Persimmon Proanthocyanidins with Different Degrees of Polymerization Possess Distinct Activities in Models of High Fat Diet Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalytic Hydrogenolysis of P-PCs
2.2. In Vivo Animal Study
2.2.1. Animals and Dosage Regimen
2.2.2. Glucose Tolerance Tests (GTTs)
2.2.3. Tissue Sample Collection
2.2.4. Biochemical Analyses and Histology
2.3. DNA Extraction and Information Analysis of Gut Microbiota
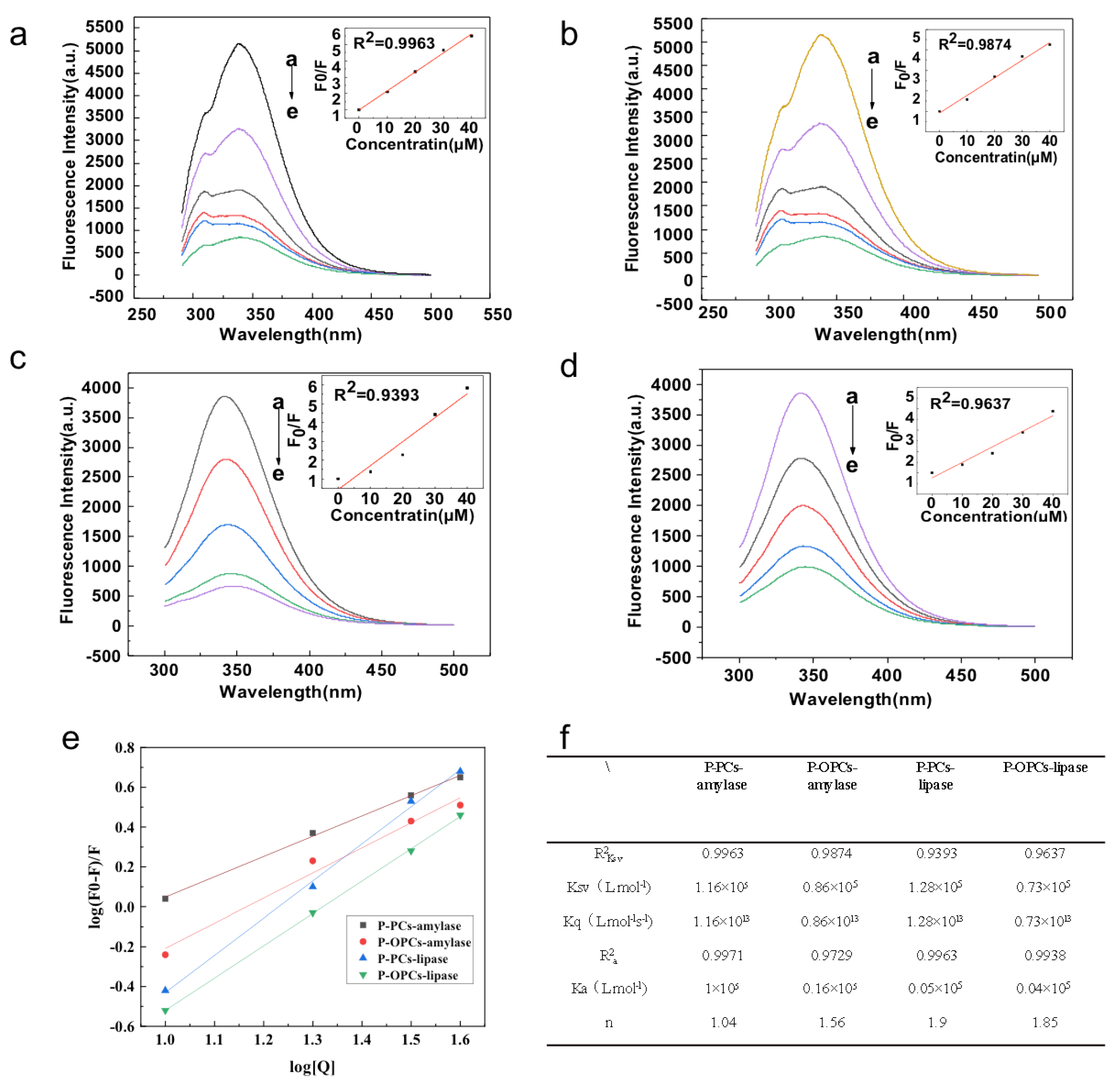
2.4. Enzyme Activity Assay
Fluorescence Quenching Measurements
2.5. Statistical Analysis
3. Results
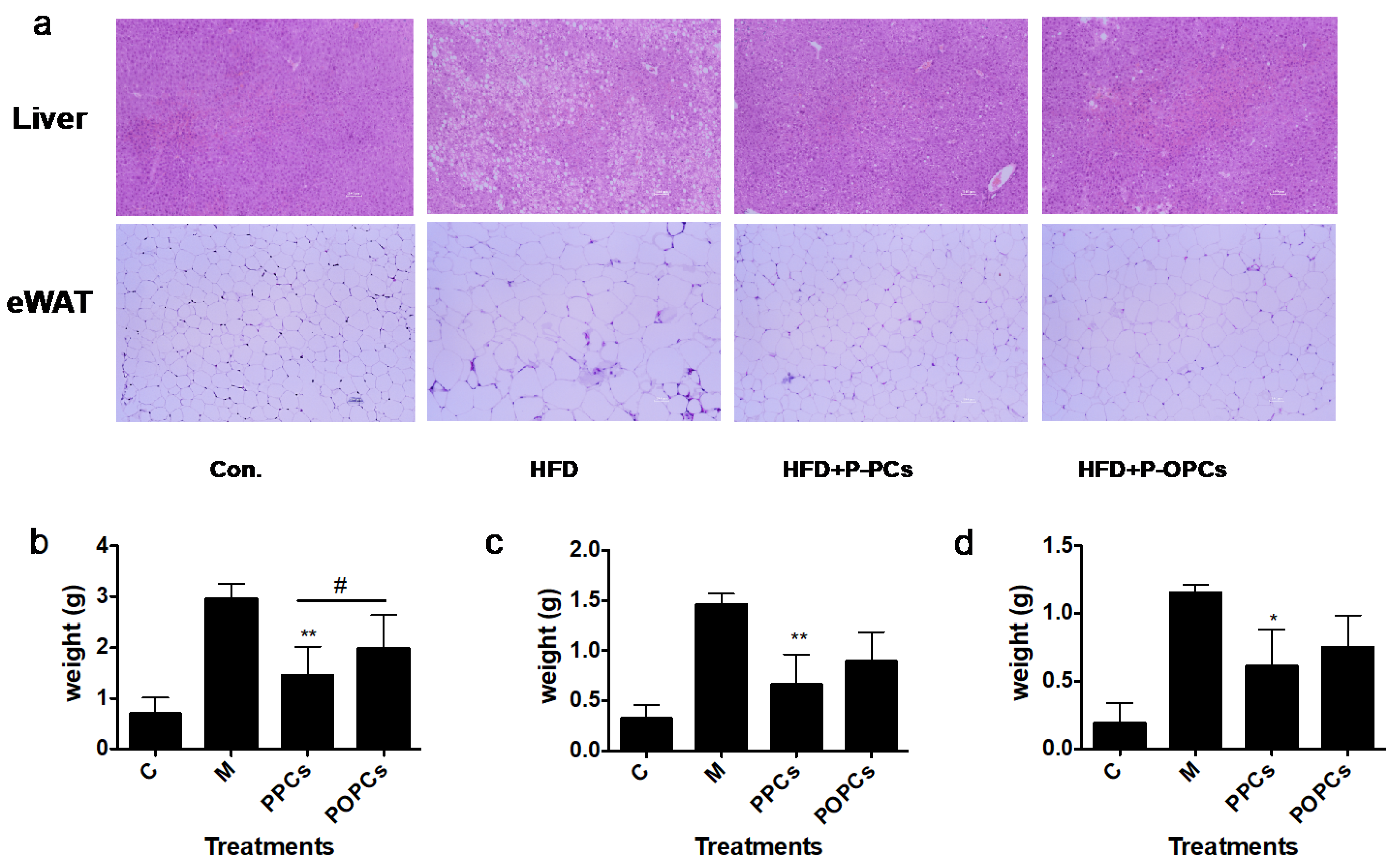
3.1. P-PCs Administration Showed Stronger Potential on HFD Induced Weight Gain Compared with P-OPCs
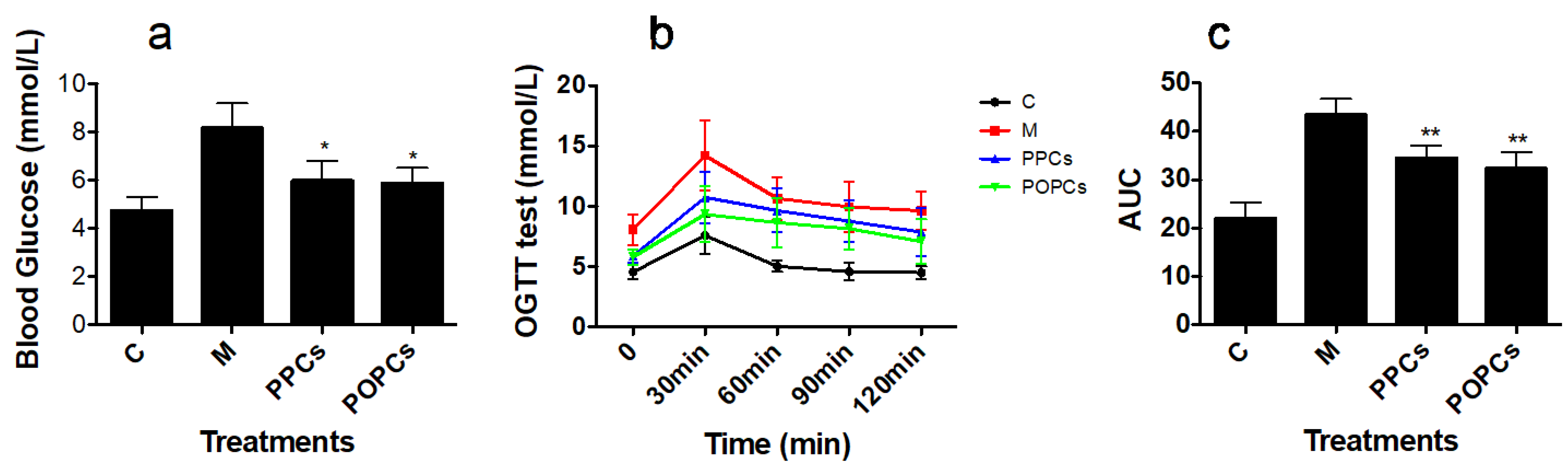
3.2. P-PCs and P-OPCs Administration Improved Insulin Resistance
3.3. Dietary P-PCs and P-OPCs Reduce Lipid Accumulation in HFD Induced Obese Mice
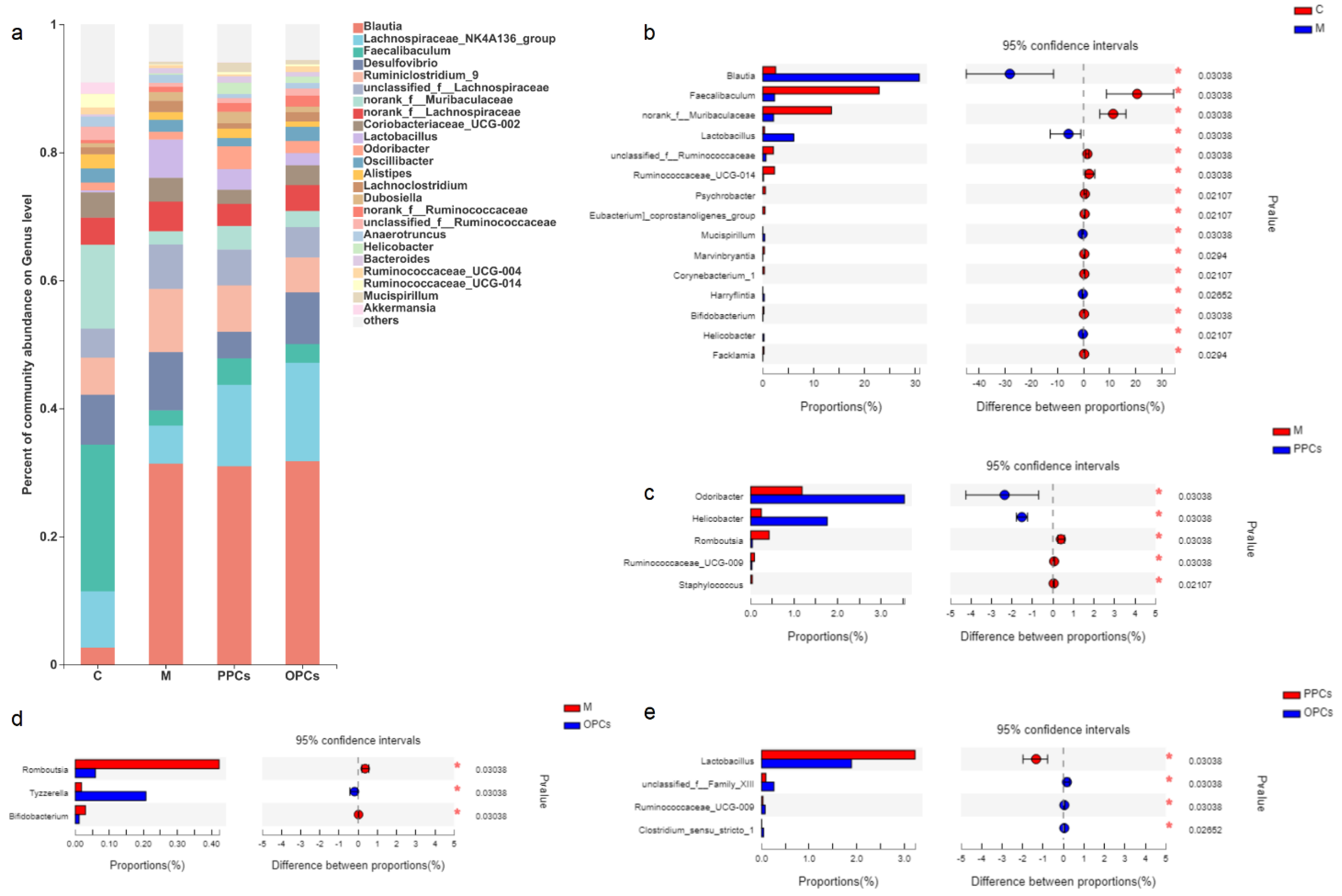
3.4. Effect of P-PCs and P-OPCs on Gut Microbiota Diversity
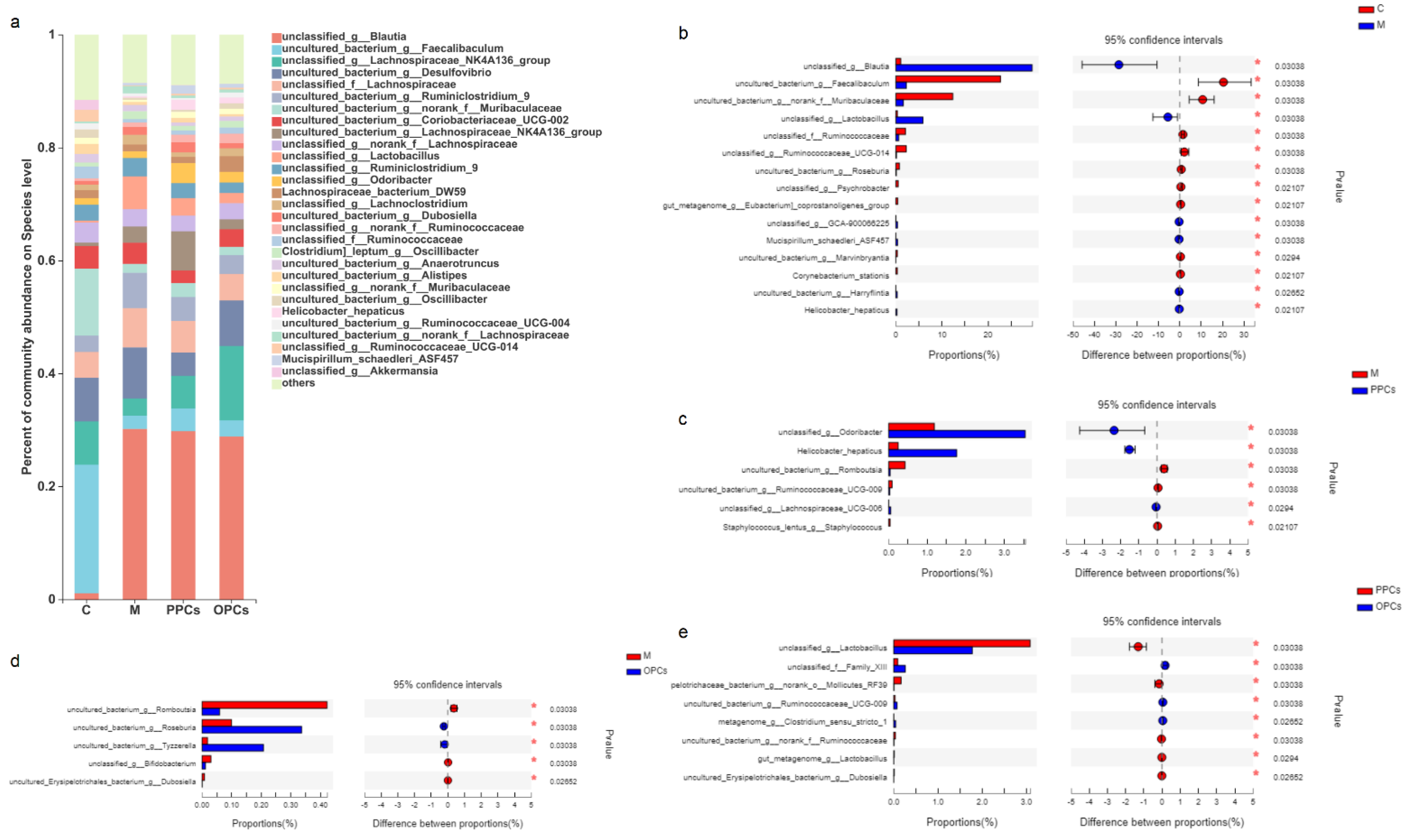
3.5. Composition of the Gut Microbiota
3.5.1. Genus Level
3.5.2. Species Level
3.6. Inhibitory Activities of P-PCs and P-OPCs on Digestive Enzymes Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, And Mortality from Cancer In A Prospectively Studied Cohort of U.S. Adults New Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, H.V.; Sharma, D.; Dodrill, M.W.; Nawab, A.; Sharma, N.; Cottrill, C.L.; Shapiro, J.I.; Sodhi, K. Phenotypic Alteration of Hepatocytes in Non-Alcoholic Fatty Liver Disease. Int. J. Med. Sci. 2018, 15, 1591–1599. [Google Scholar] [CrossRef]
- Dadarlat-Pop, A.; Sitar-Taut, A.; Zdrenghea, D.; Caloian, B.; Tomoaia, R.; Pop, D.; Buzoianu, A. Profile of Obesity and Comorbidities in Elderly Patients with Heart Failure. Clin. Interv. Aging 2020, 15, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Jia, Y.; Hu, T.; Du, J.; Wang, Y.; Cheng, B.; Li, K. GC-(4→8)-GCG, A Proanthocyanidin Dimer from Camellia ptilophylla, Modulates Obesity and Adipose Tissue Inflammation in High-Fat Diet Induced Obese Mice. Mol. Nutr. Food Res. 2019, 63, 1900082. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Choi, B.S.Y.; Dyck, J.R.B.; Schertzer, J.D.; Marette, A. Host-Microbe Interplay in the Cardiometabolic Benefits of Dietary Polyphenols. Trends Endocrinol. Metab. 2019, 30, 384–395. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Effect of food structure and processing on (Poly) phenol–gut microbiota interactions and the effects on human health. Annu. Rev. Food Sci. Technol. 2019, 10, 221–238. [Google Scholar] [CrossRef]
- Li, X.; Jiao, W.; Zhang, W.; Xu, Y.; Cao, J.; Jiang, W. Characterizing the interactions of dietary condensed tannins with bile salts. J. Agric. Food Chem. 2019, 67, 9543–9550. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Song, L.; Li, T.; Cui, S.; Zhang, L.; Jia, Y. Antimicrobial activity and mechanism of Larch bark procyanidins against Staphylococcus aureus. Acta Biochim. Biophys. Sin. 2017, 49, 1058–1066. [Google Scholar] [CrossRef]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Pierini, R.; Kroon, P.A.; Guyot, S.I.; Johnson, T.; Belshaw, N.J. The procyanidin-mediated induction of apoptosis and cell-cycle arrest in esophageal adenocarcinoma cells is not dependent on p21(Cip1/WAF1). Cancer Lett. 2008, 270, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Guerrero, L.; Fernández-Vallinas, S.; Pons, Z.; Arola, L.; Aleixandre, A.; Muguerza, B. Involvement of nitric oxide and prostacyclin in the antihypertensive effect of low-molecular-weight procyanidin rich grape seed extract in male spontaneously hypertensive rats. J. Funct. Foods 2014, 6, 419–427. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Glisan, S.L.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Lambert, J.D.; Neilson, A.P. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J. Nutr. Biochem. 2015, 26, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.G.; Mateus, N.; Freitas, V.D. Inhibition of α-amylase activity by condensed tannins. Food Chem. 2011, 125, 665–672. [Google Scholar]
- Li, K.; Yao, F.; Du, J.; Deng, X.; Li, C.M. Persimmon Tannin Decreased the Glycemic Response through Decreasing the Digestibility of Starch and Inhibiting α-Amylase, α-Glucosidase, and Intestinal Glucose Uptake. J. Agric. Food Chem. 2018, 66, 1629–1637. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Jia, Y.; Zhang, M.; Li, K. Persimmon Oligomeric Proanthocyanidins Exert Antibacterial Activity through Damaging the Cell Membrane and Disrupting the Energy Metabolism of Staphylococcus aureus. ACS Food Sci. Technol. 2021, 1, 35–44. [Google Scholar] [CrossRef]
- Zeller, W.E.; Reinhardt, L.A.; Robe, J.T.; Sullivan, M.L.; Panke-Buisse, K. Comparison of Protein Precipitation Ability of Structurally Diverse Procyanidin-Rich Condensed Tannins in Two Buffer Systems. J. Agric. Food Chem. 2020, 68, 2016–2023. [Google Scholar] [CrossRef]
- Shi, X.; Shang, F.; Zhang, Y.; Wang, R.; Jia, Y.; Li, K. Persimmon oligomeric proanthocyanidins alleviate ultraviolet B-induced skin damage by regulating oxidative stress and inflammatory responses. Free Radic Res. 2020, 54, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Khalifa, I.; Wang, R.; Li, C. Persimmon highly galloylated-tannins in vitro mitigated alpha-amylase and alpha-glucosidase via statically binding with their catalytic-closed sides and altering their secondary structure elements. J. Food Biochem. 2020, 44, e13234. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Tian, R.; Xu, W.; Li, L.; Cui, Y.; Zhang, S. Novel Catechin-Tiopronin Conjugates Derived from Grape Seed Proanthocyanidin Degradation: Process Optimization, High-Speed Counter-Current Chromatography Preparation, as Well as Antibacterial Activity. J. Agric. Food Chem. 2019, 67, 11508–11517. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.A.; Medina Larque, S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonne, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S. Grape Polyphenols Prevent Fructose-Induced Oxidative Stress and Insulin Resistance in First-Degree Relatives of Type 2 Diabetic Patients. Diabetes Care 2013, 36, 1454–1461. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D.; Li, Y.; Zhao, S.; Chen, C.; Ning, D. Alleviating effects of walnut green husk extract on disorders of lipid levels and gut bacteria flora in high fat diet-induced obesity rats. J. Funct. Foods 2019, 52, 576–586. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Carmelo-Luna, F.J.; Astiazarán-García, H.; Mata-Haro, V.; Espinosa-Plascencia, A.; María, B.; Rascón-Durán, M. Absorption of dimers, trimers and tetramers of procyanidins present in apple skin by IEC-18 cell monolayers. J. Funct. Foods 2016, 27, 386–391. [Google Scholar] [CrossRef]
- Cerqueira, F.M.; Photenhauer, A.L.; Pollet, R.M.; Brown, H.A.; Koropatkin, N.M. Starch Digestion by Gut Bacteria: Crowdsourcing for Carbs. Trends Microbiol. 2020, 28, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yamashita, Y.; Komeda, S.; Saito, A.; Ashida, H. Absorption, metabolism, distribution and faecal excretion of B-type procyanidin oligomers in mice after a single oral administration of black soybean seed coat extract. Food Funct. 2018, 9, 5362–5370. [Google Scholar] [CrossRef]
- Masumoto, S.; Aoki, S.; Miura, T.; Shoji, T. Flavan-3-ol/Procyanidin Metabolomics in Rat Urine Using HPLC-Quadrupole TOF/MS. Mol. Nutr. Food Res. 2018, 62, e1700867. [Google Scholar] [CrossRef]
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015, 59, 610–621. [Google Scholar] [CrossRef]
- Mkandawire, N.L.; Kaufman, R.C.; Bean, S.R.; Weller, C.L.; Jackson, D.S.; Rose, D.J. Effects of sorghum (Sorghum bicolor (L.) Moench) tannins on alpha-amylase activity and in vitro digestibility of starch in raw and processed flours. J. Agric. Food Chem. 2013, 61, 4448–4454. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Hurst, W.J.; Stuart, D.A.; Lambert, J.D. Inhibition of Key Digestive Enzymes by Cocoa Extracts and Procyanidins. J. Agric. Food Chem. 2011, 59, 5305–5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Chen, P.; Li, X.; Shen, S.; Li, K. Persimmon Proanthocyanidins with Different Degrees of Polymerization Possess Distinct Activities in Models of High Fat Diet Induced Obesity. Nutrients 2022, 14, 3718. https://doi.org/10.3390/nu14183718
Yu Y, Chen P, Li X, Shen S, Li K. Persimmon Proanthocyanidins with Different Degrees of Polymerization Possess Distinct Activities in Models of High Fat Diet Induced Obesity. Nutrients. 2022; 14(18):3718. https://doi.org/10.3390/nu14183718
Chicago/Turabian StyleYu, Ying, Ping Chen, Xiaofang Li, Shanshan Shen, and Kaikai Li. 2022. "Persimmon Proanthocyanidins with Different Degrees of Polymerization Possess Distinct Activities in Models of High Fat Diet Induced Obesity" Nutrients 14, no. 18: 3718. https://doi.org/10.3390/nu14183718
APA StyleYu, Y., Chen, P., Li, X., Shen, S., & Li, K. (2022). Persimmon Proanthocyanidins with Different Degrees of Polymerization Possess Distinct Activities in Models of High Fat Diet Induced Obesity. Nutrients, 14(18), 3718. https://doi.org/10.3390/nu14183718





