Does Methionine Status Influence the Outcome of Selenomethinione Supplementation? A Comparative Study of Metabolic and Selenium Levels in HepG2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Culture and Treatment of HepG2 Cells
2.3. Cell Viability Assay
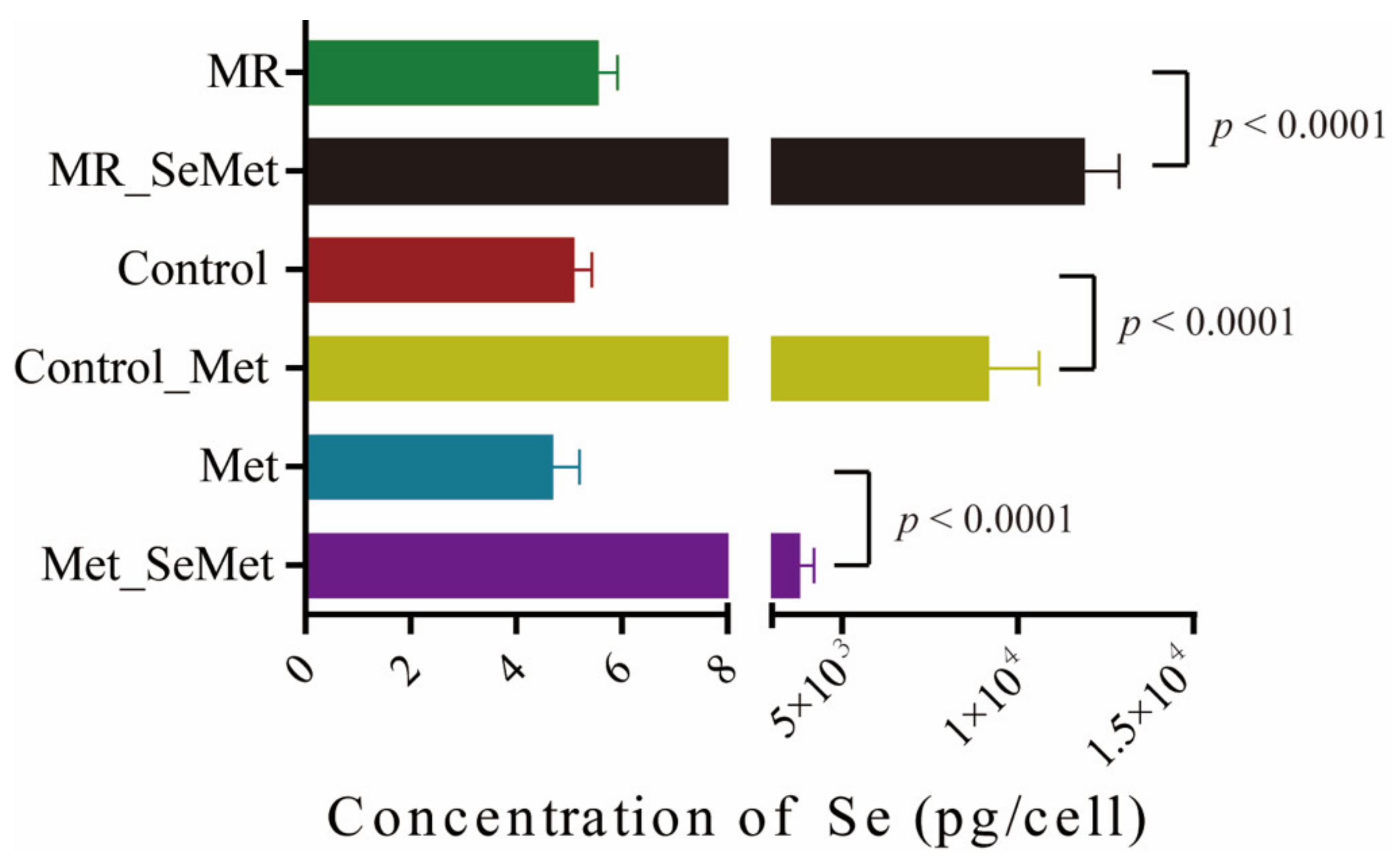
2.4. Quantification of Total Selenium in HepG2 Cells
2.5. Extraction of Intracellular Metabolites
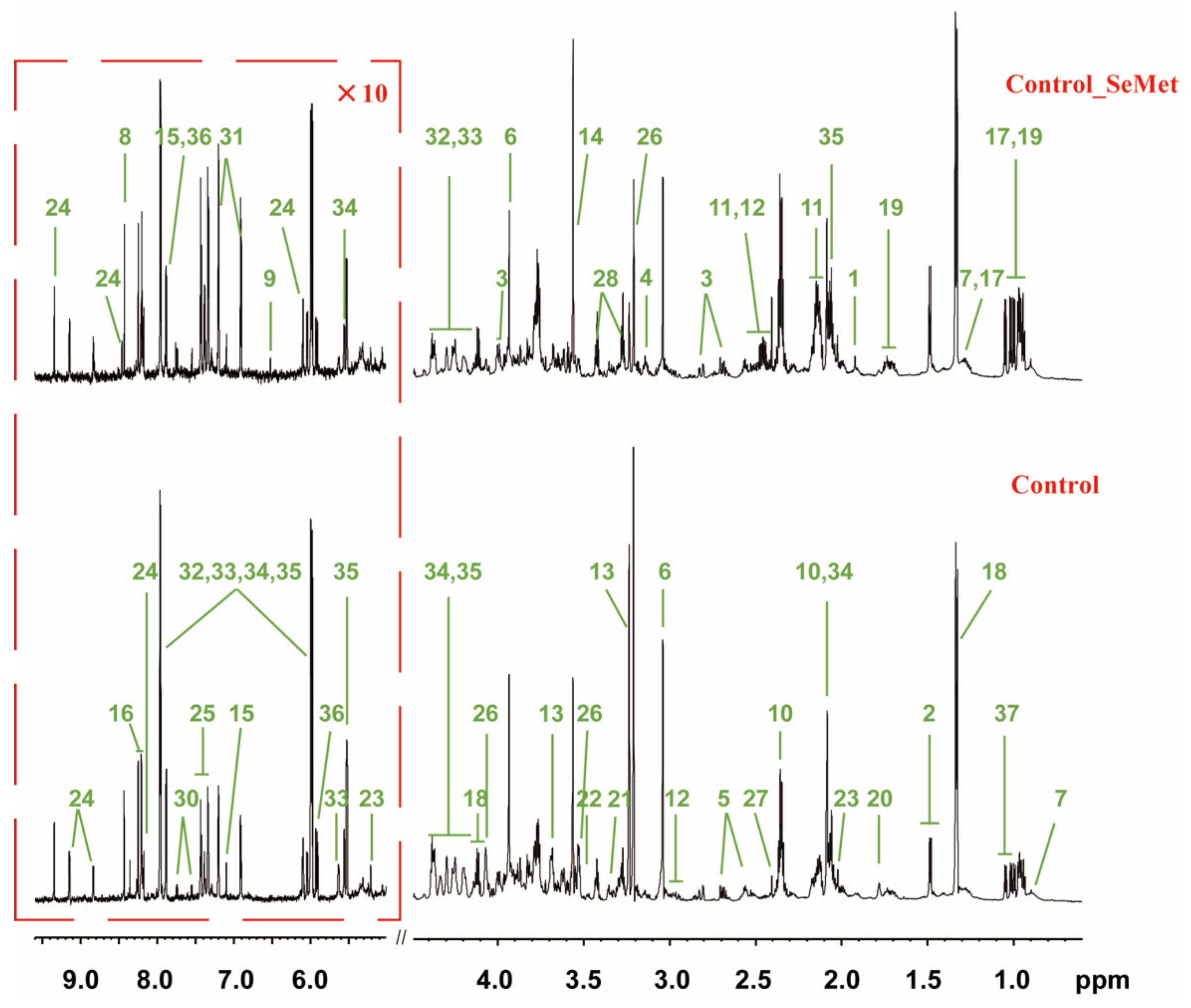
2.6. NMR Measurements of Cell Extracts
2.7. NMR Data Processing and Multivariate Data Analysis
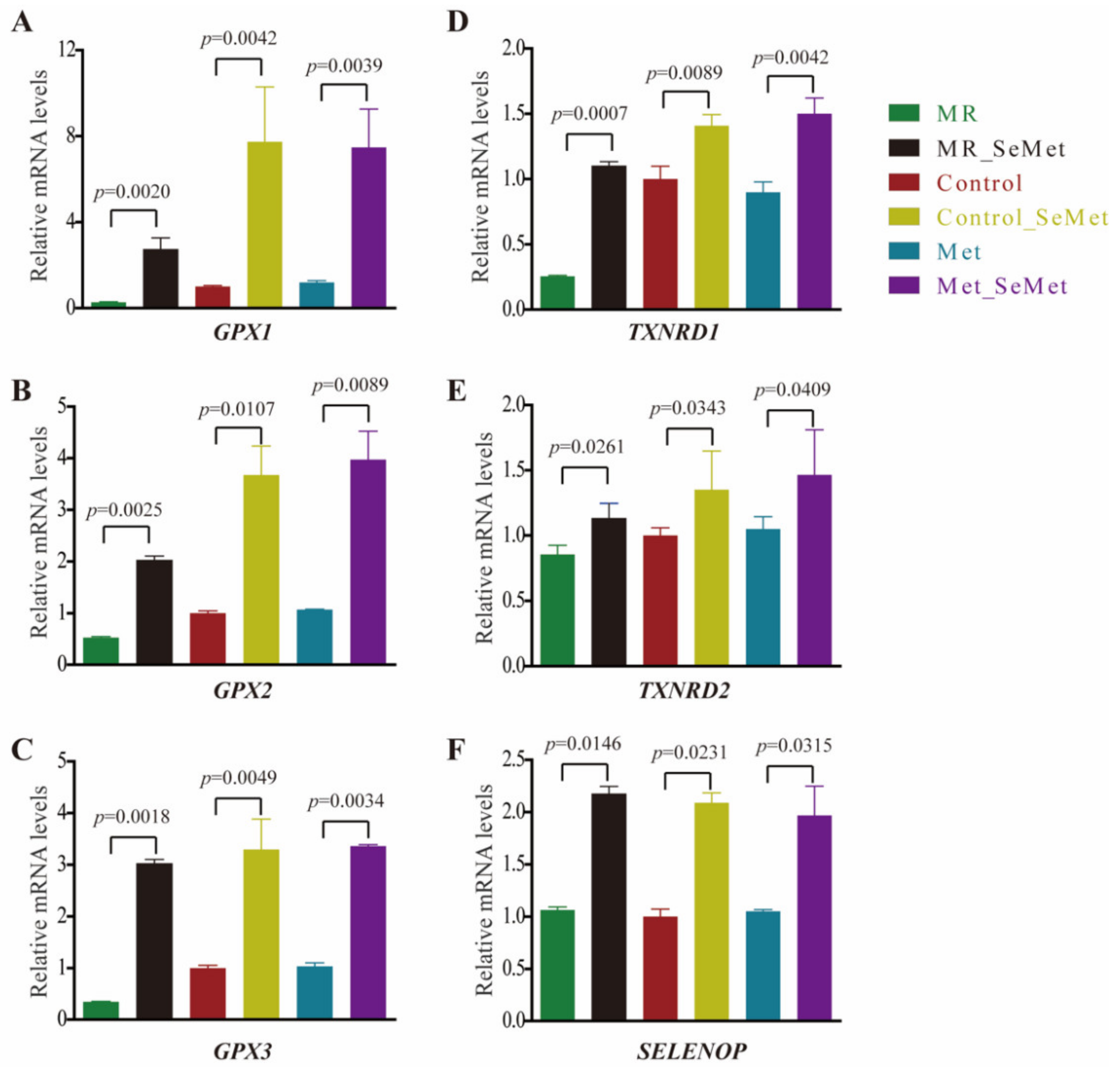
2.8. Quantitative Real-Time PCR Analysis
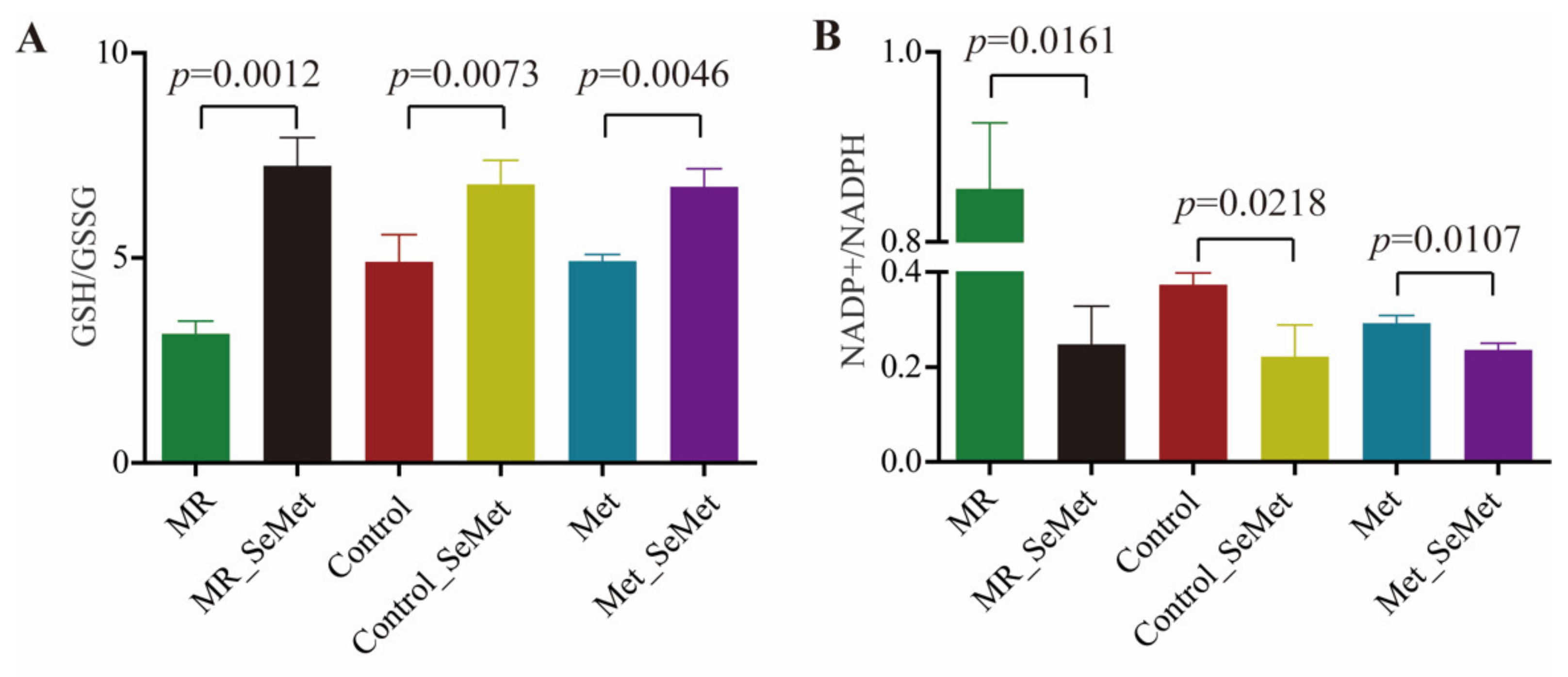
2.9. Cellular GSH/GSSG and NADP+/NADPH Assays
2.10. Statistical Analysis
3. Results
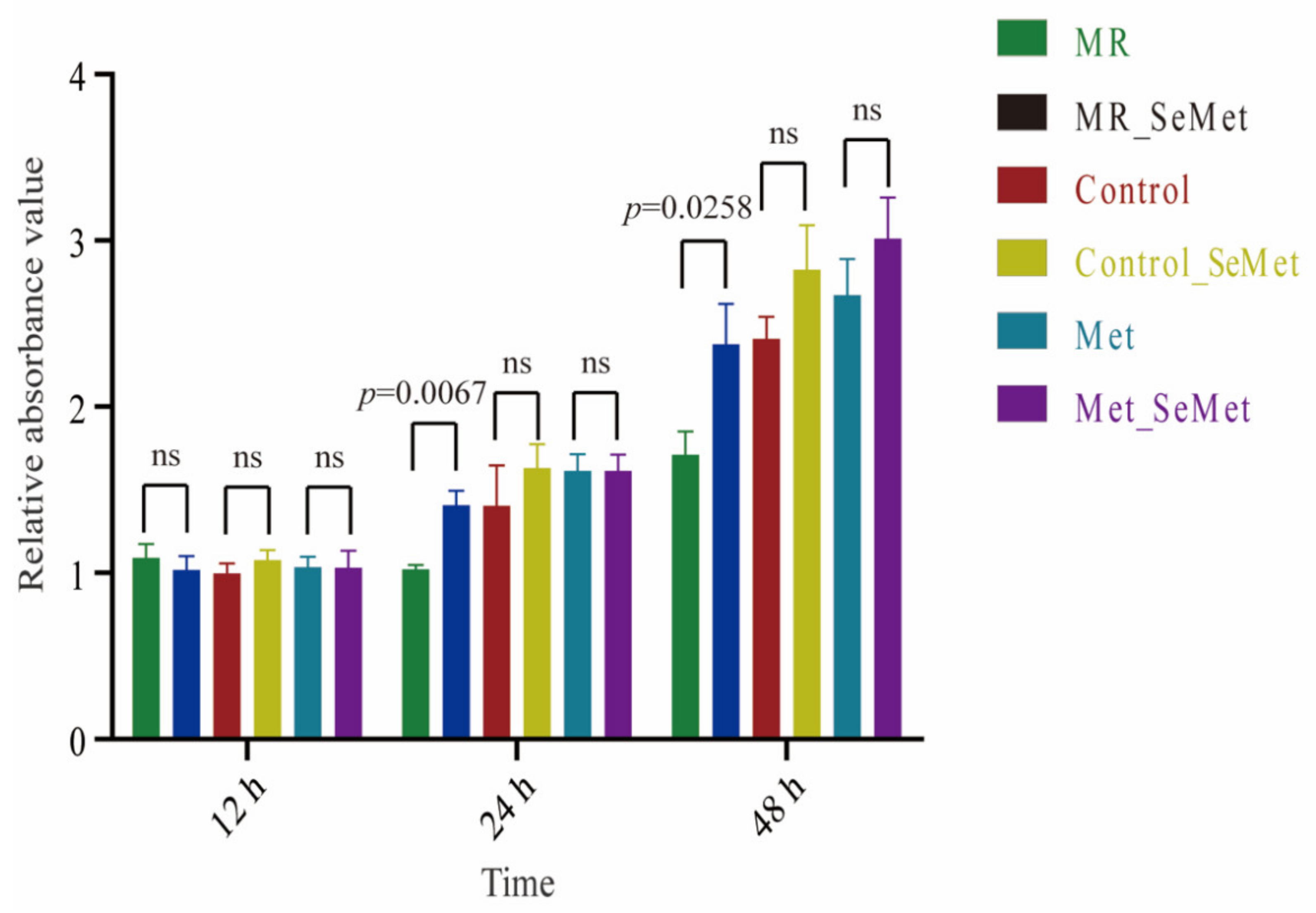
3.1. Effects of Increasing SeMet and Met Supplementation on Cell Viability and Proliferation
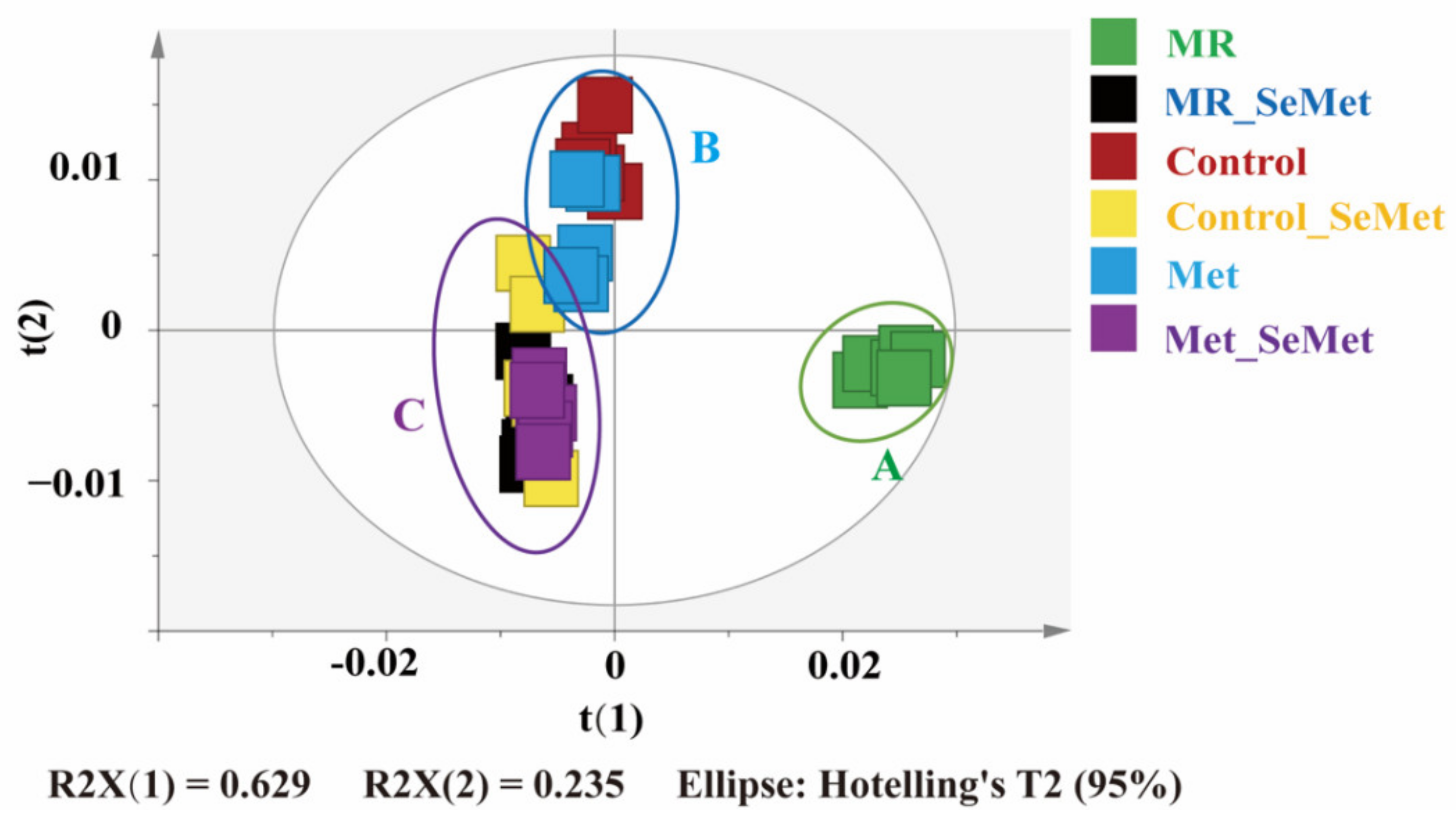
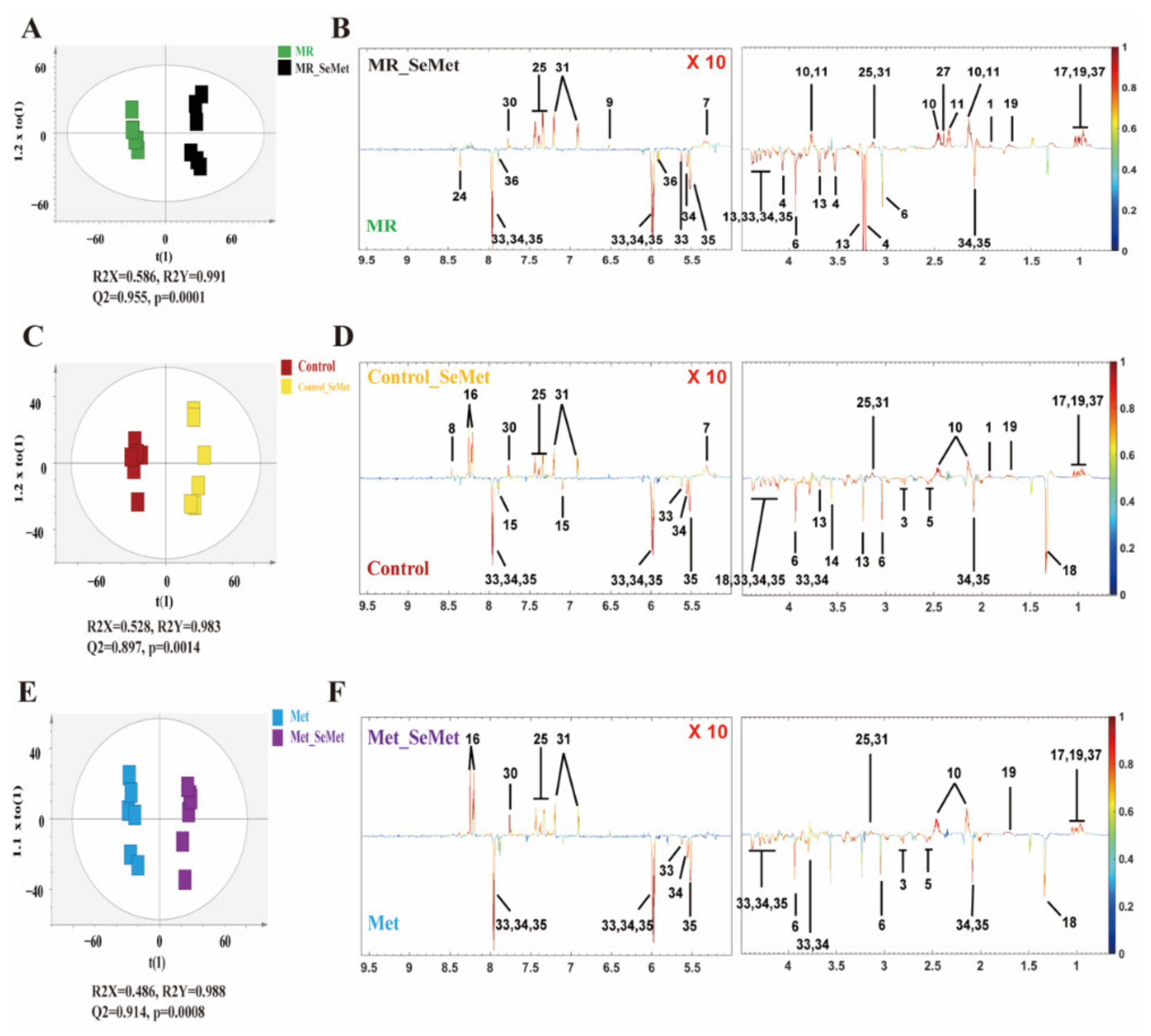
3.2. Effects of SeMet Supplementation on Metabolic Phenotypes of HepG2 Cells under Different Met Supply Conditions
3.3. Selenium Uptake, Relative Transcriptional Levels of Selenoprotein Genes, and Redox Status of HepG2 Cells with SeMet Supplementation under Different Met Supply Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combs, G.F. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H.E. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Tan, J.A.; Zhu, W.Y.; Wang, W.Y.; Li, R.B.; Hou, S.F.; Wang, D.C.; Yang, L.S. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907. [Google Scholar] [CrossRef]
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Karaye, K.M.; Sa’idu, H.; Balarabe, S.A.; Ishaq, N.A.; Sanni, B.; Abubakar, H.; Mohammed, B.L.; Abdulsalam, T.; Tukur, J.; Mohammed, I.Y. Selenium supplementation in patients with peripartum cardiomyopathy: A proof-of-concept trial. BMC Cardiovasc. Disord. 2020, 20, 457. [Google Scholar] [CrossRef]
- Zhang, Y.; Cartland, S.P.; Henriquez, R.; Patel, S.; Gammelgaard, B.; Flouda, K.; Hawkins, C.L.; Rayner, B.S. Selenomethionine supplementation reduces lesion burden, improves vessel function and modulates the inflammatory response within the setting of atherosclerosis. Redox Biol. 2020, 29, 101409. [Google Scholar] [CrossRef]
- Nasim, M.J.; Zuraik, M.M.; Abdin, A.Y.; Ney, Y.; Jacob, C. Selenomethionine: A Pink Trojan Redox Horse with Implications in Aging and Various Age-Related Diseases. Antioxidants 2021, 10, 882. [Google Scholar] [CrossRef]
- Kigawa, T.; Yamaguchi-Nunokawa, E.; Kodama, K.; Matsuda, T.; Yabuki, T.; Matsuda, N.; Ishitani, R.; Nureki, O.; Yokoyama, S. Selenomethionine incorporation into a protein by cell-free synthesis. J. Struct. Funct. Genom. 2002, 2, 29–35. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Selenomethionine: A review of its nutritional significance, metabolism and toxicity. J. Nutr. 2000, 130, 1653–1656. [Google Scholar] [CrossRef]
- Mishra, B.; Sharma, A.; Naumov, S.; Priyadarsini, K.I. Novel Reactions of One-Electron Oxidized Radicals of Selenomethionine in Comparison with Methionine. J. Phys. Chem. B 2009, 113, 7709–7715. [Google Scholar] [CrossRef]
- Ogra, Y.; Kitaguchi, T.; Suzuki, N.; Suzuki, K.T. In vitro translation with S-34 -labeled methionine, selenomethionine, and telluromethionine. Anal. Bioanal. Chem. 2008, 390, 45–51. [Google Scholar] [CrossRef]
- Bakke, A.M.; Tashjian, D.H.; Wang, C.F.; Lee, S.H.; Bai, S.C.; Hung, S.S.O. Competition between selenomethionine and methionine absorption in the intestinal tract of green sturgeon (Acipenser medirostris). Aquat. Toxicol. 2010, 96, 62–69. [Google Scholar] [CrossRef]
- Barton, W.A.; Tzvetkova-Robev, D.; Erdjument-Bromage, H.; Tempst, P.; Nikolov, D.B. Highly efficient selenomethionine labeling of recombinant proteins produced in mammalian cells. Protein Sci. 2006, 15, 2008–2013. [Google Scholar] [CrossRef]
- Butler, J.A.; Beilstein, M.A.; Whanger, P.D. Influence of dietary methionine on the metabolism of selenomethionine in rats. J. Nutr. 1989, 119, 1001–1009. [Google Scholar] [CrossRef]
- Wanders, D.; Hobson, K.; Ji, X. Methionine Restriction and Cancer Biology. Nutrients 2020, 12, 684. [Google Scholar] [CrossRef]
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P., Jr.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019, 572, 397–401. [Google Scholar] [CrossRef]
- Sun, L.; Akha, A.A.S.; Miller, R.A.; Harper, J.M. Life-Span Extension in Mice by Preweaning Food Restriction and by Methionine Restriction in Middle Age. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2009, 64, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Lees, E.K.; Krol, E.; Grant, L.; Shearer, K.; Wyse, C.; Moncur, E.; Bykowska, A.S.; Mody, N.; Gettys, T.W.; Delibegovic, M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 2014, 13, 817–827. [Google Scholar] [CrossRef]
- Markley, J.L.; Bruschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef]
- Oldham, W.M.; Clish, C.B.; Yang, Y.; Loscalzo, J. Hypoxia-Mediated Increases in L-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress. Cell Metab. 2015, 22, 291–303. [Google Scholar] [CrossRef]
- Boon, R.; Kumar, M.; Tricot, T.; Elia, I.; Ordovas, L.; Jacobs, F.; One, J.; De Smedt, J.; Eelen, G.; Bird, M.; et al. Amino acid levels determine metabolism and CYP450 function of hepatocytes and hepatoma cell lines. Nat. Commun. 2020, 11, 1393. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xu, W.; Li, H.; Lei, H.; Zhang, L.; Hao, F.; Duan, Y.; Yan, X.; Zhao, Y.; Wu, J.; et al. High-Fat Diet Induces Dynamic Metabolic Alterations in Multiple Biological Matrices of Rats. J. Proteome Res. 2013, 12, 3755–3768. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Saebo, S. A review of variable selection methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Zou, X.; Holmes, E.; Nicholson, J.K.; Loo, R.L. Automatic Spectroscopic Data Categorization by Clustering Analysis (ASCLAN): A Data-Driven Approach for Distinguishing Discriminatory Metabolites for Phenotypic Subclasses. Anal. Chem. 2016, 88, 5670–5679. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Chamchoy, K.; Pakotiprapha, D.; Pumirat, P.; Leartsakulpanich, U.; Boonyuen, U. Application of WST-8 based colorimetric NAD(P)H detection for quantitative dehydrogenase assays. BMC Biochem. 2019, 20, 4. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, Z.; Liu, C. Identification and verification of the role of crucial genes through which methionine restriction inhibits the progression of colon cancer cells. Oncol. Lett. 2022, 24, 274. [Google Scholar] [CrossRef]
- Schrauzer, G.N. The nutritional significance, metabolism and toxicology of selenomethionine. Adv. Food Nutr. Res. 2003, 47, 73–112. [Google Scholar] [CrossRef]
- Marschall, T.A.; Bornhorst, J.; Kuehnelt, D.; Schwerdtle, T. Differing cytotoxicity and bioavailability of selenite, methylselenocysteine, selenomethionine, selenosugar 1 and trimethylselenonium ion and their underlying metabolic transformations in human cells. Mol. Nutr. Food Res. 2016, 60, 2622–2632. [Google Scholar] [CrossRef]
- Kajander, E.O.; Harvima, R.J.; Eloranta, T.O.; Martikainen, H.; Kantola, M.; Karenlampi, S.O.; Akerman, K. Metabolism, cellular actions, and cytotoxicity of selenomethionine in cultured-cells. Biol. Trace Elem. Res. 1991, 28, 39–45. [Google Scholar] [CrossRef]
- Lu, Y.; Odowd, B.F.; Orrego, H.; Israel, Y. Cloning and nucleotide-sequence of human liver cdna-encoding for cystathionine gamma-lyase. Biochem. Biophys. Res. Commun. 1992, 189, 749–758. [Google Scholar] [CrossRef]
- Mihara, H.; Kurihara, T.; Watanabe, T.; Yoshimura, T.; Esaki, N. cDNA cloning, purification, and characterization of mouse liver selenocysteine lyase—Candidate for selenium delivery protein in selenoprotein synthesis. J. Biol. Chem. 2000, 275, 6195–6200. [Google Scholar] [CrossRef]
- Machacek, M.; Saunders, H.; Zhang, Z.; Tan, E.P.; Li, J.; Li, T.; Villar, M.T.; Artigues, A.; Lydic, T.; Cork, G.; et al. Elevated O-GlcNAcylation enhances pro-inflammatory Th17 function by altering the intracellular lipid microenvironment. J. Biol. Chem. 2019, 294, 8973–8990. [Google Scholar] [CrossRef]
- Gonzalez-Rellan, M.J.; Fondevila, M.F.; Fernandez, U.; Rodriguez, A.; Varela-Rey, M.; Veyrat-Durebex, C.; Seoane, S.; Bernardo, G.; Lopitz-Otsoa, F.; Fernandez-Ramos, D.; et al. O-GlcNAcylated p53 in the liver modulates hepatic glucose production. Nat. Commun. 2021, 12, 5068. [Google Scholar] [CrossRef]
- Bik, D. Influence of sodium selenite and emulsigen injection on the concentration of mineral elements and activity of creatine kinase and asparagine and alanine transaminases in sheep serum. Bull. Vet. Inst. Pulawy 1996, 40, 105–110. [Google Scholar]
- Cui, Q.; Tao, H.; Zhao, Y.; Li, H.; Zhou, W.; Zhou, Y. Effects of selenium yeast tablets combined with sodium levothyroxine on serum myocardial enzymes, creatine kinase, hcy and lp(a) in patients with hypothyroidism. Acta Med. Mediterr. 2020, 36, 1339–1343. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef] [PubMed]
- Feron, O. Pyruvate into lactate and back: From the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 2009, 92, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Lugokenski, T.H.; Mueller, L.G.; Taube, P.S.; Rocha, J.B.T.; Pereira, M.E. Inhibitory effect of ebselen on lactate dehydrogenase activity from mammals: A comparative study with diphenyl diselenide and diphenyl ditelluride. Drug Chem. Toxicol. 2011, 34, 66–76. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Chung, T.-W.; Han, C.W.; Park, S.Y.; Park, K.H.; Jang, S.B.; Ha, K.-T. A Novel Lactate Dehydrogenase Inhibitor, 1-(Phenylseleno)-4-(trifluoromethyl) Benzene, Suppresses Tumor Growth through Apoptotic Cell Death. Sci. Rep. 2019, 9, 3969. [Google Scholar] [CrossRef]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite Profiling Identifies a Key Role for Glycine in Rapid Cancer Cell Proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef]
- Mukha, D.; Fokra, M.; Feldman, A.; Sarvin, B.; Sarvin, N.; Nevo-Dinur, K.; Besser, E.; Hallo, E.; Aizenshtein, E.; Schug, Z.T.; et al. Glycine decarboxylase maintains mitochondrial protein lipoylation to support tumor growth. Cell Metab. 2022, 34, 775–782. [Google Scholar] [CrossRef]
- Lozoya, O.A.; Martinez-Reyes, I.; Wang, T.; Grenet, D.; Bushel, P.; Li, J.; Chandel, N.; Woychik, R.P.; Santos, J.H. Mitochondrial nicotinamide adenine dinucleotide reduced (NADH) oxidation links the tricarboxylic acid (TCA) cycle with methionine metabolism and nuclear DNA methylation. PLoS Biol. 2018, 16, e2005707. [Google Scholar] [CrossRef]
- Pons, D.G.; Moran, C.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. Micronutrients Selenomethionine and Selenocysteine Modulate the Redox Status of MCF-7 Breast Cancer Cells. Nutrients 2020, 12, 865. [Google Scholar] [CrossRef]
- Souba, W.W.; Pacitti, A.J. How amino-acids get into cells—Mechanisms, models, menus, and mediators. J. Parenter. Enter. Nutr. 1992, 16, 569–578. [Google Scholar] [CrossRef]
- Batey, R.T.; Gilbert, S.D.; Montange, R.K. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature 2004, 432, 411–415. [Google Scholar] [CrossRef]
- Xiao, W.; Loscalzo, J. Metabolic Responses to Reductive Stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef] [Green Version]
| No. | Metabolites | Correlation Coefficients | ||
|---|---|---|---|---|
| Model 1 a | Model 2 b | Model 3 c | ||
| 1 | Acetate | 0.885 | 0.883 | −0.065 |
| 3 | Aspartate (Asp) | −0.789 | −0.844 | −0.933 |
| 4 | Choline | −0.990 | −0.062 | −0.051 |
| 5 | Citrate | −0.491 | −0.985 | −0.954 |
| 6 | Creatine | −0.983 | −0.964 | −0.979 |
| 8 | Formate | 0.708 | 0.889 | 0.106 |
| 9 | Fumarate | 0.888 | 0.345 | 0.650 |
| 10 | Glutamate (Glu) | 0.961 | 0.922 | 0.894 |
| 11 | Glutamine (Gln) | 0.890 | 0.632 | 0.389 |
| 13 | Glycerophosphocholine (GPC) | −0.980 | −0.814 | −0.629 |
| 14 | Glycine (Gly) | 0.583 | −0.737 | −0.825 |
| 15 | Histidine (His) | −0.421 | −0.829 | −0.736 |
| 16 | Hypoxanthine | 0.045 | 0.829 | 0.918 |
| 17 | Isoleucine (Ile) | 0.964 | 0.928 | 0.843 |
| 18 | Lactate | −0.717 | −0.901 | −0.875 |
| 19 | Leucine (Leu) | 0.979 | 0.904 | 0.823 |
| 24 | NAD | −0.801 | −0.342 | −0.287 |
| 25 | Phenylalanine (Phe) | 0.983 | 0.849 | 0.737 |
| 27 | Succinate | 0.944 | 0.405 | 0.162 |
| 30 | Tryptophan (Trp) | 0.844 | 0.922 | 0.975 |
| 31 | Tyrosine (Tyr) | 0.977 | 0.879 | 0.777 |
| 33 | UDP-Glucuronate | −0.964 | −0.950 | −0.948 |
| 34 | UDP-N-acetylgalactosamine | −0.976 | −0.969 | −0.950 |
| 35 | UDP-N-acetylglucosamine | −0.989 | −0.944 | −0.951 |
| 36 | Uridine | −0.814 | −0.176 | −0.062 |
| 37 | Valine (Val) | 0.956 | 0.925 | 0.849 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Chai, X.; Men, J.; Rao, S.; Cong, X.; Cheng, S.; Qiao, Z. Does Methionine Status Influence the Outcome of Selenomethinione Supplementation? A Comparative Study of Metabolic and Selenium Levels in HepG2 Cells. Nutrients 2022, 14, 3705. https://doi.org/10.3390/nu14183705
Hu Y, Chai X, Men J, Rao S, Cong X, Cheng S, Qiao Z. Does Methionine Status Influence the Outcome of Selenomethinione Supplementation? A Comparative Study of Metabolic and Selenium Levels in HepG2 Cells. Nutrients. 2022; 14(18):3705. https://doi.org/10.3390/nu14183705
Chicago/Turabian StyleHu, Yili, Xiaocui Chai, Jun Men, Shen Rao, Xin Cong, Shuiyuan Cheng, and Zhixian Qiao. 2022. "Does Methionine Status Influence the Outcome of Selenomethinione Supplementation? A Comparative Study of Metabolic and Selenium Levels in HepG2 Cells" Nutrients 14, no. 18: 3705. https://doi.org/10.3390/nu14183705
APA StyleHu, Y., Chai, X., Men, J., Rao, S., Cong, X., Cheng, S., & Qiao, Z. (2022). Does Methionine Status Influence the Outcome of Selenomethinione Supplementation? A Comparative Study of Metabolic and Selenium Levels in HepG2 Cells. Nutrients, 14(18), 3705. https://doi.org/10.3390/nu14183705






