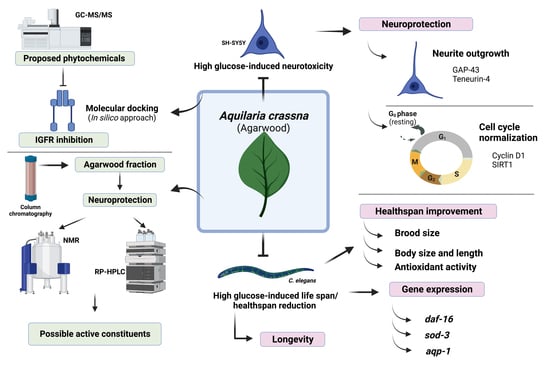
Aquilaria crassna Leaf Extract Ameliorates Glucose-Induced Neurotoxicity In Vitro and Improves Lifespan in Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Extraction
2.3. Cell Line
2.4. Antioxidant Determination
2.4.1. Folin–Ciocalteu Phenol Assay (FCP)
2.4.2. Determination of Total Flavonoid
2.4.3. Radical Scavenging Activity Assays
2.5. MTT Assay
2.6. Intracellular Reactive Oxygen Species (ROS) Assay
2.7. Neurite Outgrowth Assay and Scoring of Neurite Length and Neurite-Bearing Cells
2.8. Cell Cycle Analysis
2.9. Western Blot Analysis
2.10. C. elegans Strain, Maintenance, Synchronization, and Treatment
2.11. Brood Size, Body Length, and Body Size
2.12. Intracellular ROS Accumulation
2.13. Lifespan Assay
2.14. RT-qPCR
2.15. Phytochemical Constituent Analysis of Extracts by Gas Chromatograph-Mass Spectrometer/Mass Spectrometer (GC-MS/MS) Analysis
2.16. Plant Extract Isolation and Identification
2.17. Reverse-Phase High-Pressure Liquid Chromatography (RP-HPLC)
2.18. Molecular Docking
2.18.1. Ligand Preparation
2.18.2. Protein Preparation
2.18.3. Molecular Docking
2.19. Statistical Analysis
3. Results
3.1. Antioxidant Properties and Total Phenolic and Flavonoid Contents
3.2. Effects of ACH on Cell Viability and High Glucose-Induced Reactive Oxygen Species (ROS)
3.3. Effects of ACH on Neurite Outgrowth
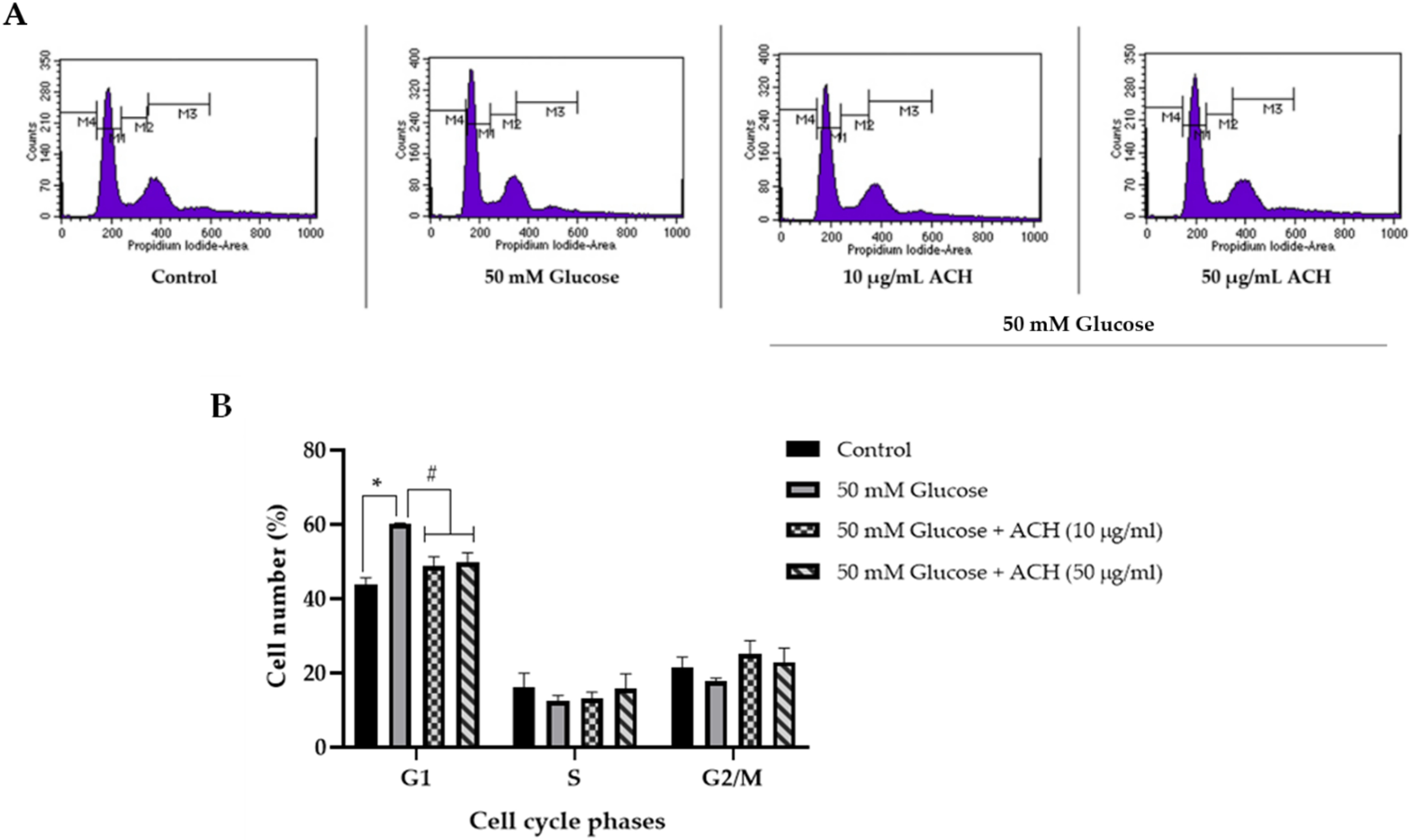
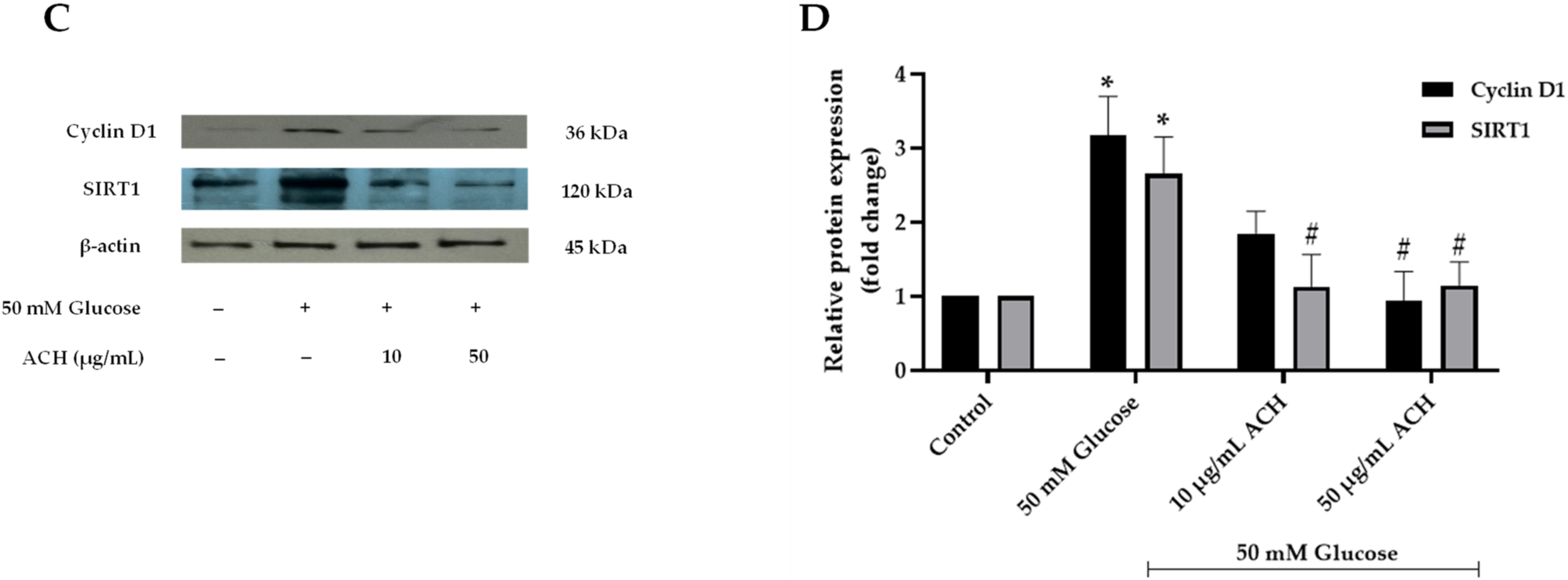
3.4. Effects of ACH on Cell Cycle Delay
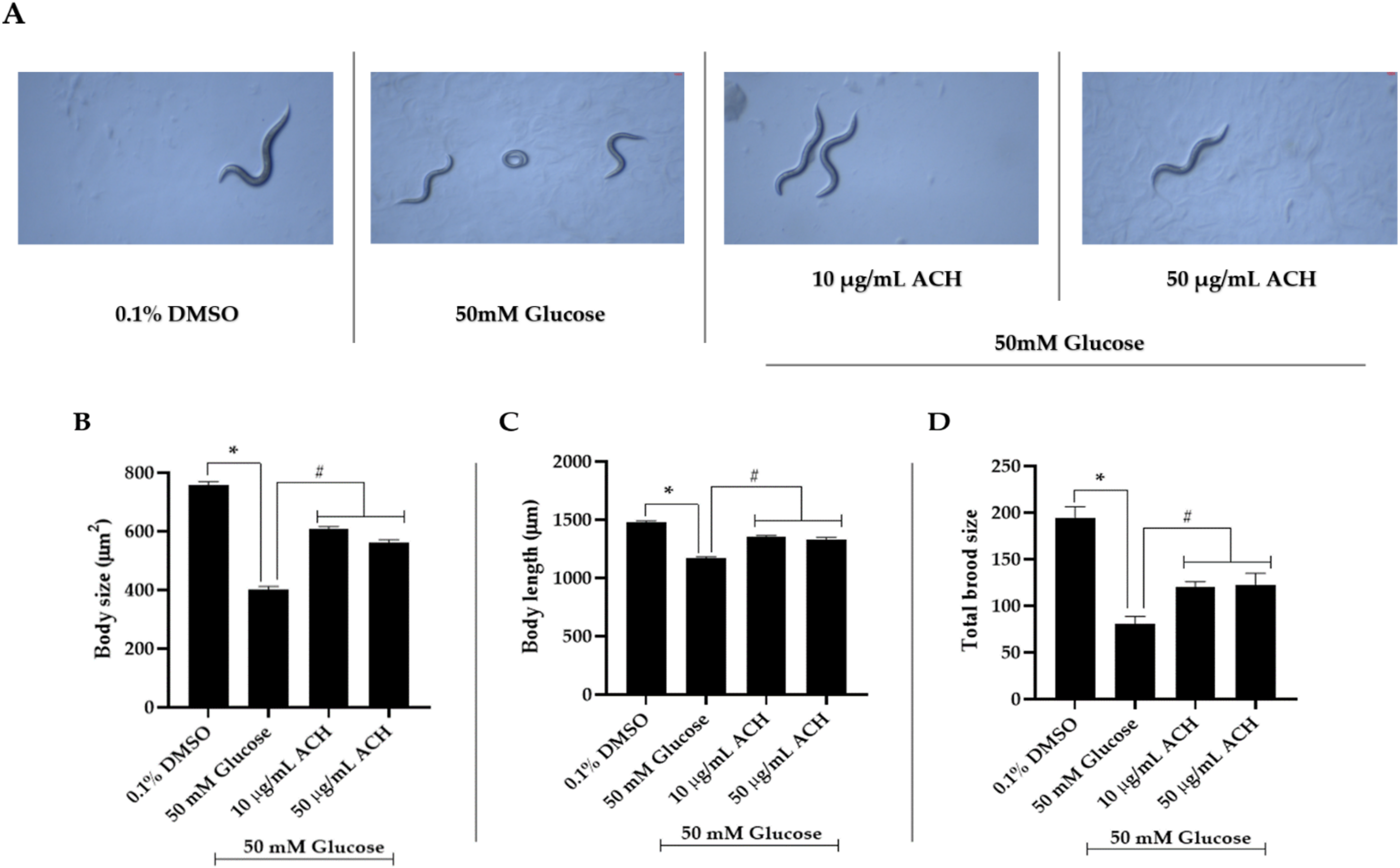
3.5. ACH Extract Attenuated the High Glucose-Induced Reduction of Body Length and Size and Brood Size
3.6. ACH Extract against High Glucose-Induced Oxidative Stress in C. elegans
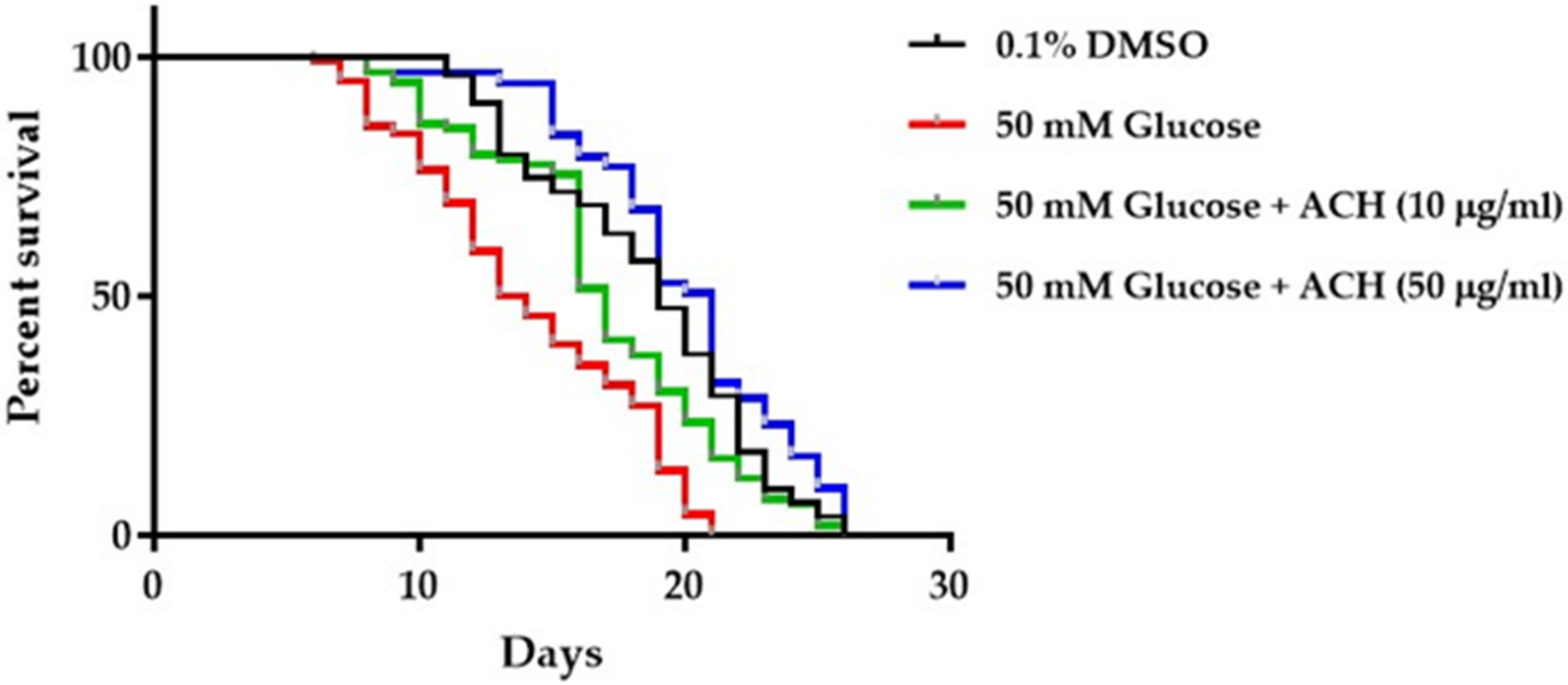
3.7. ACH Extracts Extend Lifespan in High Glucose-Fed Worms
3.8. ACH Extracts Mediated Extension of Lifespan and Healthspan in High Glucose-Fed Worms through DAF-16/FoxO and aqp-1
3.9. Phytochemical Constituents of ACH
3.10. The Ability of ACH-Derived Phytochemical Constituents as Inhibitors of IGFR Using an In Silico Approach
3.11. Isolation and Chemical Characterization of Active Compounds in ACH Extract
3.12. Compound 1 and 2 (A Mixture of Stigmastrol and β-Sitosterol)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Aquilaria crassna |
| ACH | AC hexane extract |
| GAP-43 | Growth-associated protein 43 |
| SIRT1 | Surtuin-1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| DAF-16/FoxO | Forkhead box protein O |
| sod-3 | Superoxide dismutase-3 |
| aqp-1 | Aquaporin-1 |
| ROS | Reactive oxygen species |
| H2DCFDA | 2,7-Dichlorofluorescein diacetate |
| ABTS | 2,2-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | Diammonium salt, 2,2-diphenyl-1-picrylhydrazyl |
| DMSO | Dimethyl sulfoxide |
| GC-MS/MS | Gas chromatography mass spectrometry/mass spectrometry |
| NMR | Nuclear magnetic resonance |
| RP-HPLC | Reverse-phase high-performance liquid chromatography |
| TLC | Thin layer chromatography |
| VCEAC | Vitamin C equivalent antioxidant capacity |
| IGFR | Insulin-like growth factor 1 receptor |
| EGCG | Epigallocatechin gallate |
References
- Schartner, E.; Sabbir, M.G.; Saleh, A.; Silva, R.V.; Chowdhury, S.R.; Smith, D.R.; Fernyhough, P. High glucose concentration suppresses a SIRT2 regulated pathway that enhances neurite outgrowth in cultured adult sensory neurons. Exp. Neurol. 2018, 309, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Saal, K.A.; Galter, D.; Roeber, S.; Bähr, M.; Tönges, L.; Lingor, P. Altered Expression of Growth Associated Protein-43 and Rho Kinase in Human Patients with Parkinson’s Disease. Brain Pathol. 2017, 27, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.N.; Negi, G.; Kharatmal, S.B.; Mule, N.K.; Sharma, D.; Sharma, S.S. Short-term extracellular glucose exposure alters neurite outgrowth and intracellular reactive oxygen species without altering viability in neuronal cells. Biology 2017, 55, 648–654. [Google Scholar]
- Schlotterer, A.; Kukudov, G.; Bozorgmehr, F.; Hutter, H.; Du, X.; Oikonomou, D.; Ibrahim, Y.; Pfisterer, F.; Rabbani, N.; Thornalley, P. C. elegans as model for the study of high glucose-mediated life span reduction. Diabetes 2009, 58, 2450–2456. [Google Scholar] [CrossRef]
- Barden, A.; Anak, N.A.; Mulliken, T.; Song, M. Heart of the matter: Agarwood use and trade and CITES implementation for Aquilaria malaccensis. In Traffic International; Traffic: Cambridge, UK, 2000; pp. 1–52. [Google Scholar]
- Persoon, G.A.; Beek, H. Growing ‘the wood of the gods’: Agarwood production in southeast Asia. In Smallholder Tree Growing for Rural Development and Environmental Services; Springer: Berlin/Heidelberg, Germany, 2008; pp. 245–262. [Google Scholar]
- Hashim, Y.Z.H.-Y.; Kerr, P.G.; Abbas, P.; Salleh, H.M. Aquilaria spp.(agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 189, 331–360. [Google Scholar] [CrossRef]
- Peng, D.-Q.; Yu, Z.-X.; Wang, C.-H.; Gong, B.; Liu, Y.-Y.; Wei, J.-H. Chemical Constituents and Anti-Inflammatory Effect of Incense Smoke from Agarwood Determined by GC-MS. Int. J. Anal. Chem. 2020, 2020, 4575030. [Google Scholar] [CrossRef]
- Jermsri, P.; Jiraviriyakul, A.; Unajak, S.; Kumphune, S. Effect of Aquilaria crassna crude extract on simulated ischemia induced cardiac cell death. Int. J. Pharm. Bio Sci. 2012, 3, 604–613. [Google Scholar]
- Jermsri, P.; Kumphune, S. Ethylacetate extract of Aquilaria crassna preserve actin cytoskeleton on simulated ischemia induced cardiac cell death. J. Med. Plants Res. 2012, 6, 4057–4062. [Google Scholar]
- Kim, Y.; Lee, E.; Lee, Y.; Kim, H.; Song, B.; Lee, E.; Kim, H. Effect of the aqueous extract of Aquilaria agallocha stems on the immediate hypersensitivity reactions. J. Ethnopharmacol. 1997, 58, 31–38. [Google Scholar] [CrossRef]
- Vakati, K.; Rahman, H.; Eswaraiah, M.C.; Dutta, A. Evaluation of hepatoprotective activity of ethanolic extract of Aquilaria agallocha leaves (EEAA) against CCl4 induced hepatic damage in rat. Sch. J. App. Med. Sci. 2013, 1, 9–12. [Google Scholar]
- Kamonwannasit, S.; Nantapong, N.; Kumkrai, P.; Luecha, P.; Kupittayanant, S.; Chudapongse, N. Antibacterial activity of Aquilaria crassna leaf extract against Staphylococcus epidermidis by disruption of cell wall. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 1–7. [Google Scholar] [CrossRef]
- Dahham, S.S.; Ahamed, M.B.K.; Saghir, S.M.; Alsuede, F.S.; Iqbal, M.A.; Majid, A.M.S.A. Bioactive essential oils from Aquilaria crassna for cancer prevention and treatment. Glob. J. Adv. Pure Appl. Sci. 2014, 4, 26–31. [Google Scholar]
- Supasuteekul, C.; Tadtong, S.; Putalun, W.; Tanaka, H.; Likhitwitayawuid, K.; Tengamnuay, P.; Sritularak, B. Neuritogenic and neuroprotective constituents from Aquilaria crassna leaves. J. Food Biochem. 2017, 41, e12365. [Google Scholar] [CrossRef]
- Pranakhon, R.; Pannangpetch, P.; Aromdee, C. Antihyperglycemic activity of agarwood leaf extracts in STZ-induced diabetic rats and glucose uptake enhancement activity in rat adipocytes. Songklanakarin J. Sci. Technol. 2011, 33, 405–410. [Google Scholar]
- Prasansuklab, A.; Meemon, K.; Sobhon, P.; Tencomnao, T. Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans. BMC Complement. Altern. Med. 2017, 17, 551. [Google Scholar] [CrossRef]
- Rangsinth, P.; Prasansuklab, A.; Duangjan, C.; Gu, X.; Meemon, K.; Wink, M.; Tencomnao, T. Leaf extract of Caesalpinia mimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 164. [Google Scholar] [CrossRef] [Green Version]
- Pattarachotanant, N.; Prasansuklab, A.; Tencomnao, T. Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E. Nutrients 2021, 13, 2368. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Tencomnao, T. Citrus hystrix extracts protect human neuronal cells against high glucose-induced senescence. Pharmaceuticals 2020, 13, 283. [Google Scholar] [CrossRef]
- Wong, K.-H.; Sabaratnam, V.; Naidu, M.; Keynes, R. Activity of aqueous extracts of lion’s mane mushroom Hericium erinaceus (Bull.: Friday) Pers.(Aphyllophoromycetideae) on the neural cell line NG108-15. Int. J. Med. Mushrooms 2007, 9, 57–65. [Google Scholar] [CrossRef]
- Nasu, R.; Furukawa, A.; Suzuki, K.; Takeuchi, M.; Koriyama, Y. The effect of glyceraldehyde-derived advanced glycation end products on β-tubulin-inhibited neurite outgrowth in sh-sy5y human neuroblastoma cells. Nutrients 2020, 12, 2958. [Google Scholar] [CrossRef]
- Ren, M.; Feng, H.; Fu, Y.; Land, M.; Rubin, C.S. Protein kinase D is an essential regulator of C. elegans innate immunity. Immunity 2009, 30, 521–532. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Wink, M.; Tencomnao, T. Lifespan extending and oxidative stress resistance properties of a leaf extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxidative Med. Cell. Longev. 2019, 2019, 9012396. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.-Y.; Hsiang, C.-Y.; Ho, S.-C.; Ho, T.-Y.; Lee, K.-T. Chemical profiles of incense smoke ingredients from agarwood by headspace gas chromatography-tandem mass spectrometry. Molecules 2018, 23, 2969. [Google Scholar] [CrossRef]
- Yusof, S.; Tajuddin, S.N.; Mansor, R.; Cheng, P.W.; Ramli, A.N.M. Gas chromatography analysis of artificially inoculated agarwood compounds related to high quality agarwood from Malaysia plantation. Chem. Adv. Mater. 2018, 3, 60–66. [Google Scholar]
- Rangsinth, P.; Duangjan, C.; Sillapachaiyaporn, C.; Isidoro, C.; Prasansuklab, A.; Tencomnao, T. Caesalpinia mimosoides Leaf Extract Promotes Neurite Outgrowth and Inhibits BACE1 Activity in Mutant APP-Overexpressing Neuronal Neuro2a Cells. Pharmaceuticals 2021, 14, 901. [Google Scholar] [CrossRef]
- Tao, M.; Li, R.; Zhang, Z.; Wu, T.; Xu, T.; Zogona, D.; Huang, Y.; Pan, S.; Xu, X. Vitexin and Isovitexin Act Through Inhibition of Insulin Receptor to Promote Longevity and Fitness in Caenorhabditis elegans. Mol. Nutr. Food Res. 2022, 66, e2100845. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. J. Tradit. Complement. Med. 2021, 11, 158–172. [Google Scholar] [CrossRef]
- Lam, C.T.; Gong, A.G.; Lam, K.Y.; Zhang, L.M.; Chen, J.-P.; Dong, T.T.; Lin, H.-Q.; Tsim, K.W. Jujube-containing herbal decoctions induce neuronal differentiation and the expression of anti-oxidant enzymes in cultured PC12 cells. J. Ethnopharmacol. 2016, 188, 275–283. [Google Scholar] [CrossRef]
- Suzuki, N.; Numakawa, T.; Chou, J.; de Vega, S.; Mizuniwa, C.; Sekimoto, K.; Adachi, N.; Kunugi, H.; Arikawa-Hirasawa, E.; Yamada, Y. Teneurin-4 promotes cellular protrusion formation and neurite outgrowth through focal adhesion kinase signaling. FASEB J. 2014, 28, 1386–1397. [Google Scholar] [CrossRef]
- Zhang, S.; Duangjan, C.; Tencomnao, T.; Liu, J.; Lin, J.; Wink, M. Neuroprotective effects of oolong tea extracts against glutamate-induced toxicity in cultured neuronal cells and β-amyloid-induced toxicity in Caenorhabditis elegans. Food Funct. 2020, 11, 8179–8192. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Hara, Y.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 334, 947–953. [Google Scholar] [CrossRef]
- Arablou, T.; Delbandi, A.A.; Khodaverdi, S.; Arefi, S.; Kolahdouz-Mohammadi, R.; Heidari, S.; Mohammadi, T.; Aryaeian, N. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother. Res. 2019, 33, 1044–1054. [Google Scholar] [CrossRef]
- Hioki, T.; Kawabata, T.; Sakai, G.; Fujita, K.; Kuroyanagi, G.; Matsushima-Nishiwaki, R.; Kim, W.; Otsuka, T.; Iida, H.; Tokuda, H. Resveratrol suppresses insulin-like growth factor I-induced osteoblast migration: Attenuation of the p44/p42 MAP kinase pathway. Biosci. Biotechnol. Biochem. 2020, 84, 2428–2439. [Google Scholar] [CrossRef]
- Vanamala, J.; Reddivari, L.; Radhakrishnan, S.; Tarver, C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer 2010, 10, 238. [Google Scholar] [CrossRef]
- Shindo, H.; Thomas, T.P.; Larkin, D.D.; Karihaloo, A.K.; Inada, H.; Onaya, T.; Stevens, M.J.; Greene, D.A. Modulation of basal nitric oxide-dependent cyclic-GMP production by ambient glucose, myo-inositol, and protein kinase C in SH-SY5Y human neuroblastoma cells. J. Clin. Investig. 1996, 97, 736–745. [Google Scholar] [CrossRef]
- Takano, T.; Funahashi, Y.; Kaibuchi, K. Neuronal polarity: Positive and negative feedback signals. Front. Cell Dev. Biol. 2019, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Demura, T.; Morita, M.; Banker, G.A.; Tanii, T.; Nakamura, S. Differential neurite outgrowth is required for axon specification by cultured hippocampal neurons. J. Neurochem. 2012, 123, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Benowitz, L.I.; Routtenberg, A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997, 20, 84–91. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, J.-H.; Lee, H.-Y.; Min, K.-J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24. [Google Scholar] [CrossRef]
- Lamichane, S.; Baek, S.H.; Kim, Y.-J.; Park, J.H.; Dahal Lamichane, B.; Jang, W.B.; Ji, S.; Lee, N.K.; Dehua, L.; Kim, D.Y. MHY2233 attenuates replicative cellular senescence in human endothelial progenitor cells via SIRT1 signaling. Oxidative Med. Cell. Longev. 2019, 2019, 6492029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Y.; Vanhoutte, P.M. SIRT1 and AMPK in regulating mammalian senescence: A critical review and a working model. FEBS Lett. 2011, 585, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zheng, Z.; Wang, Y.; Zhou, Q.; Cai, W.; Jia, W.; Yang, L.; Dong, M.; Zhu, X.; Su, L. SIRT1 is a regulator in high glucose-induced inflammatory response in RAW264. 7 cells. PLoS ONE 2015, 10, e0120849. [Google Scholar]
- Oberdoerffer, P.; Michan, S.; McVay, M.; Mostoslavsky, R.; Vann, J.; Park, S.-K.; Hartlerode, A.; Stegmuller, J.; Hafner, A.; Loerch, P. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 2008, 135, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef]
- Mendler, M.; Schlotterer, A.; Morcos, M.; Nawroth, P. Understanding diabetic polyneuropathy and longevity: What can we learn from the nematode Caenorhabditis elegans? Exp. Clin. Endocrinol. Diabetes 2012, 120, 182–183. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Brimson, J.M.; Chuchawankul, S.; Sukprasansap, M.; Tencomnao, T. Antiaging, stress resistance, and neuroprotective efficacies of Cleistocalyx nervosum var. paniala fruit extracts using Caenorhabditis elegans model. Oxidative Med. Cell. Longev. 2019, 2019, 7024785. [Google Scholar] [CrossRef]
- Katic, M.; Kahn, C. The role of insulin and IGF-1 signaling in longevity. Cell. Mol. Life Sci. CMLS 2005, 62, 320–343. [Google Scholar] [CrossRef]
- Salih, D.A.; Brunet, A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 2008, 20, 126–136. [Google Scholar] [CrossRef]
- Jensen, V.L.; Gallo, M.; Riddle, D.L. Targets of DAF-16 involved in Caenorhabditis elegans adult longevity and dauer formation. Exp. Gerontol. 2006, 41, 922–927. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef]
- Lee, S.-J.; Murphy, C.T.; Kenyon, C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009, 10, 379–391. [Google Scholar] [CrossRef]
- Barbieri, M.; Bonafè, M.; Franceschi, C.; Paolisso, G. Insulin/IGF-I-signaling pathway: An evolutionarily conserved mechanism of longevity from yeast to humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1064–E1071. [Google Scholar] [CrossRef]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef]
- Mao, K.; Quipildor, G.F.; Tabrizian, T.; Novaj, A.; Guan, F.; Walters, R.O.; Delahaye, F.; Hubbard, G.B.; Ikeno, Y.; Ejima, K. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Pratiwi, R.; Nantasenamat, C.; Ruankham, W.; Suwanjang, W.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Mechanisms and neuroprotective activities of stigmasterol against oxidative stress-induced neuronal cell death via sirtuin family. Front. Nutr. 2021, 8, 648995. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Liu, J.; Pan, X.; Zhao, Q. Stigmasterol exerts neuro-protective effect against ischemic/reperfusion injury through reduction of oxidative stress and inactivation of autophagy. Neuropsychiatr. Dis. Treat. 2019, 15, 2991. [Google Scholar] [CrossRef] [Green Version]
- Burg, V.K.; Grimm, H.S.; Rothhaar, T.L.; Grösgen, S.; Hundsdörfer, B.; Haupenthal, V.J.; Zimmer, V.C.; Mett, J.; Weingärtner, O.; Laufs, U. Plant sterols the better cholesterol in Alzheimer’s disease? A mechanistical study. J. Neurosci. 2013, 33, 16072–16087. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol alleviates inflammatory response via inhibiting the activation of ERK/p38 and NF-κB pathways in LPS-exposed BV2 cells. BioMed Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef]
- Reddy, P.H. Role of mitochondria in neurodegenerative diseases: Mitochondria as a therapeutic target in Alzheimer’s disease. CNS Spectr. 2009, 14.S7, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bari, W.U.; Zahoor, M.; Zeb, A.; Khan, I.; Nazir, Y.; Khan, A.; Rehman, N.U.; Ullah, R.; Shahat, A.A.; Mahmood, H.M. Anticholinesterase, antioxidant potentials, and molecular docking studies of isolated bioactive compounds from Grewia optiva. Int. J. Food Prop. 2019, 22, 1386–1396. [Google Scholar] [CrossRef]
- Koga, T.; Sakamoto, T.; Sakuradani, E.; Tai, A. Neurite Outgrowth-Promoting Activity of Compounds in PC12 Cells from Sunflower Seeds. Molecules 2020, 25, 4748. [Google Scholar] [CrossRef] [PubMed]



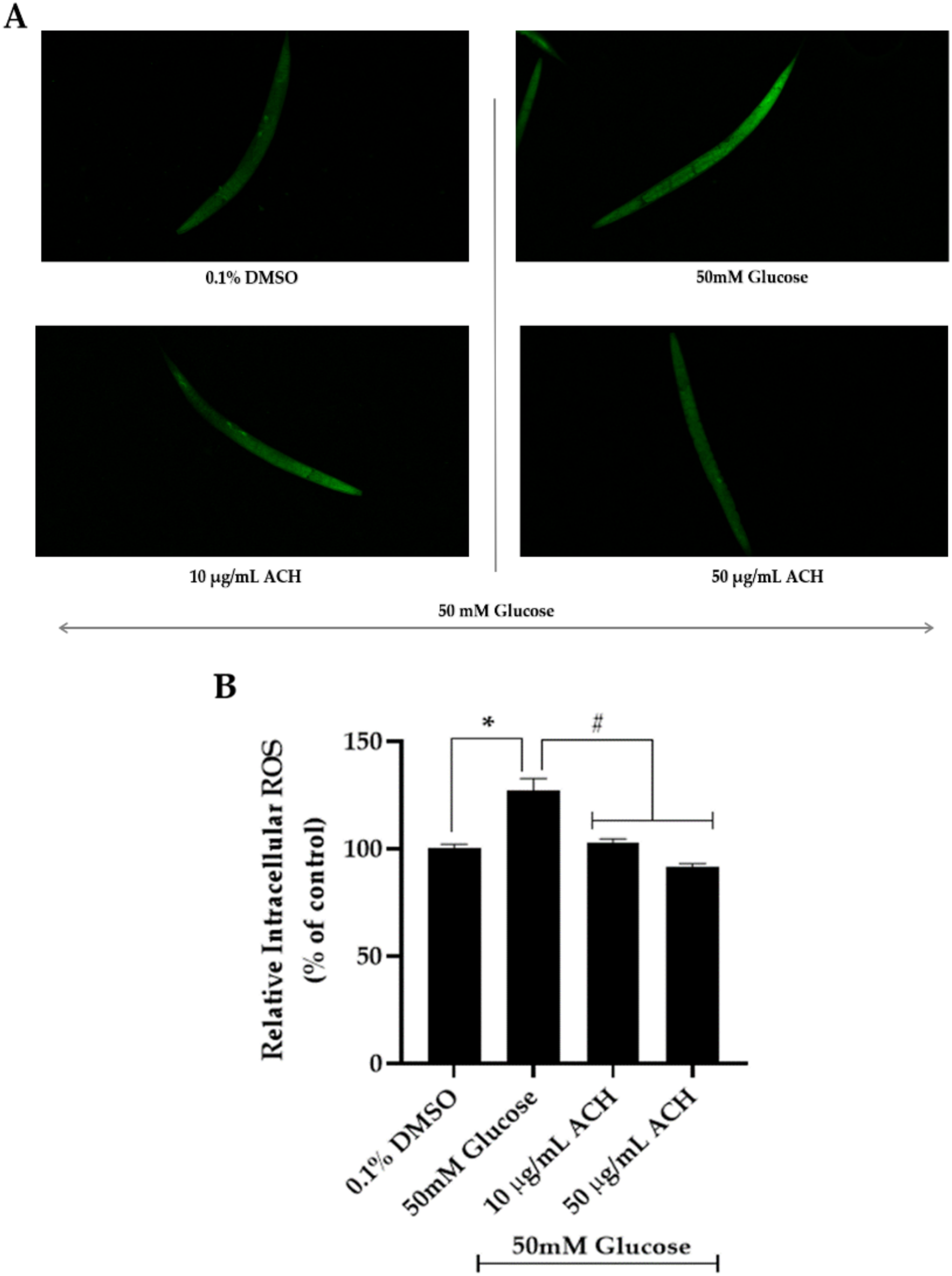
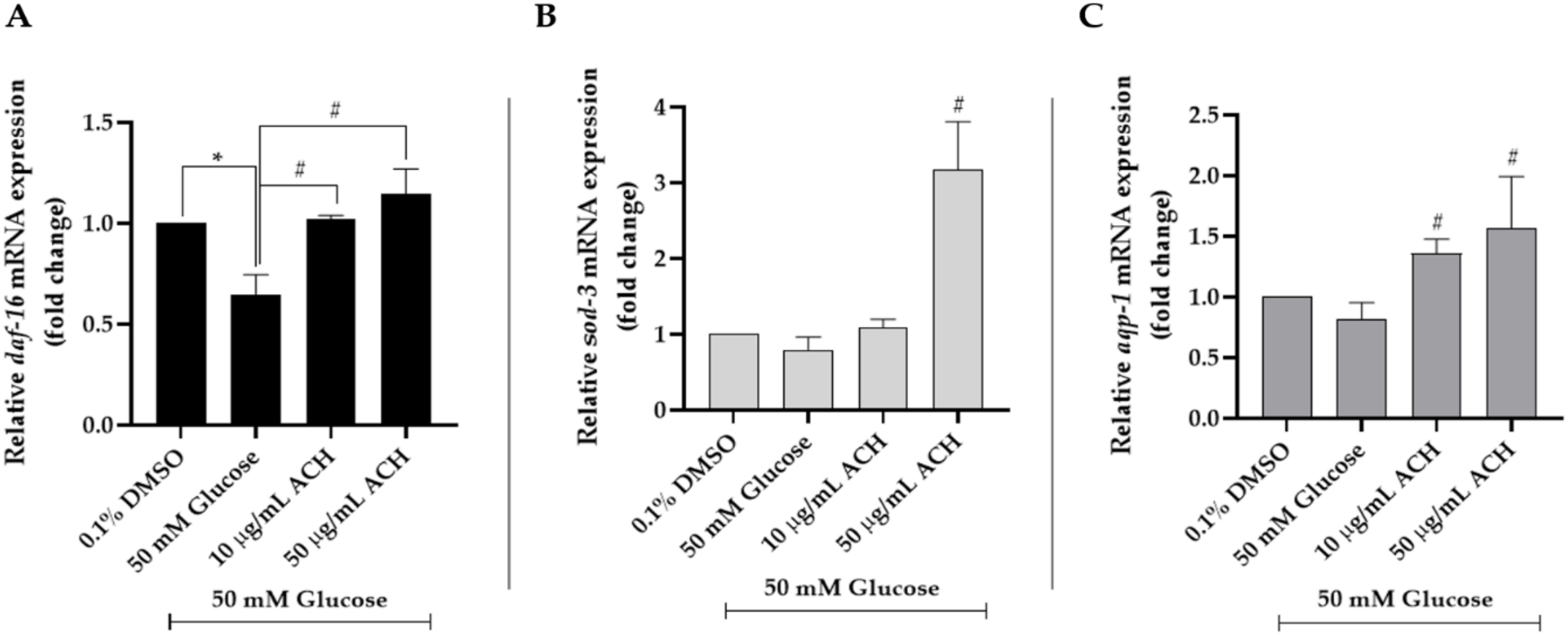
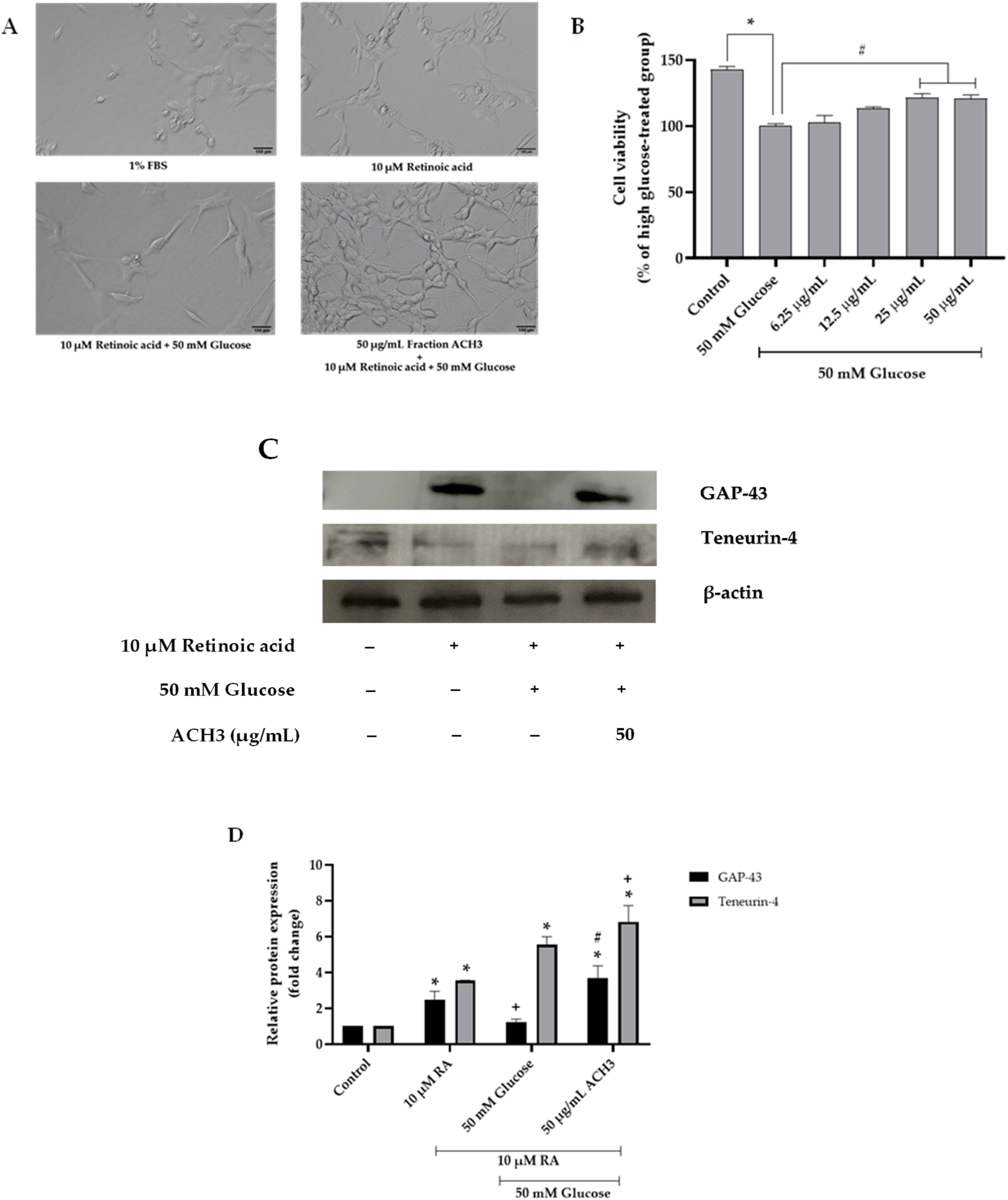
| Primer | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| daf-16 | TTTCCGTCCCCGAACTCAA | ATTCGCCAACCCATGATGG |
| sod-3 | TTCGAAAGGGAATCTAAAAGAAG | GCCAAGTTGGTCCAGAAGATAG |
| aqp-1 | TTTTGGCAAGGAACCTCATC | GCTGTTGCCATAACTGCAAA |
| act-1 | AGACAATGGATCCGGAATGT | CATCCCAGTTGGTGACGATA |
| Groups | Mean Lifespan | p-Value (vs. Control) | p-Value (vs. 50 mM Glucose) | Number of Worms | |
|---|---|---|---|---|---|
| Day ± SEM | % Increase (vs. 50 mM Glucose) | ||||
| 0.1% DMSO | 18.55 ± 0.42 | 31.00 | - | 0.0001 | 102 |
| 50 mM Glucose | 14.16 ± 0.40 | - | 0.0001 | - | 118 |
| 50 mM Glucose + 10 μg/mL ACH | 17.01 ± 0.47 | 20.13 | 0.0379 | 0.0001 | 93 |
| 50 mM Glucose + 50 μg/mL ACH | 19.93 ± 0.43 | 40.75 | 0.0192 | 0.0001 | 91 |
| Compound | RT | Area (%) | MF | MW |
|---|---|---|---|---|
| tetradecane | 22.867 | 0.15 | C14H30 | 198 |
| hexadecane | 30.730 | 0.12 | C16H34 | 226 |
| phytol | 39.231 | 0.3 | C20H40O | 296 |
| n-hexadecanoic acid | 43.3 | 0.9 | C16H32O2 | 256 |
| oleic acid | 48.638 | 0.57 | C18H34O2 | 282 |
| oleamide (9-Octadecenamide, (Z)-) | 54.799 | 0.97 | C18H35NO | 281 |
| squalene | 66.506 | 13.55 | C30H50 | 410 |
| nonacosane | 68.036 | 0.49 | C29H60 | 408 |
| 9,19-cyclolanost-24-en-3-ol, acetate, (3.β.) | 68.794 | 0.38 | C32H52O2 | 468 |
| 2,2,4-trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetraenyl)-cyclohexanol | 69.037 | 0.4 | C30H52O | 428 |
| ϒ-tocopherol | 71.233 | 0.69 | C28H48O2 | 416 |
| hentriacontane | 72.371 | 2.68 | C31H64 | 436 |
| vitamin E | 73 | 8.94 | C29H50O2 | 430 |
| stigmasterol | 75.305 | 1.17 | C29H48O | 412 |
| D-friedoolean-14-en-3-one | 76.122 | 1.25 | C30H48O | 424 |
| tritriacontane | 76.463 | 9.38 | C33H68 | 464 |
| β-amyrin | 76.547 | 5.17 | C30H50O | 426 |
| olean-12-en-3-one | 77.411 | 2.23 | C30H48O | 424 |
| lupenone (lup-20(29)-en-3-one) | 77.498 | 3.31 | C30H48O | 424 |
| α-amyrin | 77.878 | 1.94 | C30H50O | 426 |
| lup-20(29)-en-3-ol, acetate, (3.β.) | 78.077 | 0.93 | C32H52O2 | 468 |
| D:A-friedooleanan-7-one, 3-hydroxy | 78.41 | 1.19 | C30H50O2 | 442 |
| ursa-9(11),12-dien-3-ol | 78.65 | 1.16 | C30H48O | 424 |
| 9,19-cyclolanostan-3-ol,24,24-epoxymethano, acetate | 78.796 | 3.74 | C33H54O3 | 498 |
| betulin | 79.05 | 1.00 | C31H52O | 440 |
| friedelan-3-one | 79.473 | 0.42 | C30H50O | 426 |
| D:A-friedooleanan-3-ol, (3.α.) | 80.004 | 10.21 | C30H52O | 428 |
| pentatriacontane | 80.197 | 1.42 | C35H72 | 492 |
| 24-methylenecycloartan-3-one | 80.474 | 14.17 | C31H50O | 438 |
| No. | Compound | MW | Binding Energy (kcal/mol) | Inhibition Constant | Amino Acid Interaction | ||
|---|---|---|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Bond | Electrostatic Bond | |||||
| EGCG (positive control) | 458.4 | −6.54 | 16.18 μM | ASP1086 SER1089 GLU1015 | LEU1005 (2) ARG1084 (2) GLY1085 | ||
| Resveratrol (positive control) | 228.24 | −6.57 | 15.39 μM | MET1082 MET1082 GLU1080 GLN1007 | VAL1013 ALA1031 MET1156 | ||
| 1 | 24-Methylenecycloartan-3-one | 438.7 | −5.13 | 172.22 μM | GLY1085 | LEU1005 VAL1013 (2) ALA1031 LYS1033 MET1079 LEU1081 (2) MET1082 MET1142 MET1156 (2) | |
| 2 | Squalene | 410.7 | −6.37 | 21.57 μM | ARG1003 LEU1005 (3) VAL1013 ALA1031 (2) LYS1033 VAL1063 MET1079 LEU1081 (2) MET1142 (2) MET1156 (2) | ||
| 3 | D:A-Friedooleanan-3-ol, (3.alpha.)- | 428.7 | −5.90 | 47.43 μM | GLU1080 | VAL1013 ALA1031 ALA1031 MET1142 VAL1063 MET1079 MET1142 VAL1013 LYS1033 MET1079 VAL1013 MET1156 | |
| 4 | Friedelan-3-one | 426.7 | −7.88 | 1.66 μM | VAL1013 (3) ALA1031 (3) LYS1033 (2) MET1079 MET1142 MET1156 | ||
| 5 | Stigmasterol | 412.7 | −9.32 | 146.8 nM | ARG1003 | LEU1005 (3) VAL1013 (2) LEU1081 MET1142 (2) | |
| 6 | Tritriacontane | 464.9 | −3.51 | 2.69 mM | LEU1005 (3) ALA1031 (2) LYS1033 MET1079 MET1142 (4) MET1156 (2) ILE1160 | ||
| 7 | Vitamin E | 430.7 | −7.92 | 1.56 μM | GLY1085 ASP1086 SER1089 | VAL1013 (3) ALA1031 (2) LYS1033 MET1079 MET1082 MET1142 (2) MET1156 ILE1160 | ASP1086 |
| 8 | D-Friedoolean-14-en-3-one | 424.7 | −8.88 | 355.27 nM | LEU1005 (4) VAL1013 ALA1031 LEU1081 (2) MET1142 | ||
| 9 | 9,19-Cyclolanostan-3-ol,24,24-epoxymethano-, acetate | 498.8 | −7.30 | 4.49 μM | SER1089 GLY1085 MET1082 | LEU1005 VAL1013 (2) ALA1031 (2) LYS1033 MET1142 (2) MET1156 (2) ILE1160 MET1079 (2) LEU1081 | |
| 10 | D:A-Friedooleanan-7-one, 3-hydroxy- | 442.7 | −5.37 | 116.19 μM | GLU1080 | LEU1005 VAL1013 ALA1031 MET1079 MET1082 MET1142 (2) | |
| 11 | Beta-amyrin | 426.7 | −9.02 | 245.77 nM | THR1083 | LEU1005 VAL1013 (4) ALA1031 LYS1033 (3) MET1142 (2) MET1156 (2) | |
| 12 | Olean-12-en-3-one | 424.7 | −9.77 | 68.61 nM | LEU1005 VAL1013 (3) ALA1031 (3) LYS1033 (2) MET1079 (2) MET1142 (3) MET1156 | ||
| 13 | Lup-20(29)-en-3-ol, acetate, (3.beta.)- | 468.8 | −7.36 | 4.02 μM | LEU1005 VAL1013 ALA1031 (3) VAL1063 MET1079 LEU1081 MET1142 (3) | ||
| 14 | Lupenone (Lup-20(29)-en-3-one) | 424.7 | −9.56 | 97.57 nM | SER1089 | LEU1005 VAL1013 ALA1031 (2) LYS1033 (2) MET1142 (3) MET1156 (2) VAL1063 MET1079 LEU1081 MET1082 | |
| 15 | Gamma-Tocopherol | 416.7 | −7.75 | 2.08 μM | LEU1005 (3) VAL1013 (2) LYS1033 MET1079 LEU1081 MET1142 MET1156 (2) | ||
| 16 | Hentriacontane | 436.8 | −3.39 | 3.25 mM | LEU1005 (2) VAL1013 (2) ALA1031 (2) LYS1033 LEU1081 MET1142 (2) MET1156 ILE1160 (2) TYR1161 | ||
| 17 | Oleamide (9-Octadecenamide, (Z)-) | 281.5 | −4.51 | 490.34 μM | GLU1080 | LEU1005 (2) VAL1013 (3) ALA1031 LYS1033 (2) MET1079 MET1156 | |
| 18 | Alpha-amyrin | 426.7 | −9.21 | 178.76 nM | LEU1005 VAL1013 (2) ALA1031 (3) LYS1033 MET1079 MET1142 (2) MET1156 | ||
| 19 | Pentatriacontane | 492.9 | −1.59 | 68.88 mM | LEU1005 (5) VAL1013 ALA1031 ARG1084 MET1142 | ||
| 20 | Ursa-9(11),12-dien-3-ol | 424.7 | −8.53 | 554.63 nM | VAL1013 (2) ALA1031 LYS1033 ILE1160 VAL1063 LEU1081 MET1082 (2) MET1142 (2) MET1156 | ||
| 21 | Betulin | 442.7 | −8.86 | 322.86 nM | LEU1005 (2) ALA1031 (3) VAL1063 MET1079 MET1082 MET1142 (4) MET1156 | ||
| 22 | n-Hexadecanoic acid | 256.42 | −3.76 | 1.75 mM | MET1082 GLU1080 | LEU1005 VAL1013 (3) ALA1031 LYS1033 (2) MET1079 MET1156 (2) | |
| 23 | Oleic acid | 282.5 | −4.43 | 564.46 μM | SER1089 ASP1086 | VAL1013 (2) ALA1031 (2) LYS1033 MET1142 (2) MET1156 (2) | |
| 24 | Nonacosane | 408.8 | −3.85 | 1.52 mM | LEU1005 VAL1013 (3) ALA1031 (2) LYS1033 (2) VAL1063 MET1079 (2) MET1142 MET1156 (2) | ||
| 25 | 2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetraenyl)-cyclohexanol | 428.7 | −6.75 | 11.23 μM | SER1089 | LEU1005 (2) VAL1013 (2) ALA1031 (3) LYS1033 MET1079 MET1142 (2) MET1156 (2) | |
| 26 | 9,19-Cyclolanost-24-en-3-ol, acetate, (3.beta.)- | 468.8 | −5.29 | 132.45 μM | LEU1005 (3) ALA1031 (2) MET1079 ARG1084 TYR1090 MET1142 (2) | ||
| 27 | Phytol | 296.5 | −5.17 | 161.26 μM | ASP1086 | LEU1005 (2) VAL1013 (3) ALA1031 (2) LYS1033 (2) LEU1081 MET1082 MET1142 (2) MET1156 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattarachotanant, N.; Sornkaew, N.; Warayanon, W.; Rangsinth, P.; Sillapachaiyaporn, C.; Vongthip, W.; Chuchawankul, S.; Prasansuklab, A.; Tencomnao, T. Aquilaria crassna Leaf Extract Ameliorates Glucose-Induced Neurotoxicity In Vitro and Improves Lifespan in Caenorhabditis elegans. Nutrients 2022, 14, 3668. https://doi.org/10.3390/nu14173668
Pattarachotanant N, Sornkaew N, Warayanon W, Rangsinth P, Sillapachaiyaporn C, Vongthip W, Chuchawankul S, Prasansuklab A, Tencomnao T. Aquilaria crassna Leaf Extract Ameliorates Glucose-Induced Neurotoxicity In Vitro and Improves Lifespan in Caenorhabditis elegans. Nutrients. 2022; 14(17):3668. https://doi.org/10.3390/nu14173668
Chicago/Turabian StylePattarachotanant, Nattaporn, Nilubon Sornkaew, Watis Warayanon, Panthakarn Rangsinth, Chanin Sillapachaiyaporn, Wudtipong Vongthip, Siriporn Chuchawankul, Anchalee Prasansuklab, and Tewin Tencomnao. 2022. "Aquilaria crassna Leaf Extract Ameliorates Glucose-Induced Neurotoxicity In Vitro and Improves Lifespan in Caenorhabditis elegans" Nutrients 14, no. 17: 3668. https://doi.org/10.3390/nu14173668
APA StylePattarachotanant, N., Sornkaew, N., Warayanon, W., Rangsinth, P., Sillapachaiyaporn, C., Vongthip, W., Chuchawankul, S., Prasansuklab, A., & Tencomnao, T. (2022). Aquilaria crassna Leaf Extract Ameliorates Glucose-Induced Neurotoxicity In Vitro and Improves Lifespan in Caenorhabditis elegans. Nutrients, 14(17), 3668. https://doi.org/10.3390/nu14173668