.50 ± 0.25 | 3.60 ± 0.01 | 2.52 ± 0.34 |
| His | 3.03 ± 0.10 | 3.14 ± 0.00 | 3.34 ± 0.09 | 3.15 ± 0.02 | 2.77 ± 0.13 | 3.15 ± 0.24 | 2.93 ± 0.18 | 3.38 ± 0.20 | 3.29 ± 0.18 | 3.28 ± 0.25 | 3.55 ± 0.01 | 2.94 ± 0.10 |
| Arg | 4.24 ± 0.25 | 4.38 ± 0.54 | 4.19 ± 0.03 | 3.90 ± 0.32 | 4.08 ± 0.07 | 4.36 ± 0.11 | 4.08 ± 0.12 | 4.34 ± 0.00 | 4.37 ± 0.01 | 3.44 ± 0.23 | 4.39 ± 0.01 | 3.95 ± 0.38 |
| Pro | 8.50 ± 0.38 | 8.27 ± 0.62 | 8.34 ± 1.02 | 8.58 ± 0.68 | 7.30 ± 0.57 | 7.82 ± 0.71 | 8.11 ± 0.03 | 7.78 ± 0.17 | 7.97 ± 0.21 | 8.82 ± 0.23 | 8.59 ± 0.01 | 8.56 ± 0.24 |
| Trp | 0.67 ± 0.03 | – | 0.58 ± 0.06 | – | 0.58 ± 0.01 | – | 0.52 ± 0.02 | – | – | – | – | – |
| Inner Mongolia | Jilin | Heilongjiang | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 2–1 | 2–2 | 2–3 | 2–4 | 3–1 | 3–2 | 3–3 | |
| EAAs (mg/g) | 13.12 ± 0.37 ab | 13.40 ± 0.40 ab | 12.52 ± 0.50 bc | 12.74 ± 0.04 b | 12.83 ± 0.03 b | 9.44 ± 0.73 f | 11.50 ± 0.49 cd | 10.66 ± 0.05 de | 10.35 ± 0.24 ef | 9.64 ± 0.21 ef | 9.66 ± 0.13 ef | 13.99 ± 1.22 a |
| EAA/TAA | 0.360 ± 0.001 abc | 0.356 ± 0.003 cde | 0.351 ± 0.008 def | 0.357 ± 0.004 cde | 0.350 ± 0.002 ef | 0.353 ± 0.005 cde | 0.345 ± 0.001 f | 0.356 ± 0.000 cde | 0.359 ± 0.002 bcd | 0.360 ± 0.003 abc | 0.368 ± 0.001 a | 0.366 ± 0.003 ab |
| EAA/NEAA | 0.557 ± 0.003 abc | 0.546 ± 0.006 cde | 0.537 ± 0.018 def | 0.549 ± 0.010 cde | 0.534 ± 0.006 ef | 0.541 ± 0.011 cde | 0.521 ± 0.001 f | 0.546 ± 0.000 cde | 0.554 ± 0.005 bcd | 0.560 ± 0.008 abc | 0.576 ± 0.003 a | 0.573 ± 0.006 ab |
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 2–1 | 2–2 | 2–3 | 2–4 | 3–1 | 3–2 | 3–3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ile | 0.61 ± 0.62 bc | 0.61 ± 0.65 bc | 0.69 ± 0.63 ab | 0.61 ± 0.63 bc | 0.63 ± 0.60 bc | 0.54 ± 0.06 cd | 0.61 ± 0.04 bc | 0.66 ± 0.01 ab | 0.62 ± 0.01 bc | 0.49 ± 0.01 d | 0.62 ± 0.02 bc | 0.74 ± 0.11 a |
| Leu | 1.29 ± 1.41 bcd | 1.43 ± 1.42 bc | 1.53 ± 1.44 b | 1.42 ± 1.36 bc | 1.29 ± 1.24 bcd | 1.09 ± 0.13 d | 1.28 ± 0.03 bcd | 1.27 ± 0.00 bcd | 1.23 ± 0.08 cd | 1.23 ± 0.04 c | 1.30 ± 0.05 bcd | 1.83 ± 0.29 a |
| Lys | 0.50 ± 0.48 bc | 0.45 ± 0.49 cde | 0.49 ± 0.43 bcd | 0.41 ± 0.48 ef | 0.53 ± 0.49 ab | 0.44 ± 0.03 de | 0.50 ± 0.03 bc | 0.57 ± 0.02 a | 0.52 ± 0.00 b | 0.27 ± 0.02 g | 0.46 ± 0.02 cde | 0.39 ± 0.00 f |
| Met | 0.34 ± 0.34 abc | 0.34 ± 0.34 abc | 0.35 ± 0.34 ab | 0.33 ± 0.34 bc | 0.32 ± 0.34 bc | 0.33 ± 0.04 bc | 0.29 ± 0.01 c | 0.34 ± 0.01 abc | 0.34 ± 0.00 abc | 0.23 ± 0.00 d | 0.39 ± 0.01 a | 0.39 ± 0.04 a |
| Phe + Tyr | 0.73 ± 0.73 de | 0.73 ± 0.75 e | 0.80 ± 0.79 cde | 0.75 ± 0.74 de | 0.76 ± 0.77 de | 0.73 ± 0.05 de | 0.85 ± 0.07 abcd | 0.92 ± 0.05 ab | 0.91 ± 0.05 abc | 0.73 ± 0.00 de | 0.84 ± 0.05 bcde | 0.96 ± 0.11 a |
| Thr | 0.72 ± 0.75 def | 0.75 ± 0.77 bcde | 0.80 ± 0.75 abc | 0.72 ± 0.74 f | 0.77 ± 0.73 abcde | 0.66 ± 0.04 f | 0.75 ± 0.02 bce | 0.82 ± 0.02 ab | 0.80 ± 0.01 abce | 0.55 ± 0.01 f | 0.73 ± 0.03 cef | 0.83 ± 0.09 a |
| Val | 0.76 ± 0.75 ab | 0.73 ± 0.77 ab | 0.82 ± 0.76 a | 0.72 ± 0.73 ab | 0.74 ± 0.73 ab | 0.66 ± 0.06 b | 0.74 ± 0.04 ab | 0.78 ± 0.00 a | 0.74 ± 0.02 ab | 0.54 ± 0.00 c | 0.72 ± 0.03 ab | 0.82 ± 0.11 a |
| Trp | 0.37 ± 0.41 ac | 0.43 ± 0.40 a | 0.36 ± 0.35 ac | 0.36 ± 0.34 ac | 0.33 ± 0.33 cd | 0.32 ± 0.01 cd | 0.32 ± 0.01 cd | 0.37 ± 0.01 a | 0.36 ± 0.02 ac | 0.21 ± 0.04 e | 0.36 ± 0.02 ac | 0.29 ± 0.02 d |
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 2–1 | 2–2 | 2–3 | 2–4 | 3–1 | 3–2 | 3–3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ile | 110.32 ± 4.82 bc | 110.08 ± 8.87 bc | 124.44 ± 6.45 ab | 110.67 ± 3.43 bc | 112.75 ± 0.76 bc | 96.99 ± 11.32 cd | 110.23 ± 6.65 bc | 118.24 ± 1.53 ab | 111.24 ± 2.20 bc | 88.25 ± 1.99 d | 111.89 ± 4.16 bc | 133.44 ± 20.42 a |
| Leu | 246.19 ± 15.99 bcd | 273.92 ± 42.76 bc | 292.64 ± 10.03 b | 271.58 ± 8.51 bc | 245.74 ± 5.35 bcd | 207.6 ± 25.64 d | 243.92 ± 5.92 bcd | 242.48 ± 0.80 bcd | 234.56 ± 14.78 cd | 234.36 ± 8.25 c | 249.12 ± 9.63 bcd | 350.21 ± 55.55 a |
| Lys | 77.98 ± 4.95 bc | 70.28 ± 4.18 cde | 75.59 ± 5.51 bcd | 63.90 ± 2.68 ef | 82.80 ± 0.78 ab | 68.62 ± 3.91 de | 77.44 ± 5.13 bc | 89.21 ± 2.94 a | 81.07 ± 0.17 b | 41.23 ± 3.42 g | 72.08 ± 2.70 cde | 60.23 ± 0.71 f |
| Met | 88.50 ± 8.35 abc | 87.91 ± 6.66 abc | 91.92 ± 1.69 ab | 85.99 ± 6.72 bc | 83.45 ± 0.28 bc | 85.35 ± 10.41 bc | 75.44 ± 2.84 | 88.33 ± 2.30 abc | 87.24 ± 0.99 abc | 59.06 ± 0.22 d | 100.83 ± 3.75 a | 100.87 ± 11.06 a |
| Phe + Tyr | 178.57 ± 2.70 de | 177.63 ± 2.75 e | 196.96 ± 5.19 cde | 184.22 ± 1.95 de | 186.33 ± 7.54 de | 178.34 ± 12.64 de | 207.15 ± 15.93 abcd | 225.53 ± 13.17 ab | 223.22 ± 11.79 abc | 178.26 ± 1.13 de | 204.42 ± 11.18 bcde | 234.86 ± 27.30 a |
| Thr | 147.53 ± 1.62 cef | 152.57 ± 5.68 bce | 164.05 ± 1.80 ab | 146.5 ± 1.75 ef | 156.7 ± 1.50 abce | 135.47 ± 9.19 f | 152.36 ± 4.63 bce | 166.88 ± 4.06 ab | 162.64 ± 2.13 abc | 113.14 ± 1.71 g | 150.04 ± 5.65 bcef | 168.83 ± 17.39 |
| Val | 128.75 ± 6.07 ab | 124.25 ± 5.61 ab | 138.34 ± 3.86 a | 122.68 ± 2.26 ab | 124.94 ± 3.92 ab | 112.08 ± 10.57 b | 124.8 ± 6.39 ab | 132.43 ± 0.37 a | 125.06 ± 3.21 ab | 92.08 ± 0.37 c | 121.76 ± 4.59 ab | 139.34 ± 18.45 a |
| Trp | 103.49 ± 4.37 bc | 121.51 ± 4.85 a | 102.57 ± 7.98 bc | 102.08 ± 0.14 bc | 92.23 ± 2.94 cd | 91.85 ± 2.73 cd | 91.74 ± 3.70 cd | 106.07 ± 3.23 b | 103.26 ± 5.71 bc | 59.24 ± 11.42 e | 102.03 ± 5.73 bc | 81.76 ± 4.37 d |
| His | 182.66 ± 0.06 efg | 197.02 ± 16.23 bcdef | 226.76 ± 6.11 a | 193.39 ± 6.37 cdef | 173.17 ± 10.33 fg | 172.42 ± 0.50 fg | 185.32 ± 20.14 defg | 221.59 ± 10.00 ab | 205.51 ± 6.97 abcde | 162.43 ± 9.28 g | 213.26 ± 8.02 abc | 211.73 ± 18.73 abcd |
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 2–1 | 2–2 | 2–3 | 2–4 | 3–1 | 3–2 | 3–3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EAAI | 83.23 ± 1.77 | 84.99 ± 4.75 | 90.29 ± 2.98 | 81.34 ± 2.37 | 83.03 ± 1.45 | 74.18 ± 6.60 | 81.55 ± 4.51 | 90.67 ± 0.61 | 86.59 ± 1.65 | 58.4 ± 1.02 | 85.22 ± 4.1 | 91.59 ± 10.67 |
| BV | 79.02 ± 1.93 | 80.94 ± 5.18 | 86.71 ± 3.25 | 76.96 ± 2.58 | 78.80 ± 1.58 | 69.16 ± 7.19 | 77.19 ± 4.92 | 87.13 ± 0.67 | 82.68 ± 1.8 | 51.95 ± 1.11 | 81.19 ± 4.47 | 88.13 ± 11.63 |
| U | 0.89 ± 0.00 | 0.87 ± 0.01 | 0.86 ± 0.00 | 0.87 ± 0.00 | 0.87 ± 0.00 | 0.88 ± 0.01 | 0.85 ± 0.01 | 0.85 ± 0.01 | 0.86 ± 0.00 | 0.83 ± 0.01 | 0.86 ± 0.01 | 0.81 ± 0.03 |
| NI | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.025 ± 0.023 | 0.030 ± 0.028 | 0.028 ± 0.028 | 0.027 ± 0.026 | 0.021 ± 0.021 | 0.025 ± 0.025 | 0.034 ± 0.031 |
| F | 3.87 ± 0.00 | 4.07 ± 0.25 | 3.82 ± 0.10 | 3.98 ± 0.02 | 3.75 ± 0.04 | 3.50 ± 0.01 | 3.70 ± 0.01 | 3.50 ± 0.05 | 3.46 ± 0.16 | 4.03 ± 0.10 | 3.38 ± 0.00 | 4.17 ± 0.17 |
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 2–1 | 2–2 | 2–3 | 2–4 | 3–1 | 3–2 | 3–3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PER 1 | 1.20 ± 0.10 | 1.41 ± 0.23 | 1.28 ± 0.12 | 1.33 ± 0.01 | 1.16 ± 0.01 | 0.6 ± 0.15 | 0.95 ± 0.02 | 0.72 ± 0.01 | 0.69 ± 0.10 | 0.93 ± 0.00 | 0.70 ± 0.01 | 1.70 ± 0.29 |
| PER 2 | 1.43 ± 0.12 | 1.64 ± 0.25 | 1.48 ± 0.10 | 1.55 ± 0.02 | 1.36 ± 0.00 | 0.81 ± 0.14 | 1.15 ± 0.03 | 0.92 ± 0.01 | 0.89 ± 0.10 | 1.15 ± 0.01 | 0.91 ± 0.01 | 1.92 ± 0.29 |
| PER 3 | 1.07 ± 0.27 | 1.44 ± 0.45 | 1.08 ± 0.13 | 1.26 ± 0.09 | 0.86 ± 0.01 | 0.11 ± 0.20 | 0.49 ± 0.05 | 0.19 ± 0.02 | 0.15 ± 0.18 | 0.56 ± 0.03 | 0.25 ± 0.02 | 1.67 ± 0.69 |
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 2–1 | 2–2 | 2–3 | 2–4 | 3–1 | 3–2 | 3–3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ile | 93.77 ± 4.10 | 93.57 ± 7.54 | 105.78 ± 5.48 | 94.07 ± 2.92 | 95.84 ± 0.65 | 82.44 ± 9.62 | 93.70 ± 5.65 | 100.51 ± 1.3 | 94.56 ± 1.87 | 75.02 ± 1.69 | 95.11 ± 3.53 | 113.42 ± 17.36 |
| Leu | 209.26 ± 13.59 | 232.83 ± 36.34 | 248.74 ± 8.52 | 230.84 ± 7.23 | 208.88 ± 4.54 | 176.46 ± 21.79 | 207.33 ± 5.03 | 206.11 ± 0.68 | 199.37 ± 12.56 | 199.21 ± 7.02 | 211.75 ± 8.19 | 297.68 ± 47.22 |
| Lys | 66.28 ± 4.20 | 59.74 ± 3.56 | 64.25 ± 4.68 | 54.31 ± 2.28 | 70.38 ± 0.66 | 58.32 ± 3.32 | 65.82 ± 4.36 | 75.83 ± 2.50 | 68.91 ± 0.15 | 35.05 ± 2.91 | 61.27 ± 2.30 | 51.2 ± 0.60 |
| Met + Cys | 75.23 ± 7.10 | 74.73 ± 5.66 | 78.13 ± 1.44 | 73.09 ± 5.71 | 70.93 ± 0.24 | 72.54 ± 8.85 | 64.13 ± 2.42 | 75.08 ± 1.95 | 74.15 ± 0.84 | 50.2 ± 0.19 | 85.71 ± 3.19 | 85.74 ± 9.40 |
| Phe + Tyr | 151.79 ± 2.30 | 150.99 ± 2.34 | 167.42 ± 4.41 | 156.59 ± 1.66 | 158.38 ± 6.41 | 151.59 ± 10.75 | 176.08 ± 13.54 | 191.7 ± 11.20 | 189.74 ± 10.02 | 151.52 ± 0.96 | 173.76 ± 9.50 | 199.63 ± 23.21 |
| Thr | 125.40 ± 1.38 | 129.68 ± 4.83 | 139.45 ± 1.53 | 124.53 ± 1.49 | 133.2 ± 1.27 | 115.15 ± 7.81 | 129.51 ± 3.93 | 141.85 ± 3.45 | 138.25 ± 1.81 | 96.17 ± 1.46 | 127.54 ± 4.80 | 143.5 ± 14.78 |
| Val | 109.44 ± 5.16 | 105.61 ± 4.76 | 117.59 ± 3.28 | 104.28 ± 1.92 | 106.2 ± 3.33 | 95.27 ± 8.98 | 106.08 ± 5.43 | 112.56 ± 0.31 | 106.3 ± 2.73 | 78.27 ± 0.31 | 103.5 ± 3.90 | 118.44 ± 15.68 |
| Trp | 87.96 ± 3.72 | 103.28 ± 4.12 | 87.18 ± 6.78 | 86.77 ± 0.12 | 78.39 ± 2.50 | 78.07 ± 2.32 | 77.97 ± 3.14 | 90.16 ± 2.75 | 87.77 ± 4.85 | 50.36 ± 9.71 | 86.73 ± 4.87 | 69.49 ± 3.72 |
| His | 155.26 ± 0.05 | 167.47 ± 13.79 | 192.75 ± 5.19 | 164.38 ± 5.41 | 147.2 ± 8.78 | 146.56 ± 0.42 | 157.52 ± 17.12 | 188.35 ± 8.50 | 174.68 ± 5.93 | 138.07 ± 7.88 | 181.28 ± 6.81 | 179.97 ± 15.92 |
| Ile | Leu | Lys | Met | Phe + Tyr | Thr | Val | Trp | ||
|---|---|---|---|---|---|---|---|---|---|
| 1–1 | RAA | 0.83 ± 0.04 | 1.58 ± 0.10 | 0.64 ± 0.04 | 0.56 ± 0.05 | 1.13 ± 0.02 | 0.85 ± 0.01 | 1.00 ± 0.05 | 0.62 ± 0.03 |
| RC | 0.92 ± 0.02 | 1.76 ± 0.07 | 0.71 ± 0.06 | 0.62 ± 0.04 | 1.26 ± 0.01 | 0.94 ± 0.03 | 1.11 ± 0.02 | 0.69 ± 0.05 | |
| SRC | 62.35 ± 2.64 | ||||||||
| 1–2 | RAA | 0.83 ± 0.07 | 1.76 ± 0.27 | 0.58 ± 0.03 | 0.55 ± 0.04 | 1.13 ± 0.02 | 0.88 ± 0.03 | 0.97 ± 0.04 | 0.73 ± 0.03 |
| RC | 0.89 ± 0.02 | 1.89 ± 0.18 | 0.62 ± 0.08 | 0.60 ± 0.01 | 1.22 ± 0.06 | 0.95 ± 0.03 | 1.05 ± 0.02 | 0.79 ± 0.02 | |
| SRC | 58.21 ± 5.93 | ||||||||
| 1–3 | RAA | 0.93 ± 0.05 | 1.88 ± 0.06 | 0.62 ± 0.05 | 0.58 ± 0.01 | 1.25 ± 0.03 | 0.94 ± 0.01 | 1.08 ± 0.03 | 0.62 ± 0.05 |
| RC | 0.95 ± 0.02 | 1.91 ± 0.02 | 0.63 ± 0.03 | 0.59 ± 0.03 | 1.26 ± 0.07 | 0.96 ± 0.01 | 1.09 ± 0.00 | 0.62 ± 0.03 | |
| SRC | 55.9 ± 0.51 | ||||||||
| 1–4 | RAA | 0.83 ± 0.03 | 1.75 ± 0.05 | 0.52 ± 0.02 | 0.54 ± 0.04 | 1.17 ± 0.01 | 0.84 ± 0.01 | 0.96 ± 0.02 | 0.61 ± 0.00 |
| RC | 0.92 ± 0.01 | 1.93 ± 0.02 | 0.58 ± 0.01 | 0.60 ± 0.03 | 1.29 ± 0.04 | 0.93 ± 0.01 | 1.06 ± 0.00 | 0.68 ± 0.01 | |
| SRC | 55.00 ± 0.32 | ||||||||
| 1–5 | RAA | 0.85 ± 0.01 | 1.58 ± 0.03 | 0.68 ± 0.01 | 0.52 ± 0.00 | 1.18 ± 0.05 | 0.90 ± 0.01 | 0.97 ± 0.03 | 0.55 ± 0.02 |
| RC | 0.94 ± 0.02 | 1.75 ± 0.01 | 0.75 ± 0.02 | 0.58 ± 0.01 | 1.30 ± 0.03 | 1.00 ± 0.01 | 1.08 ± 0.01 | 0.61 ± 0.01 | |
| SRC | 61.25 ± 0.82 | ||||||||
| 2–1 | RAA | 0.73 ± 0.08 | 1.33 ± 0.16 | 0.56 ± 0.03 | 0.54 ± 0.07 | 1.13 ± 0.08 | 0.78 ± 0.05 | 0.87 ± 0.08 | 0.55 ± 0.02 |
| RC | 0.89 ± 0.03 | 1.64 ± 0.06 | 0.69 ± 0.02 | 0.66 ± 0.03 | 1.39 ± 0.02 | 0.96 ± 0.02 | 1.08 ± 0.01 | 0.68 ± 0.08 | |
| SRC | 64.15 ± 2.18 | ||||||||
| 2–2 | RAA | 0.83 ± 0.05 | 1.57 ± 0.04 | 0.63 ± 0.04 | 0.47 ± 0.02 | 1.31 ± 0.10 | 0.88 ± 0.03 | 0.97 ± 0.05 | 0.55 ± 0.02 |
| RC | 0.92 ± 0.01 | 1.74 ± 0.04 | 0.70 ± 0.01 | 0.53 ± 0.01 | 1.45 ± 0.04 | 0.97 ± 0.02 | 1.08 ± 0.00 | 0.61 ± 0.00 | |
| SRC | 58.01 ± 0.38 | ||||||||
| 2–3 | RAA | 0.89 ± 0.01 | 1.56 ± 0.01 | 0.73 ± 0.02 | 0.56 ± 0.01 | 1.43 ± 0.08 | 0.96 ± 0.02 | 1.03 ± 0.00 | 0.64 ± 0.02 |
| RC | 0.91 ± 0.02 | 1.60 ± 0.01 | 0.75 ± 0.03 | 0.57 ± 0.02 | 1.47 ± 0.07 | 0.99 ± 0.01 | 1.06 ± 0.01 | 0.65 ± 0.01 | |
| SRC | 62.94 ± 1.52 | ||||||||
| 2–4 | RAA | 0.83 ± 0.02 | 1.51 ± 0.10 | 0.66 ± 0.00 | 0.55 ± 0.01 | 1.41 ± 0.07 | 0.94 ± 0.01 | 0.98 ± 0.03 | 0.62 ± 0.03 |
| RC | 0.89 ± 0.00 | 1.61 ± 0.08 | 0.71 ± 0.01 | 0.59 ± 0.00 | 1.51 ± 0.10 | 1.00 ± 0.00 | 1.04 ± 0.01 | 0.66 ± 0.03 | |
| SRC | 61.75 ± 0.37 | ||||||||
| 3–1 | RAA | 0.66 ± 0.01 | 1.51 ± 0.05 | 0.34 ± 0.03 | 0.37 ± 0.00 | 1.13 ± 0.01 | 0.65 ± 0.01 | 0.72 ± 0.00 | 0.36 ± 0.07 |
| RC | 0.92 ± 0.03 | 2.10 ± 0.04 | 0.47 ± 0.05 | 0.52 ± 0.01 | 1.58 ± 0.01 | 0.91 ± 0.03 | 1.00 ± 0.01 | 0.50 ± 0.09 | |
| SRC | 42.24 ± 0.72 | ||||||||
| 3–2 | RAA | 0.84 ± 0.03 | 1.6 ± 0.06 | 0.59 ± 0.02 | 0.63 ± 0.02 | 1.29 ± 0.07 | 0.86 ± 0.03 | 0.95 ± 0.04 | 0.61 ± 0.03 |
| RC | 0.91 ± 0.00 | 1.74 ± 0.01 | 0.64 ± 0.00 | 0.69 ± 0.00 | 1.40 ± 0.02 | 0.93 ± 0.00 | 1.03 ± 0.00 | 0.66 ± 0.01 | |
| SRC | 61.12 ± 0.07 | ||||||||
| 3–3 | RAA | 1.00 ± 0.15 | 2.25 ± 0.36 | 0.49 ± 0.01 | 0.63 ± 0.07 | 1.49 ± 0.17 | 0.97 ± 0.10 | 1.09 ± 0.14 | 0.49 ± 0.03 |
| RC | 0.95 ± 0.03 | 2.14 ± 0.08 | 0.47 ± 0.06 | 0.60 ± 0.01 | 1.41 ± 0.01 | 0.92 ± 0.02 | 1.03 ± 0.01 | 0.47 ± 0.03 | |
| SRC | 44.05 ± 3.64 | ||||||||
| Ile | Leu | Lys | Met | Phe + Tyr | Thr | Val | Trp | His | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–1 | RAA | 1.32 ± 0.06 | 2.01 ± 0.13 | 0.69 ± 0.04 | 0.78 ± 0.07 | 1.44 ± 0.02 | 1.26 ± 0.01 | 1.57 ± 0.07 | 0.89 ± 0.04 | 1.52 ± 0.00 |
| RC | 1.04 ± 0.02 | 1.58 ± 0.07 | 0.54 ± 0.05 | 0.61 ± 0.04 | 1.13 ± 0.01 | 0.99 ± 0.03 | 1.23 ± 0.03 | 0.70 ± 0.05 | 1.19 ± 0.03 | |
| SRC | 66.38 ± 2.16 | |||||||||
| 1–2 | RAA | 1.32 ± 0.11 | 2.24 ± 0.35 | 0.62 ± 0.04 | 0.77 ± 0.06 | 1.44 ± 0.02 | 1.30 ± 0.05 | 1.51 ± 0.07 | 1.04 ± 0.04 | 1.64 ± 0.14 |
| RC | 1.00 ± 0.01 | 1.69 ± 0.15 | 0.47 ± 0.06 | 0.59 ± 0.01 | 1.09 ± 0.06 | 0.98 ± 0.03 | 1.15 ± 0.02 | 0.79 ± 0.02 | 1.24 ± 0.02 | |
| SRC | 63.43 ± 4.62 | |||||||||
| 1–3 | RAA | 1.49 ± 0.08 | 2.39 ± 0.08 | 0.67 ± 0.05 | 0.81 ± 0.01 | 1.59 ± 0.04 | 1.40 ± 0.02 | 1.69 ± 0.05 | 0.88 ± 0.07 | 1.89 ± 0.05 |
| RC | 1.05 ± 0.04 | 1.68 ± 0.03 | 0.47 ± 0.03 | 0.57 ± 0.02 | 1.12 ± 0.05 | 0.98 ± 0.01 | 1.18 ± 0.01 | 0.62 ± 0.04 | 1.33 ± 0.06 | |
| SRC | 60.56 ± 0.69 | |||||||||
| 1–4 | RAA | 1.33 ± 0.04 | 2.22 ± 0.07 | 0.56 ± 0.02 | 0.76 ± 0.06 | 1.49 ± 0.02 | 1.25 ± 0.01 | 1.50 ± 0.03 | 0.87 ± 0.00 | 1.61 ± 0.05 |
| RC | 1.03 ± 0.01 | 1.73 ± 0.01 | 0.44 ± 0.01 | 0.59 ± 0.03 | 1.16 ± 0.04 | 0.97 ± 0.01 | 1.16 ± 0.01 | 0.68 ± 0.02 | 1.25 ± 0.01 | |
| SRC | 60.75 ± 0.22 | |||||||||
| 1–5 | RAA | 1.35 ± 0.01 | 2.01 ± 0.04 | 0.73 ± 0.01 | 0.73 ± 0.00 | 1.51 ± 0.06 | 1.33 ± 0.01 | 1.52 ± 0.05 | 0.79 ± 0.03 | 1.44 ± 0.09 |
| RC | 1.07 ± 0.03 | 1.58 ± 0.00 | 0.58 ± 0.02 | 0.58 ± 0.01 | 1.19 ± 0.02 | 1.05 ± 0.01 | 1.20 ± 0.01 | 0.62 ± 0.01 | 1.14 ± 0.04 | |
| SRC | 65.67 ± 0.66 | |||||||||
| 2–1 | RAA | 1.16 ± 0.14 | 1.70 ± 0.21 | 0.61 ± 0.03 | 0.75 ± 0.09 | 1.44 ± 0.10 | 1.15 ± 0.08 | 1.37 ± 0.13 | 0.79 ± 0.02 | 1.44 ± 0.00 |
| RC | 1.01 ± 0.04 | 1.47 ± 0.07 | 0.52 ± 0.01 | 0.65 ± 0.03 | 1.25 ± 0.00 | 1.00 ± 0.01 | 1.18 ± 0.02 | 0.68 ± 0.07 | 1.25 ± 0.09 | |
| SRC | 67.74 ± 1.25 | |||||||||
| 2–2 | RAA | 1.32 ± 0.08 | 2.00 ± 0.05 | 0.68 ± 0.05 | 0.66 ± 0.03 | 1.67 ± 0.13 | 1.30 ± 0.04 | 1.52 ± 0.08 | 0.79 ± 0.03 | 1.54 ± 0.17 |
| RC | 1.04 ± 0.00 | 1.56 ± 0.05 | 0.54 ± 0.01 | 0.52 ± 0.01 | 1.31 ± 0.03 | 1.02 ± 0.03 | 1.19 ± 0.01 | 0.62 ± 0.01 | 1.21 ± 0.06 | |
| SRC | 63.01 ± 0.07 | |||||||||
| 2–3 | RAA | 1.42 ± 0.02 | 1.98 ± 0.01 | 0.79 ± 0.03 | 0.78 ± 0.02 | 1.82 ± 0.11 | 1.42 ± 0.03 | 1.61 ± 0.00 | 0.91 ± 0.03 | 1.85 ± 0.08 |
| RC | 1.01 ± 0.02 | 1.42 ± 0.00 | 0.56 ± 0.02 | 0.56 ± 0.02 | 1.30 ± 0.07 | 1.02 ± 0.02 | 1.15 ± 0.00 | 0.65 ± 0.02 | 1.32 ± 0.06 | |
| SRC | 66.25 ± 0.45 | |||||||||
| 2–4 | RAA | 1.33 ± 0.03 | 1.92 ± 0.12 | 0.72 ± 0.00 | 0.77 ± 0.01 | 1.80 ± 0.10 | 1.39 ± 0.02 | 1.52 ± 0.04 | 0.89 ± 0.05 | 1.71 ± 0.06 |
| RC | 1.00 ± 0.01 | 1.43 ± 0.08 | 0.53 ± 0.01 | 0.57 ± 0.00 | 1.35 ± 0.08 | 1.03 ± 0.00 | 1.14 ± 0.02 | 0.66 ± 0.03 | 1.28 ± 0.05 | |
| SRC | 65.91 ± 0.59 | |||||||||
| 3–1 | RAA | 1.06 ± 0.02 | 1.92 ± 0.07 | 0.36 ± 0.03 | 0.52 ± 0.00 | 1.44 ± 0.01 | 0.96 ± 0.01 | 1.12 ± 0.00 | 0.51 ± 0.10 | 1.35 ± 0.08 |
| RC | 1.03 ± 0.03 | 1.87 ± 0.06 | 0.35 ± 0.03 | 0.51 ± 0.00 | 1.40 ± 0.00 | 0.94 ± 0.02 | 1.09 ± 0.00 | 0.49 ± 0.09 | 1.32 ± 0.08 | |
| SRC | 50.59 ± 0.01 | |||||||||
| 3–2 | RAA | 1.34 ± 0.05 | 2.04 ± 0.08 | 0.64 ± 0.02 | 0.89 ± 0.03 | 1.65 ± 0.09 | 1.28 ± 0.05 | 1.48 ± 0.06 | 0.87 ± 0.05 | 1.78 ± 0.07 |
| RC | 1.01 ± 0.00 | 1.53 ± 0.00 | 0.48 ± 0.00 | 0.67 ± 0.00 | 1.24 ± 0.02 | 0.96 ± 0.00 | 1.12 ± 0.00 | 0.66 ± 0.01 | 1.34 ± 0.01 | |
| SRC | 65.16 ± 0.06 | |||||||||
| 3–3 | RAA | 1.60 ± 0.25 | 2.87 ± 0.45 | 0.53 ± 0.01 | 0.89 ± 0.10 | 1.90 ± 0.22 | 1.44 ± 0.15 | 1.70 ± 0.22 | 0.70 ± 0.04 | 1.76 ± 0.16 |
| RC | 1.07 ± 0.04 | 1.92 ± 0.08 | 0.36 ± 0.05 | 0.60 ± 0.00 | 1.28 ± 0.00 | 0.97 ± 0.01 | 1.14 ± 0.02 | 0.47 ± 0.03 | 1.19 ± 0.04 | |
| SRC | 52.04 ± 3.11 | |||||||||
| Ile | Leu | Lys | Phe + Tyr | Thr | Val | Trp | ||
|---|---|---|---|---|---|---|---|---|
| 1–1 | RAA | 0.50 ± 0.02 | 1.52 ± 0.10 | 0.64 ± 0.04 | 0.68 ± 0.01 | 0.67 ± 0.01 | 0.78 ± 0.04 | 0.39 ± 0.02 |
| RC | 0.68 ± 0.02 | 2.05 ± 0.09 | 0.86 ± 0.07 | 0.92 ± 0.00 | 0.90 ± 0.03 | 1.06 ± 0.03 | 0.53 ± 0.03 | |
| SRC | 50.36 ± 4.10 | |||||||
| 1–2 | RAA | 0.50 ± 0.04 | 1.69 ± 0.26 | 0.58 ± 0.03 | 0.68 ± 0.01 | 0.69 ± 0.03 | 0.76 ± 0.03 | 0.46 ± 0.02 |
| RC | 0.66 ± 0.01 | 2.21 ± 0.20 | 0.76 ± 0.10 | 0.89 ± 0.05 | 0.90 ± 0.03 | 0.99 ± 0.02 | 0.60 ± 0.02 | |
| SRC | 44.87 ± 8.26 | |||||||
| 1–3 | RAA | 0.57 ± 0.03 | 1.80 ± 0.06 | 0.62 ± 0.05 | 0.75 ± 0.02 | 0.74 ± 0.01 | 0.84 ± 0.02 | 0.38 ± 0.03 |
| RC | 0.69 ± 0.01 | 2.21 ± 0.01 | 0.76 ± 0.03 | 0.92 ± 0.05 | 0.91 ± 0.02 | 1.03 ± 0.00 | 0.47 ± 0.02 | |
| SRC | 43.39 ± 0.28 | |||||||
| 1–4 | RAA | 0.50 ± 0.02 | 1.67 ± 0.05 | 0.52 ± 0.02 | 0.70 ± 0.01 | 0.66 ± 0.01 | 0.75 ± 0.01 | 0.38 ± 0.00 |
| RC | 0.68 ± 0.01 | 2.26 ± 0.03 | 0.70 ± 0.02 | 0.94 ± 0.03 | 0.89 ± 0.01 | 1.01 ± 0.00 | 0.52 ± 0.01 | |
| SRC | 41.96 ± 0.92 | |||||||
| 1–5 | RAA | 0.51 ± 0.00 | 1.51 ± 0.03 | 0.34 ± 0.48 | 0.71 ± 0.03 | 0.71 ± 0.01 | 0.76 ± 0.02 | 0.35 ± 0.01 |
| RC | 0.74 ± 0.05 | 2.18 ± 0.22 | 0.46 ± 0.65 | 1.02 ± 0.12 | 1.02 ± 0.09 | 1.09 ± 0.12 | 0.50 ± 0.05 | |
| SRC | 39.62 ± 16.03 | |||||||
| 2–1 | RAA | 0.44 ± 0.05 | 1.28 ± 0.16 | 0.56 ± 0.03 | 0.68 ± 0.05 | 0.61 ± 0.04 | 0.68 ± 0.06 | 0.34 ± 0.01 |
| RC | 0.67 ± 0.02 | 1.94 ± 0.08 | 0.86 ± 0.02 | 1.03 ± 0.01 | 0.93 ± 0.01 | 1.04 ± 0.01 | 0.53 ± 0.06 | |
| SRC | 54.26 ± 3.60 | |||||||
| 2–2 | RAA | 0.50 ± 0.03 | 1.50 ± 0.04 | 0.63 ± 0.04 | 0.79 ± 0.06 | 0.69 ± 0.02 | 0.76 ± 0.04 | 0.34 ± 0.01 |
| RC | 0.67 ± 0.01 | 2.02 ± 0.05 | 0.85 ± 0.02 | 1.06 ± 0.03 | 0.92 ± 0.01 | 1.02 ± 0.00 | 0.46 ± 0.00 | |
| SRC | 50.54 ± 1.58 | |||||||
| 2–3 | RAA | 0.54 ± 0.01 | 1.49 ± 0.00 | 0.73 ± 0.02 | 0.86 ± 0.05 | 0.75 ± 0.02 | 0.81 ± 0.00 | 0.40 ± 0.01 |
| RC | 0.67 ± 0.02 | 1.88 ± 0.01 | 0.92 ± 0.04 | 1.08 ± 0.05 | 0.94 ± 0.01 | 1.01 ± 0.01 | 0.50 ± 0.01 | |
| SRC | 56.35 ± 0.19 | |||||||
| 2–4 | RAA | 0.51 ± 0.01 | 1.45 ± 0.09 | 0.66 ± 0.00 | 0.85 ± 0.04 | 0.73 ± 0.01 | 0.76 ± 0.02 | 0.39 ± 0.02 |
| RC | 0.66 ± 0.00 | 1.89 ± 0.08 | 0.87 ± 0.02 | 1.11 ± 0.08 | 0.96 ± 0.01 | 1.00 ± 0.01 | 0.51 ± 0.02 | |
| SRC | 55.45 ± 2.17 | |||||||
| 3–1 | RAA | 0.40 ± 0.01 | 1.44 ± 0.05 | 0.34 ± 0.03 | 0.68 ± 0.00 | 0.51 ± 0.01 | 0.56 ± 0.00 | 0.22 ± 0.04 |
| RC | 0.68 ± 0.02 | 2.43 ± 0.05 | 0.57 ± 0.05 | 1.14 ± 0.01 | 0.86 ± 0.02 | 0.95 ± 0.01 | 0.37 ± 0.07 | |
| SRC | 31.85 ± 1.68 | |||||||
| 3–2 | RAA | 0.51 ± 0.02 | 1.54 ± 0.06 | 0.59 ± 0.02 | 0.78 ± 0.04 | 0.68 ± 0.03 | 0.74 ± 0.03 | 0.38 ± 0.02 |
| RC | 0.68 ± 0.00 | 2.06 ± 0.01 | 0.79 ± 0.00 | 1.04 ± 0.01 | 0.91 ± 0.00 | 1.00 ± 0.00 | 0.51 ± 0.01 | |
| SRC | 49.68 ± 0.26 | |||||||
| 3–3 | RAA | 0.61 ± 0.09 | 2.16 ± 0.34 | 0.49 ± 0.01 | 0.89 ± 0.10 | 0.76 ± 0.08 | 0.85 ± 0.11 | 0.31 ± 0.02 |
| RC | 0.70 ± 0.02 | 2.48 ± 0.09 | 0.57 ± 0.08 | 1.03 ± 0.01 | 0.88 ± 0.02 | 0.98 ± 0.01 | 0.36 ± 0.02 | |
| SRC | 30.33 ± 4.30 | |||||||
| Umami Amino Acids | Sweet Amino Acids | Bitter Amino Acids | |
|---|---|---|---|
| Umami amino acids | 1 | ||
| Sweet amino acids | 0.931 ** | 1 | |
| Bitter amino acids | 0.973 ** | 0.904 ** | 1 |
| Amino Acids | Taste Threshold (mg/g) | TVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–1 | 1–2 | 1–3 | 1–4 | 1–5 | 2–1 | 2–2 | 2–3 | 2–4 | 3–1 | 3–2 | 3–3 | ||
| Umami amino acids | |||||||||||||
| Asp | 1.0 | 2.45 ± 0.02 | 2.33 ± 0.07 | 2.24 ± 0.12 | 2.18 ± 0.04 | 2.62 ± 0.02 | 2.25 ± 0.12 | 2.58 ± 0.11 | 2.29 ± 0.05 | 2.22 ± 0.06 | 1.82 ± 0.02 | 1.88 ± 0.02 | 2.50 ± 0.17 |
| Glu | 0.3 | 24.55 ± 1.4 | 26.11 ± 2.43 | 24.67 ± 0.03 | 24.17 ± 0.62 | 24.29 ± 0.02 | 16.77 ± 1.49 | 21.62 ± 0.64 | 18.6 ± 0.03 | 19.01 ± 0.78 | 18.82 ± 0.07 | 16.33 ± 0.15 | 27.78 ± 3.25 |
| Sweet amino acids | |||||||||||||
| Gly | 1.3 | 1.05 ± 0.02 | 1.07 ± 0.01 | 0.97 ± 0.05 | 0.93 ± 0.02 | 1.01 ± 0.03 | 0.79 ± 0.03 | 0.91 ± 0.04 | 0.87 ± 0.02 | 0.82 ± 0.04 | 0.65 ± 0.04 | 0.85 ± 0.01 | 0.99 ± 0.01 |
| Ala | 0.6 | 5.96 ± 0.11 | 6.55 ± 0.00 | 6.07 ± 0.17 | 6.70 ± 0.07 | 7.47 ± 0.01 | 4.83 ± 0.31 | 6.60 ± 0.30 | 5.46 ± 0.16 | 4.33 ± 0.14 | 4.06 ± 0.09 | 3.79 ± 0.03 | 5.39 ± 0.60 |
| Ser | 1.5 | 1.21 ± 0.01 | 1.25 ± 0.06 | 1.19 ± 0.05 | 1.14 ± 0.04 | 1.22 ± 0.01 | 0.81 ± 0.03 | 1.04 ± 0.01 | 0.97 ± 0.01 | 1.00 ± 0.03 | 0.87 ± 0.00 | 0.84 ± 0.01 | 1.21 ± 0.03 |
| Pro | 3.0 | 1.03 ± 0.07 | 1.04 ± 0.12 | 0.99 ± 0.10 | 1.02 ± 0.10 | 0.89 ± 0.08 | 0.69 ± 0.02 | 0.90 ± 0.04 | 0.78 ± 0.01 | 0.77 ± 0.04 | 0.79 ± 0.01 | 0.75 ± 0.01 | 1.09 ± 0.06 |
| Thr | 2.6 | 1.60 ± 1.52 | 1.61 ± 1.54 | 1.55 ± 1.48 | 1.53 ± 1.45 | 1.53 ± 1.38 | 1.37 ± 1.35 | 1.40 ± 1.21 | 1.41 ± 1.27 | 1.37 ± 1.22 | 1.26 ± 1.17 | 1.30 ± 1.16 | 1.31 ± 0.90 |
| Met | 0.3 | 2.61 ± 0.22 | 2.58 ± 0.08 | 2.36 ± 0.00 | 2.44 ± 0.14 | 2.40 ± 0.04 | 2.03 ± 0.22 | 1.95 ± 0.06 | 1.98 ± 0.07 | 1.97 ± 0.04 | 1.56 ± 0.04 | 2.16 ± 0.02 | 2.61 ± 0.18 |
| Bitter amino acids | |||||||||||||
| Ile | 0.9 | 1.48 ± 0.05 | 1.47 ± 0.05 | 1.45 ± 0.10 | 1.43 ± 0.01 | 1.47 ± 0.04 | 1.05 ± 0.11 | 1.29 ± 0.07 | 1.20 ± 0.03 | 1.14 ± 0.03 | 1.06 ± 0.06 | 1.09 ± 0.01 | 1.57 ± 0.18 |
| Arg | 0.5 | 3.08 ± 0.1 | 3.29 ± 0.28 | 2.98 ± 0.08 | 2.79 ± 0.19 | 2.99 ± 0.08 | 2.33 ± 0.21 | 2.72 ± 0.19 | 2.60 ± 0.01 | 2.52 ± 0.07 | 1.84 ± 0.10 | 2.31 ± 0.02 | 3.01 ± 0.05 |
| Leu | 1.9 | 2.35 ± 0.13 | 2.59 ± 0.29 | 2.43 ± 0.13 | 2.49 ± 0.02 | 2.28 ± 0.00 | 1.59 ± 0.17 | 2.03 ± 0.03 | 1.75 ± 0.01 | 1.71 ± 0.12 | 2.00 ± 0.01 | 1.72 ± 0.02 | 2.93 ± 0.34 |
| Val | 0.4 | 5.05 ± 0.19 | 4.85 ± 0.00 | 4.72 ± 0.21 | 4.64 ± 0.02 | 4.77 ± 0.04 | 3.54 ± 0.28 | 4.28 ± 0.19 | 3.94 ± 0.02 | 3.75 ± 0.13 | 3.23 ± 0.09 | 3.46 ± 0.03 | 4.80 ± 0.44 |
| Lys | 0.5 | 2.83 ± 0.20 | 2.54 ± 0.26 | 2.38 ± 0.21 | 2.23 ± 0.04 | 2.92 ± 0.09 | 2.00 ± 0.09 | 2.45 ± 0.14 | 2.45 ± 0.10 | 2.24 ± 0.01 | 1.34 ± 0.15 | 1.89 ± 0.02 | 1.92 ± 0.10 |
| His | 0.2 | 5.52 ± 0.05 | 5.91 ± 0.22 | 5.95 ± 0.06 | 5.62 ± 0.06 | 5.08 ± 0.19 | 4.19 ± 0.05 | 4.89 ± 0.49 | 5.07 ± 0.27 | 4.74 ± 0.12 | 4.39 ± 0.39 | 4.67 ± 0.04 | 5.61 ± 0.27 |
| Phe | 0.9 | 1.66 ± 0.08 | 1.63 ± 0.09 | 1.43 ± 0.09 | 1.63 ± 0.06 | 1.64 ± 0.07 | 1.41 ± 0.04 | 1.70 ± 0.18 | 1.66 ± 0.17 | 1.70 ± 0.13 | 1.56 ± 0.02 | 1.38 ± 0.06 | 2.01 ± 0.16 |
| 11–12-Year-Olds | Adults | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 1–1 | 12.68 ± 0.18 | 13.59 ± 0.20 | 11.15 ± 0.15 | 13.15 ± 0.18 |
| 1–2 | 13.09 ± 0.22 | 14.02 ± 0.24 | 11.42 ± 0.17 | 13.47 ± 0.20 |
| 1–3 | 12.06 ± 0.50 | 12.92 ± 0.53 | 10.59 ± 0.43 | 12.49 ± 0.51 |
| 1–4 | 12.31 ± 0.05 | 13.19 ± 0.06 | 10.74 ± 0.01 | 12.68 ± 0.02 |
| 1–5 | 12.30 ± 0.01 | 13.18 ± 0.01 | 10.88 ± 0.04 | 12.84 ± 0.05 |
| 2–1 | 9.25 ± 0.59 | 9.91 ± 0.63 | 8.12 ± 0.54 | 9.58 ± 0.64 |
| 2–2 | 11.16 ± 0.48 | 11.95 ± 0.51 | 9.77 ± 0.40 | 11.52 ± 0.47 |
| 2–3 | 10.46 ± 0.09 | 11.21 ± 0.10 | 9.18 ± 0.04 | 10.83 ± 0.05 |
| 2–4 | 10.20 ± 0.20 | 10.93 ± 0.21 | 8.93 ± 0.18 | 10.54 ± 0.21 |
| 3–1 | 9.14 ± 0.09 | 9.79 ± 0.09 | 7.90 ± 0.14 | 9.32 ± 0.16 |
| 3–2 | 9.49 ± 0.15 | 10.17 ± 0.16 | 8.32 ± 0.12 | 9.81 ± 0.14 |
| 3–3 | 13.00 ± 1.04 | 13.93 ± 1.11 | 11.36 ± 0.88 | 13.4 ± 1.04 |
| Asp | Thr | Ser | Glu | Gly | Ala | Val | Met | Ile | Leu | Tyr | Phe | Lys | His | Arg | Pro | Trp | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp | 1 | ||||||||||||||||
| Thr | 0.767 ** | 1 | |||||||||||||||
| Ser | 0.648 ** | 0.964 ** | 1 | ||||||||||||||
| Glu | 0.595 ** | 0.892 ** | 0.940 ** | 1 | |||||||||||||
| Gly | 0.702 ** | 0.931 ** | 0.871 ** | 0.764 ** | 1 | ||||||||||||
| Ala | 0.760 ** | 0.833 ** | 0.769 ** | 0.700 ** | 0.737 ** | 1 | |||||||||||
| Val | 0.725 ** | 0.969 ** | 0.949 ** | 0.914 ** | 0.921 ** | 0.813 ** | 1 | ||||||||||
| Met | 0.516 ** | 0.823 ** | 0.798 ** | 0.793 ** | 0.880 ** | 0.566 ** | 0.871 ** | 1 | |||||||||
| Ile | 0.675 ** | 0.947 ** | 0.947 ** | 0.959 ** | 0.866 ** | 0.756 ** | 0.968 ** | 0.857 ** | 1 | ||||||||
| Leu | 0.424 * | 0.778 ** | 0.848 ** | 0.963 ** | 0.660 ** | 0.565 ** | 0.819 ** | 0.754 ** | 0.909 ** | 1 | |||||||
| Tyr | 0.656 ** | 0.903 ** | 0.901 ** | 0.893 ** | 0.780 ** | 0.748 ** | 0.890 ** | 0.675 ** | 0.935 ** | 0.837 ** | 1 | ||||||
| Phe | 0.530 ** | 0.478 * | 0.441 * | 0.543 ** | 0.296 | 0.263 | 0.429 * | 0.301 | 0.497 * | 0.508 * | 0.489 * | 1 | |||||
| Lys | 0.735 ** | 0.736 ** | 0.625 ** | 0.388 | 0.790 ** | 0.739 ** | 0.687 ** | 0.523 ** | 0.548 ** | 0.181 | 0.530 ** | 0.094 | 1 | ||||
| His | 0.377 | 0.825 ** | 0.849 ** | 0.831 ** | 0.793 ** | 0.596 ** | 0.845 ** | 0.768 ** | 0.853 ** | 0.794 ** | 0.821 ** | 0.331 | 0.465 * | 1 | |||
| Arg | 0.723 ** | 0.936 ** | 0.873 ** | 0.773 ** | 0.954 ** | 0.740 ** | 0.912 ** | 0.823 ** | 0.847 ** | 0.656 ** | 0.794 ** | 0.371 | 0.767 ** | 0.787 ** | 1 | ||
| Pro | 0.434 * | 0.787 ** | 0.858 ** | 0.927 ** | 0.716 ** | 0.591 ** | 0.839 ** | 0.781 ** | 0.875 ** | 0.912 ** | 0.787 ** | 0.460* | 0.300 | 0.837 ** | 0.694 ** | 1 | |
| Trp | 0.424 * | 0.731 ** | 0.696 ** | 0.544 ** | 0.812 ** | 0.656 ** | 0.728 ** | 0.689 ** | 0.599 ** | 0.434 * | 0.476 * | 0.022 | 0.727 ** | 0.672 ** | 0.810 ** | 0.536 ** | 1 |
| Eigenvalue | Variance Contribution Rate (%) | Cumulative Variance Contribution Rate (%) | Eigenvalue of Rotation | Rotation Variance Contribution Rate (%) | The Cumulative Variance Contribution after Rotation (%) | |
|---|---|---|---|---|---|---|
| 1 | 12.597 | 74.103 | 74.103 | 7.832 | 46.072 | 46.072 |
| 2 | 1.744 | 10.261 | 84.363 | 5.686 | 33.448 | 79.519 |
| 3 | 1.133 | 6.663 | 91.026 | 1.956 | 11.507 | 91.026 |
| Amino Acids | Principal Component | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Asp | 0.166 | 0.739 | 0.604 |
| Thr | 0.673 | 0.670 | 0.289 |
| Ser | 0.776 | 0.534 | 0.236 |
| Glu | 0.871 | 0.305 | 0.348 |
| Gly | 0.617 | 0.745 | 0.064 |
| Ala | 0.402 | 0.739 | 0.258 |
| Val | 0.737 | 0.616 | 0.232 |
| Met | 0.766 | 0.470 | 0.005 |
| Ile | 0.813 | 0.461 | 0.316 |
| Leu | 0.930 | 0.101 | 0.296 |
| Tyr | 0.724 | 0.434 | 0.395 |
| Phe | 0.321 | 0.017 | 0.846 |
| Lys | 0.101 | 0.971 | 0.041 |
| His | 0.860 | 0.359 | 0.004 |
| Arg | 0.594 | 0.738 | 0.136 |
| Pro | 0.912 | 0.197 | 0.196 |
| Trp | 0.480 | 0.738 | –0.299 |
| Amino Acids | Weighted Score |
|---|---|
| Asp | 0.0553 |
| Thr | 0.0676 |
| Ser | 0.0653 |
| Glu | 0.0634 |
| Gly | 0.0618 |
| Ala | 0.0568 |
| Val | 0.0668 |
| Met | 0.0558 |
| Ile | 0.0662 |
| Leu | 0.0562 |
| Tyr | 0.0631 |
| Phe | 0.0393 |
| Lys | 0.0463 |
| His | 0.0556 |
| Arg | 0.0624 |
| Pro | 0.0566 |
| Trp | 0.0616 |
| Regions | Y1 | Y2 | Y3 | Composite Score | Sort |
|---|---|---|---|---|---|
| 1–1 | 0.588 | 0.952 | –0.241 | 0.620 | 3 |
| 1–2 | 1.186 | 0.804 | –0.919 | 0.780 | 1 |
| 1–3 | 1.063 | 0.144 | –0.880 | 0.480 | 5 |
| 1–4 | 0.984 | –0.092 | –0.428 | 0.410 | 6 |
| 1–5 | –0.306 | 1.459 | 0.773 | 0.480 | 4 |
| 2–1 | –1.436 | –0.082 | –0.561 | –0.830 | 11 |
| 2–2 | –0.791 | 0.600 | 1.199 | –0.030 | 7 |
| 2–3 | –0.954 | 0.355 | 0.229 | –0.325 | 8 |
| 2–4 | –0.909 | –0.149 | 0.293 | –0.480 | 9 |
| 3–1 | –0.393 | –2.251 | 0.132 | –1.010 | 12 |
| 3–2 | –0.468 | –0.795 | –1.507 | –0.720 | 10 |
| 3–3 | 1.435 | –0.944 | 1.909 | 0.620 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Hong, T.; Shen, G.; Gu, Y.; Guo, Y.; Han, J. Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet–Waxy Corn from Three Different Regions of China. Nutrients 2022, 14, 3887. https://doi.org/10.3390/nu14193887
Li Z, Hong T, Shen G, Gu Y, Guo Y, Han J. Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet–Waxy Corn from Three Different Regions of China. Nutrients. 2022; 14(19):3887. https://doi.org/10.3390/nu14193887
Chicago/Turabian StyleLi, Ziqi, Tiannuo Hong, Guohui Shen, Yanting Gu, Yanzhi Guo, and Juan Han. 2022. "Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet–Waxy Corn from Three Different Regions of China" Nutrients 14, no. 19: 3887. https://doi.org/10.3390/nu14193887
APA StyleLi, Z., Hong, T., Shen, G., Gu, Y., Guo, Y., & Han, J. (2022). Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet–Waxy Corn from Three Different Regions of China. Nutrients, 14(19), 3887. https://doi.org/10.3390/nu14193887