Sex Difference in the Associations among Hyperuricemia with New-Onset Chronic Kidney Disease in a Large Taiwanese Population Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. TWB
2.2. Laboratory, Demographic, and Medical Variables
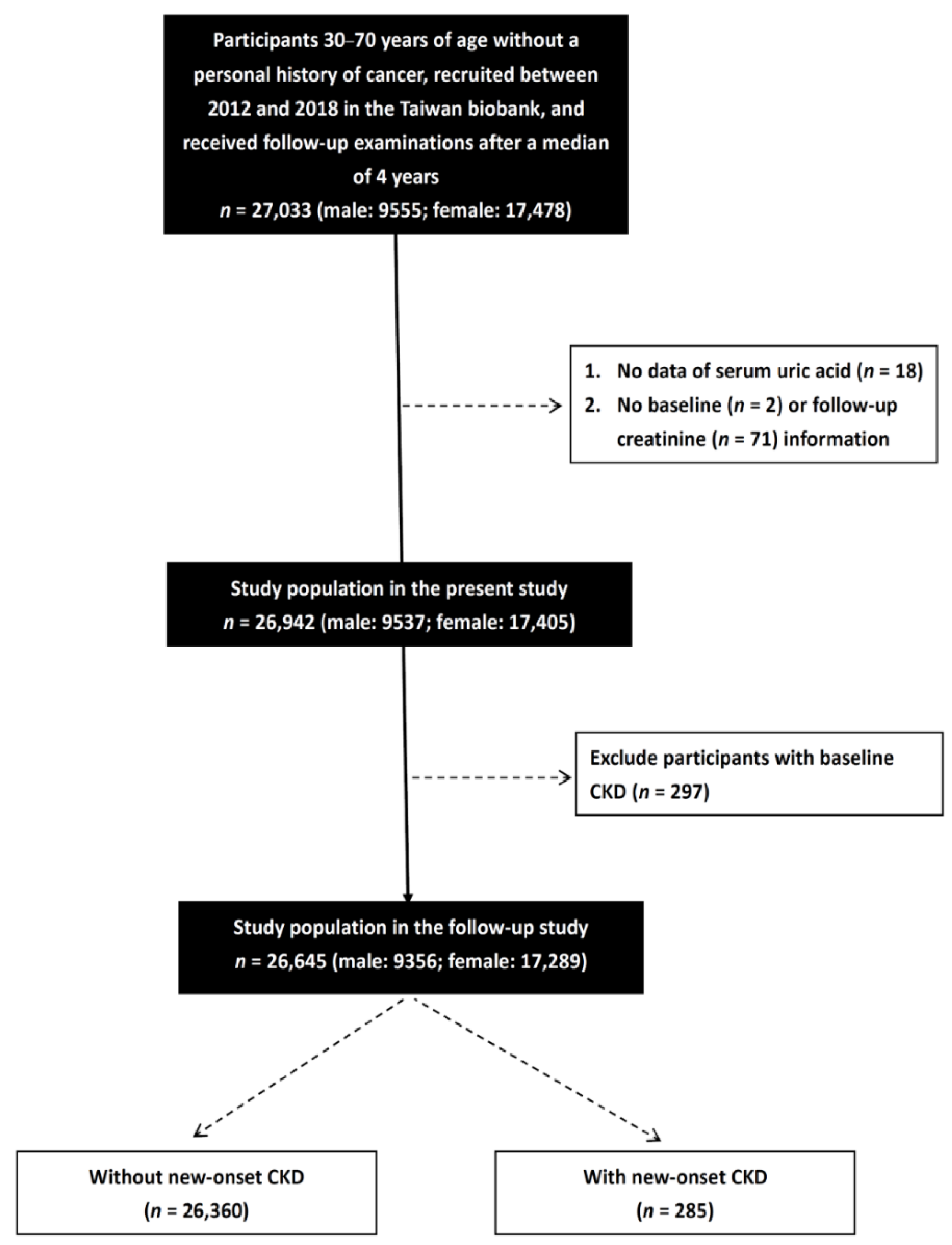
2.3. Study Participants
2.4. Definition of New-Onset CKD
2.5. Definition of Hyperuricemia
2.6. Ethics Statement
2.7. Statistical Analysis
3. Results
3.1. Comparison of Clinical Characteristics among the New-Onset CKD Groups
3.2. Determinants of New-Onset CKD
3.3. Clinical Characteristics of the Study Participants Classified by the Presence of Different Sex and Hyperuricemia
3.4. Associations of Hyperuricemia and Quartiles of Serum UA with New-Onset CKD by Sex
3.5. Interactions among Hyperuricemia, Quartiles of Serum UA and Sex on New-Onset CKD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, T.S.; Hsu, C.C.; Lin, M.H.; Wu, V.C.; Chen, Y.M. Trends in the incidence and prevalence of end-stage kidney disease requiring dialysis in Taiwan: 2010–2018. J. Formos. Med. Assoc. 2022, 121, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Yagisawa, T.; Nakai, S.; Nakayama, M.; Imai, E.; Hattori, M.; Iseki, K.; Akiba, T. Prevalence and incidence of chronic kidney disease stage G5 in Japan. Clin. Exp. Nephrol. 2015, 19, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef]
- Nelson, A.J.; Raggi, P.; Wolf, M.; Gold, A.M.; Chertow, G.M.; Roe, M.T. Targeting Vascular Calcification in Chronic Kidney Disease. JACC Basic Transl. Sci. 2020, 5, 398–412. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.L.; Dua, P.; Gurrell, R.; Loudon, P.; Pike, A.; Storer, R.I.; Vangjeli, C. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front. Med. 2018, 5, 160. [Google Scholar] [CrossRef]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef]
- Ben-Dov, Z.I.; Kark, J.D. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: The Jerusalem Lipid Research Clinic cohort study. Nephrol. Dial. Transplant. 2011, 26, 2558–2566. [Google Scholar] [CrossRef]
- Iseki, K.; Oshiro, S.; Tozawa, M.; Iseki, C.; Ikemiya, Y.; Takishita, S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens. Res. 2001, 24, 691–697. [Google Scholar] [CrossRef]
- Obermayr, R.P.; Temml, C.; Gutjahr, G.; Knechtelsdorfer, M.; Oberbauer, R.; Klauser-Braun, R. Elevated uric acid increases the risk for kidney disease. J. Am. Soc. Nephrol. 2008, 19, 2407–2413. [Google Scholar] [CrossRef]
- Weiner, D.E.; Tighiouart, H.; Elsayed, E.F.; Griffith, J.L.; Salem, D.N.; Levey, A.S. Uric acid and incident kidney disease in the community. J. Am. Soc. Nephrol. 2008, 19, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Hung, C.C.; Chen, S.C.; Yeh, S.M.; Lin, M.Y.; Chiu, Y.W.; Kuo, M.C.; Chang, J.M.; Hwang, S.J.; Chen, H.C. Association of hyperuricemia with renal outcomes, cardiovascular disease, and mortality. Clin. J. Am. Soc. Nephrol. 2012, 7, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, D.; Vlassopoulos, C.; Seitanides, B.; Contoyiannis, P.; Vassilopoulos, P. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol. 1977, 85, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Yang, J.H.; Chiang, C.W.K.; Hsiung, C.N.; Wu, P.E.; Chang, L.C.; Chu, H.W.; Chang, J.; Song, I.W.; Yang, S.L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef]
- Fan, C.T.; Hung, T.H.; Yeh, C.K. Taiwan Regulation of Biobanks. J. Law Med. Ethics 2015, 43, 816–826. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Isomaa, B.; Henricsson, M.; Almgren, P.; Tuomi, T.; Taskinen, M.R.; Groop, L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia 2001, 44, 1148–1154. [Google Scholar] [CrossRef]
- Lee, J.W.; Kwon, B.C.; Choi, H.G. Analyses of the relationship between hyperuricemia and osteoporosis. Sci. Rep. 2021, 11, 12080. [Google Scholar] [CrossRef]
- Chonchol, M.; Shlipak, M.G.; Katz, R.; Sarnak, M.J.; Newman, A.B.; Siscovick, D.S.; Kestenbaum, B.; Carney, J.K.; Fried, L.F. Relationship of uric acid with progression of kidney disease. Am. J. Kidney Dis. 2007, 50, 239–247. [Google Scholar] [CrossRef]
- Mazzali, M.; Kanellis, J.; Han, L.; Feng, L.; Xia, Y.Y.; Chen, Q.; Kang, D.H.; Gordon, K.L.; Watanabe, S.; Nakagawa, T.; et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am. J. Physiol. Renal. Physiol. 2002, 282, F991–F997. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Tapia, E.; Santamaria, J.; Avila-Casado, C.; Soto, V.; Nepomuceno, T.; Rodriguez-Iturbe, B.; Johnson, R.J.; Herrera-Acosta, J. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005, 67, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Soto, V.; Tapia, E.; Avila-Casado, C.; Sautin, Y.Y.; Nakagawa, T.; Franco, M.; Rodriguez-Iturbe, B.; Johnson, R.J. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am. J. Physiol. Renal. Physiol. 2008, 295, F1134–F1141. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.S.; Kim, M.J.; Shin, H.S.; Jang, Y.H.; Choi, H.S.; Jo, I.; Johnson, R.J.; Kang, D.H. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am. J. Physiol. Renal. Physiol. 2013, 304, F471–F480. [Google Scholar] [CrossRef]
- Sellmayr, M.; Hernandez Petzsche, M.R.; Ma, Q.; Kruger, N.; Liapis, H.; Brink, A.; Lenz, B.; Angelotti, M.L.; Gnemmi, V.; Kuppe, C.; et al. Only Hyperuricemia with Crystalluria, but not Asymptomatic Hyperuricemia, Drives Progression of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 2773–2792. [Google Scholar] [CrossRef]
- Kuwabara, M.; Niwa, K.; Nishi, Y.; Mizuno, A.; Asano, T.; Masuda, K.; Komatsu, I.; Yamazoe, M.; Takahashi, O.; Hisatome, I. Relationship between serum uric acid levels and hypertension among Japanese individuals not treated for hyperuricemia and hypertension. Hypertens. Res. 2014, 37, 785–789. [Google Scholar] [CrossRef]
- Forman, J.P.; Choi, H.; Curhan, G.C. Plasma uric acid level and risk for incident hypertension among men. J. Am. Soc. Nephrol. 2007, 18, 287–292. [Google Scholar] [CrossRef]
- Mellen, P.B.; Bleyer, A.J.; Erlinger, T.P.; Evans, G.W.; Nieto, F.J.; Wagenknecht, L.E.; Wofford, M.R.; Herrington, D.M. Serum uric acid predicts incident hypertension in a biethnic cohort: The atherosclerosis risk in communities study. Hypertension 2006, 48, 1037–1042. [Google Scholar] [CrossRef]
- Lehto, S.; Niskanen, L.; Ronnemaa, T.; Laakso, M. Serum uric acid is a strong predictor of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke 1998, 29, 635–639. [Google Scholar] [CrossRef]
- Dehghan, A.; van Hoek, M.; Sijbrands, E.J.; Hofman, A.; Witteman, J.C. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 2008, 31, 361–362. [Google Scholar] [CrossRef]
- Cicero, A.F.; Salvi, P.; D'Addato, S.; Rosticci, M.; Borghi, C.; Brisighella Heart Study group. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: Data from the Brisighella Heart Study. J. Hypertens. 2014, 32, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Testa, A.; Leonardis, D.; Tripepi, R.; Pisano, A.; Spoto, B.; Sanguedolce, M.C.; Parlongo, R.M.; Tripepi, G.; Zoccali, C. A genetic marker of uric acid level, carotid atherosclerosis, and arterial stiffness: A family-based study. Am. J. Kidney Dis. 2015, 65, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, C.; Zhao, Y.; Zeng, X.; Liu, F.; Fu, P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Nakayama, S.; Satoh, M.; Tatsumi, Y.; Murakami, T.; Muroya, T.; Hirose, T.; Ohkubo, T.; Mori, T.; Hozawa, A.; Metoki, H. Detailed association between serum uric acid levels and the incidence of chronic kidney disease stratified by sex in middle-aged adults. Atherosclerosis 2021, 330, 107–113. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, W.; Wang, Y.; Zhou, R. Gender-specific association between uric acid level and chronic kidney disease in the elderly health checkup population in China. Ren. Fail. 2019, 41, 197–203. [Google Scholar] [CrossRef]
- Wang, Y.; Charchar, F.J. Establishment of sex difference in circulating uric acid is associated with higher testosterone and lower sex hormone-binding globulin in adolescent boys. Sci. Rep. 2021, 11, 17323. [Google Scholar] [CrossRef]
- Huh, K.; Shin, U.S.; Choi, J.W.; Lee, S.I. Effect of sex hormones on lipid peroxidation in rat liver. Arch Pharm. Res. 1994, 17, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Levin, A.; Kidney, M. Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group, Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincon, A.; Arroyo, D.; Luno, J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393. [Google Scholar] [CrossRef]
- Kanbay, M.; Huddam, B.; Azak, A.; Solak, Y.; Kadioglu, G.K.; Kirbas, I.; Duranay, M.; Covic, A.; Johnson, R.J. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin. J. Am. Soc. Nephrol. 2011, 6, 1887–1894. [Google Scholar] [CrossRef]
- Doria, A.; Galecki, A.T.; Spino, C.; Pop-Busui, R.; Cherney, D.Z.; Lingvay, I.; Parsa, A.; Rossing, P.; Sigal, R.J.; Afkarian, M.; et al. Serum Urate Lowering with Allopurinol and Kidney Function in Type 1 Diabetes. N. Engl. J. Med. 2020, 382, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.V.; Pascoe, E.M.; Tiku, A.; Boudville, N.; Brown, F.G.; Cass, A.; Clarke, P.; Dalbeth, N.; Day, R.O.; de Zoysa, J.R.; et al. Effects of Allopurinol on the Progression of Chronic Kidney Disease. N. Engl. J. Med. 2020, 382, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Shrestha, P.; Sumida, K.; Thomas, F.; Sweeney, P.L.; Potukuchi, P.K.; Rhee, C.M.; Streja, E.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of Uric Acid-Lowering Therapy With Incident Chronic Kidney Disease. JAMA Netw. Open 2022, 5, e2215878. [Google Scholar] [CrossRef]
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Culleton, B.; Wilson, P.W.; Levy, D. Predictors of new-onset kidney diseas.se in a community-based population. JAMA 2004, 291, 844–850. [Google Scholar] [CrossRef]
- Yamagata, K.; Ishida, K.; Sairenchi, T.; Takahashi, H.; Ohba, S.; Shiigai, T.; Narita, M.; Koyama, A. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int. 2007, 71, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Rakheja, D.; Yu, X.; Saxena, R.; Vaziri, N.D.; Silva, F.G. The aging kidney. Kidney Int. 2008, 74, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, S.R.; Neugarten, J. The impact of gender on the progression of chronic renal disease. Am. J. Kidney Dis. 1995, 25, 515–533. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Kazancioglu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Narayan, K.M.; Weisman, D.; Golden, S.H.; Jaar, B.G. Association between prediabetes and risk of chronic kidney disease: A systematic review and meta-analysis. Diabet Med. 2016, 33, 1615–1624. [Google Scholar] [CrossRef]
- Chang, T.I.; Lim, H.; Park, C.H.; Rhee, C.M.; Moradi, H.; Kalantar-Zadeh, K.; Kang, E.W.; Kang, S.W.; Han, S.H. Associations of Systolic Blood Pressure With Incident CKD G3-G5: A Cohort Study of South Korean Adults. Am. J. Kidney Dis. 2020, 76, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Chen, Y.Y.; Lin, Y.K.; Chen, C.C.; Yen, Y.F.; Deng, C.Y. Alcohol Consumption and Risk of Chronic Kidney Disease: A Nationwide Observational Cohort Study. Nutrients 2019, 11, 2121. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Yamaguchi, M.; Ishimoto, T.; Katsuno, T.; Nobata, H.; Iwagaitsu, S.; Sugiyama, H.; Kinashi, H.; Banno, S.; Imaizumi, T.; et al. Association of alcohol consumption with the incidence of proteinuria and chronic kidney disease: A retrospective cohort study in Japan. Nutr. J. 2022, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Cho, S.; Kim, S.R. Effect of alcohol consumption on kidney function: Population-based cohort study. Sci. Rep. 2021, 11, 2381. [Google Scholar] [CrossRef]
- Chang, A.; Kramer, H. CKD progression: A risky business. Nephrol. Dial. Transplant. 2012, 27, 2607–2609. [Google Scholar] [CrossRef]
- Ejerblad, E.; Fored, C.M.; Lindblad, P.; Fryzek, J.; McLaughlin, J.K.; Nyren, O. Obesity and risk for chronic renal failure. J. Am. Soc. Nephrol. 2006, 17, 1695–1702. [Google Scholar] [CrossRef]
- Iseki, K.; Kohagura, K. Anemia as a risk factor for chronic kidney disease. Kidney Int. Suppl. 2007, 72, S4–S9. [Google Scholar] [CrossRef]
- Muntner, P.; Coresh, J.; Smith, J.C.; Eckfeldt, J.; Klag, M.J. Plasma lipids and risk of developing renal dysfunction: The atherosclerosis risk in communities study. Kidney Int. 2000, 58, 293–301. [Google Scholar] [CrossRef]
- Hadjadj, S.; Duly-Bouhanick, B.; Bekherraz, A.; BrIdoux, F.; Gallois, Y.; Mauco, G.; Ebran, J.; Marre, M. Serum triglycerides are a predictive factor for the development and the progression of renal and retinal complications in patients with type 1 diabetes. Diabetes Metab. 2004, 30, 43–51. [Google Scholar] [CrossRef]
| Characteristics | New-Onset CKD (−) (n = 26,360) | New-Onset CKD (+) (n = 285) | p |
|---|---|---|---|
| Age (year) | 51.0 ± 10.4 | 59.4 ± 6.9 | <0.001 |
| Male gender (%) | 65.2 | 38.9 | <0.001 |
| DM (%) | 4.9 | 23.2 | <0.001 |
| Hypertension (%) | 12.3 | 44.6 | <0.001 |
| Systolic BP (mmHg) | 117.2 ± 17.5 | 133.3 ± 19.9 | <0.001 |
| Diastolic BP (mmHg) | 72.4 ± 10.8 | 77.8 ± 11.6 | <0.001 |
| Smoking history (%) | 25.3 | 41.8 | <0.001 |
| Alcohol history (%) | 2.8 | 8.4 | <0.001 |
| Regular exercise habits (%) | 48.1 | 51.9 | 0.203 |
| BMI (kg/m2) | 24.0 ± 3.5 | 26.1 ± 4.0 | <0.001 |
| Menstruation in female (%) | 45.3 | 14.4 | <0.001 |
| Laboratory parameters | |||
| Uric acid (mg/dL) | 5.4 ± 1.4 | 6.7 ± 1.5 | <0.001 |
| Hyperuricemia (%) | 19.7 | 48.4 | <0.001 |
| Fasting glucose (mg/dL) | 95.9 ± 19.5 | 113.3 ± 42.3 | <0.001 |
| Hemoglobin (g/dL) | 13.7 ± 1.5 | 14.0 ± 1.6 | 0.002 |
| Triglyceride (mg/dL) | 113.1 ± 82.2 | 161.5 ± 127.5 | <0.001 |
| Total cholesterol (mg/dL) | 195.4 ± 35.3 | 196.3 ± 41.4 | 0.658 |
| HDL-cholesterol (mg/dL) | 54.4 ± 13.2 | 48.5 ± 12.4 | <0.001 |
| LDL-cholesterol (mg/dL) | 121.7 ± 31.6 | 119.9 ± 33.5 | 0.350 |
| eGFR (mL/min/1.73 m2) | 115.2 ± 24.5 | 76.6 ± 16.1 | <0.001 |
| Parameters | New-Onset CKD | ||
|---|---|---|---|
| Multivariable | |||
| OR | 95% CI | p | |
| Age (per 1 year) | 1.079 | 1.060–1.098 | <0.001 |
| Female (vs. male) | 0.340 | 0.239–0.484 | <0.001 |
| DM | 1.676 | 1.183–2.375 | 0.004 |
| Hypertension | 1.740 | 1.327–2.281 | <0.001 |
| Systolic BP (per 1 mmHg) | 1.021 | 1.012–1.031 | <0.001 |
| Diastolic BP (per 1 mmHg) | 0.996 | 0.980–1.012 | 0.595 |
| Smoking history | 1.095 | 0.804–1.491 | 0.564 |
| Alcohol history | 2.081 | 1.308–3.311 | 0.002 |
| BMI (per 1 kg/m2) | 1.065 | 1.027–1.103 | 0.001 |
| Laboratory parameters | |||
| Hyperuricemia | 2.541 | 1.970–3.276 | <0.001 |
| Fasting glucose (per 1 mg/dL) | 1.009 | 1.005–1.013 | <0.001 |
| Hemoglobin (per 1 g/dL) | 0.762 | 0.692–0.89 | <0.001 |
| Triglyceride (per 1 mg/dL) | 1.002 | 1.001–1.003 | <0.001 |
| HDL-cholesterol (per 1 mg/dL) | 1.002 | 0.990–1.014 | 0.743 |
| Characteristics | Male (n = 9356) | Female (n = 17,289) | ||||
|---|---|---|---|---|---|---|
| Hyperuricemia (−) (n = 6532) | Hyperuricemia (+) (n = 2824) | p | Hyperuricemia (−) (n = 14,773) | Hyperuricemia (+) (n = 2516) | p | |
| Age (year) | 51.9 ± 10.8 | 49.9 ± 11.0 | <0.001 | 50.5 ± 10.1 | 54.2 ± 9.3 | <0.001 |
| DM (%) | 7.5 | 4.7 | <0.001 | 3.6 | 7.8 | <0.001 |
| Hypertension (%) | 15.7 | 19.9 | <0.001 | 8.3 | 22.3 | <0.001 |
| Systolic BP (mmHg) | 121.6 ± 16.5 | 123.4 ± 15.7 | <0.001 | 113.6 ± 17.3 | 122.6 ± 18.2 | <0.001 |
| Diastolic BP (mmHg) | 76.2 ± 10.4 | 78.8 ± 10.2 | <0.001 | 69.3 ± 10.1 | 74.0 ± 10.4 | <0.001 |
| Smoking history (%) | 57.3 | 62.4 | <0.001 | 7.4 | 7.6 | 0.637 |
| Alcohol history (%) | 6.3 | 8.9 | <0.001 | 0.6 | 0.9 | 0.125 |
| Regular exercise habits (%) | 50.1 | 46.7 | 0.003 | 47.7 | 47.9 | 0.846 |
| BMI (kg/m2) | 24.5 ± 3.1 | 26.3 ± 3.4 | <0.001 | 23.1 ± 3.3 | 26.1 ± 4.1 | <0.001 |
| Menstruation in female (%) | – | – | 47.8 | 29.0 | <0.001 | |
| Laboratory parameters | ||||||
| Uric acid (mg/dL) | 5.8 ± 0.9 | 8.0 ± 0.8 | <0.001 | 4.6 ± 0.8 | 6.8 ± 0.8 | <0.001 |
| Fasting glucose (mg/dL) | 100.2 ± 24.7 | 95.4 ± 15.6 | <0.001 | 93.4 ± 17.9 | 98.5 ± 19.1 | <0.001 |
| Hemoglobin (g/dL) | 15.0 ± 1.2 | 15.2 ± 1.1 | <0.001 | 13.0 ± 1.3 | 13.4 ± 1.1 | <0.001 |
| Triglyceride (mg/dL) | 121.1 ± 89.5 | 161.4 ± 117.5 | <0.001 | 96.8 ± 65.3 | 138.9 ± 80.5 | <0.001 |
| Total cholesterol (mg/dL) | 189.6 ± 33.7 | 195.9 ± 357.8 | <0.001 | 196.0 ± 35.2 | 206.3 ± 37.2 | <0.001 |
| HDL-cholesterol (mg/dL) | 49.1 ± 11.2 | 45.6 ± 10.1 | <0.001 | 58.7 ± 13.0 | 52.1 ± 11.6 | <0.001 |
| LDL-cholesterol (mg/dL) | 121.0 ± 30.7 | 124.7 ± 32.6 | <0.001 | 119.8 ± 31.1 | 130.8 ± 33.5 | <0.001 |
| eGFR (mL/min/1.73 m2) | 102.7 ± 19.8 | 93.9 ± 18.0 | <0.001 | 117.2 ± 25.3 | 103.1 ± 22.7 | <0.001 |
| Parameters | Male (n = 9356) | Female (n = 17,289) | |||||
|---|---|---|---|---|---|---|---|
| Multivariable * | Multivariable # | ||||||
| OR | 95% CI | p | OR | 95% CI | p | Interaction p | |
| Hyperuricemia | 1.989 | 1.440–2.747 | <0.001 | 3.813 | 2.500–5.815 | <0.001 | 0.024 |
| Serum uric acid | |||||||
| Quartile 1 | Reference | Reference | 0.010 | ||||
| Quartile 2 | 0.998 | 0.616–1.618 | 0.994 | 2.577 | 0.810–8.196 | 0.109 | |
| Quartile 3 | 1.122 | 0.685–1.833 | 0.647 | 3.741 | 1.250–11.915 | 0.018 | |
| Quartile 4 | 2.279 | 1.464–3.547 | <0.001 | 12.114 | 4.278–34.305 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-H.; Tsai, C.-C.; Liu, Y.-H.; Wu, P.-Y.; Huang, J.-C.; Chung, T.-L.; Su, H.-M.; Chen, S.-C. Sex Difference in the Associations among Hyperuricemia with New-Onset Chronic Kidney Disease in a Large Taiwanese Population Follow-Up Study. Nutrients 2022, 14, 3832. https://doi.org/10.3390/nu14183832
Chen J-H, Tsai C-C, Liu Y-H, Wu P-Y, Huang J-C, Chung T-L, Su H-M, Chen S-C. Sex Difference in the Associations among Hyperuricemia with New-Onset Chronic Kidney Disease in a Large Taiwanese Population Follow-Up Study. Nutrients. 2022; 14(18):3832. https://doi.org/10.3390/nu14183832
Chicago/Turabian StyleChen, Jui-Hsin, Chun-Chi Tsai, Yi-Hsueh Liu, Pei-Yu Wu, Jiun-Chi Huang, Tung-Ling Chung, Ho-Ming Su, and Szu-Chia Chen. 2022. "Sex Difference in the Associations among Hyperuricemia with New-Onset Chronic Kidney Disease in a Large Taiwanese Population Follow-Up Study" Nutrients 14, no. 18: 3832. https://doi.org/10.3390/nu14183832
APA StyleChen, J.-H., Tsai, C.-C., Liu, Y.-H., Wu, P.-Y., Huang, J.-C., Chung, T.-L., Su, H.-M., & Chen, S.-C. (2022). Sex Difference in the Associations among Hyperuricemia with New-Onset Chronic Kidney Disease in a Large Taiwanese Population Follow-Up Study. Nutrients, 14(18), 3832. https://doi.org/10.3390/nu14183832






