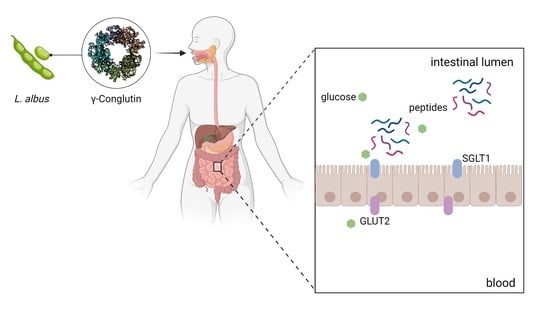
Lupinus albus γ-Conglutin: New Findings about Its Action at the Intestinal Barrier and a Critical Analysis of the State of the Art on Its Postprandial Glycaemic Regulating Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of γ-Conglutin Hydrolysate
2.2. α-Glucosidase and α-Amylase Inhibition Assay
2.3. Cells Culture Conditions
2.4. SGLT1 and GLUT2 Glucose Transporters Gene Expression
2.5. Western Blots
2.6. Ussing Chamber Experiments—IPEC-J2
2.7. Ussing Chamber Experiments—Jejunum and Ileum
2.8. Characterisation of Pig Intestinal Products
2.9. Statistical Analysis
3. Results
3.1. γ-Conglutin Hydrolysate Characterisation
3.2. Effects of γ-Conglutin on D-Glucose Active Uptake and Glucose Transporters Protein Expression in IPEC-J2 and Pig Small Intestine
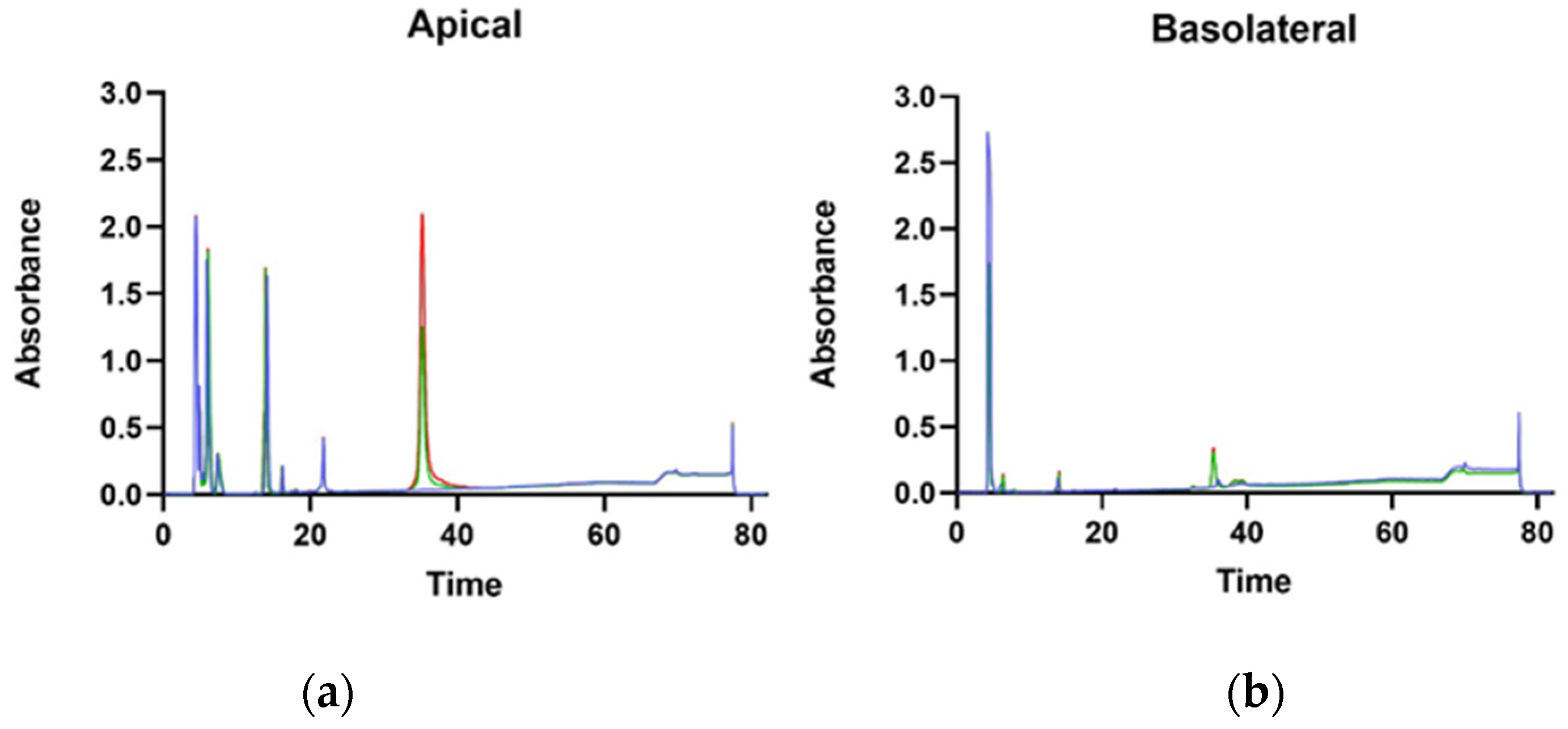
3.3. Protein Transport through Caco-2 Artificial Intestinal Barrier, Cell Viability, and Glucose Transporters Gene Expression
3.4. Inhibition of α-Amilase and α-Glucosidase Activity
4. Discussion
| Authors | Year | Cell Culture | Analysis |
|---|---|---|---|
| Sirtori et al. [40] | 2004 | HepG2 | Lipid-lowering mechanism |
| Terruzzi et al. [12] | 2011 | C2C12 | P7056 kinase, eIF-4E, PHAS-1 activation |
| Capraro et al. [21] | 2011 | Caco2 | Protein uptake |
| Lovati et al. [14] | 2012 | HepG2 | glucose consumption |
| Capraro et al. [35] | 2013 | HepG2 | Internalisation |
| Lammi et al. [41] | 2016 | Caco2 | Intestinal absorption |
| Wojciechowicz et al. [42] | 2018 | 3T3-L1 | Viability, proliferation, and adipogenic markers |
| Muñoz et al. [43] | 2018 | Caco-2, 3T3-L1 | Glucose uptake, glucose transporter, PEPCK expression |
| Barbiroli et al. [44] | 2019 | Caco-2 | Peptide transporters expression |
| Lima-Cabello et al. [45] | 2019 | PANC-1 | IRS-1, p85-PI3K, GLUT4 upregulation |
| Guzman et al. [46] | 2021 | HepG2, Min6 | Cell death induced by lipotoxicity and oxidative stress |
| Tapadia et al. [25] | 2021 | INS-1E, BRIN-BD11, Primary human skeletal muscle myoblasts | Insulin secretion, glucose uptake, glycogen content, protein synthesis |
| Gracio et al. [47] | 2021 | HepG2, rabbit erythrocytes | Glycosylated receptors binding, hemagglutination assays |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S.K. 1-Nutritional, Health, and Technological Functionality of Lupin Flour Addition to Bread and Other Baked Products: Benefits and Challenges. Crit. Rev. Food Sci. Nutr. 2016, 56, 835–857. [Google Scholar] [CrossRef]
- Kouris-Blazos, A.; Belski, R. 2-Health Benefits of Legumes and Pulses with a Focus on Australian Sweet Lupins. Asia Pac. J. Clin. Nutr. 2016, 25, 1–17. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Mafra, I. 3-Lupine Allergens: Clinical Relevance, Molecular Characterization, Cross-reactivity, and Detection Strategies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3886–3915. [Google Scholar] [CrossRef]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Obeme-Nmom, J.I.; Agboinghale, P.E.; Aguchem, R.N.; Nechi, R.N.; Lammi, C. Lupin-Derived Bioactive Peptides: Intestinal Transport, Bioavailability and Health Benefits. Nutrients 2021, 13, 3266. [Google Scholar] [CrossRef]
- Ruiz-López, M.A.; Barrientos-Ramírez, L.; García-López, P.M.; Valdés-Miramontes, E.H.; Zamora-Natera, J.F.; Rodríguez-Macias, R.; Salcedo-Pérez, E.; Bañuelos-Pineda, J.; Vargas-Radillo, J.J. Nutritional and Bioactive Compounds in Mexican Lupin Beans Species: A Mini-Review. Nutrients 2019, 11, 1785. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Legume Proteins and Peptides as Compounds in Nutraceuticals: A Structural Basis for Dietary Health Effects. Nutrients 2022, 14, 1188. [Google Scholar] [CrossRef]
- Duranti, M.; Consonni, A.; Magni, C.; Sessa, F.; Scarafoni, A. The Major Proteins of Lupin Seed: Characterisation and Molecular Properties for Use as Functional and Nutraceutical Ingredients. Trends Food Sci. Technol. 2008, 19, 624–633. [Google Scholar] [CrossRef]
- Wang, W.; de Mejia, E.G. A New Frontier in Soy Bioactive Peptides That May Prevent Age-Related Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2005, 4, 63–78. [Google Scholar] [CrossRef]
- Reyes-Díaz, A.; Del-Toro-Sánchez, C.L.; Rodríguez-Figueroa, J.C.; Valdéz-Hurtado, S.; Wong-Corral, F.J.; Borboa-Flores, J.; González-Osuna, M.F.; Perez-Perez, L.M.; González-Vega, R.I. Legume Proteins as a Promising Source of Anti-Inflammatory Peptides. Curr. Protein Pept. Sci. 2019, 20, 1204–1217. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [Green Version]
- Bertoglio, J.C.; Calvo, M.A.; Hancke, J.L.; Burgos, R.A.; Riva, A.; Morazzoni, P.; Ponzone, C.; Magni, C.; Duranti, M. Hypoglycemic Effect of Lupin Seed γ-Conglutin in Experimental Animals and Healthy Human Subjects. Fitoterapia 2011, 82, 933–938. [Google Scholar] [CrossRef]
- Terruzzi, I.; Senesi, P.; Magni, C.; Montesano, A.; Scarafoni, A.; Luzi, L.; Duranti, M. Insulin-Mimetic Action of Conglutin-γ, a Lupin Seed Protein, in Mouse Myoblasts. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 197–205. [Google Scholar] [CrossRef]
- Magni, C.; Sessa, F.; Accardo, E.; Vanoni, M.; Morazzoni, P.; Scarafoni, A.; Duranti, M. Conglutin γ, A Lupin Seed Protein, Binds Insulin in Vitro and Reduces Plasma Glucose Levels of Hyperglycemic Rats. J. Nutr. Biochem. 2004, 15, 646–650. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Parolari, A.; Magni, C.; Duranti, M. Lupin Seed γ-Conglutin Lowers Blood Glucose in Hyperglycaemic Rats and Increases Glucose Consumption of HepG2 Cells. Br. J. Nutr. 2012, 107, 67–73. [Google Scholar] [CrossRef]
- Vargas-Guerrero, B.; García-López, P.M.; Martínez-Ayala, A.L.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Administration of Lupinus Albus Gamma Conglutin (Cγ) to N5 STZ Rats Augmented Ins-1 Gene Expression and Pancreatic Insulin Content. Plant Foods Hum. Nutr. 2014, 69, 241–247. [Google Scholar] [CrossRef]
- Capraro, J.; Magni, C.; Scarafoni, A.; Caramanico, R.; Rossi, F.; Morlacchini, M.; Duranti, M. Pasta Supplemented with Isolated Lupin Protein Fractions Reduces Body Weight Gain and Food Intake of Rats and Decreases Plasma Glucose Concentration upon Glucose Overload Trial. Food Funct. 2014, 5, 375–380. [Google Scholar] [CrossRef]
- González-Santiago, A.E.; Vargas-Guerrero, B.; García-López, P.M.; Martínez-Ayala, A.L.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Lupinus Albus Conglutin Gamma Modifies the Gene Expressions of Enzymes Involved in Glucose Hepatic Production In Vivo. Plant Foods Hum. Nutr. 2017, 72, 134–140. [Google Scholar] [CrossRef]
- Sandoval-Muñíz, R.d.J.; Vargas-Guerrero, B.; Guzmán, T.J.; García-López, P.M.; Martínez-Ayala, A.L.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Lupin Gamma Conglutin Protein: Effect on Slc2a2, Gck and Pdx-1 Gene Expression and GLUT2 Levels in Diabetic Rats. Rev. Bras. Farmacogn. 2018, 28, 716–723. [Google Scholar] [CrossRef]
- Zepeda-Peña, A.C.; Gurrola-Díaz, C.M.; Domínguez-Rosales, J.A.; García-López, P.M.; Pizano-Andrade, J.C.; Hernández-Nazará, Z.H.; Vargas-Guerrero, B. Effect of Lupinus Rotundiflorus Gamma Conglutin Treatment on JNK1 Gene Expression and Protein Activation in a Rat Model of Type 2 Diabetes. Pharm. Biol. 2021, 59, 373–379. [Google Scholar] [CrossRef]
- Gomez Galan, A.M.; Brohée, M.; Scaravelli, E.; van Hengel, A.J.; Chassaigne, H. Development of Real-Time PCR Assays for the Detection of Lupin Residues in Food Products. Eur. Food Res. Technol. 2010, 230, 597–608. [Google Scholar] [CrossRef]
- Capraro, J.; Clemente, A.; Rubio, L.A.; Magni, C.; Scarafoni, A.; Duranti, M. Assessment of the Lupin Seed Glucose-Lowering Protein Intestinal Absorption by Using in Vitro and Ex Vivo Models. Food Chem. 2011, 125, 1279–1283. [Google Scholar] [CrossRef]
- Dove, E.R.; Mori, T.A.; Chew, G.T.; Barden, A.E.; Woodman, R.J.; Puddey, I.B.; Sipsas, S.; Hodgson, J.M. Lupin and Soya Reduce Glycaemia Acutely in Type 2 Diabetes. Br. J. Nutr. 2011, 106, 1045–1051. [Google Scholar] [CrossRef]
- Fornasini, M.; Castro, J.; Villacrés, E.; Narváez, L.; Villamar, M.P.; Baldeón, M.E. Hypoglycemic effect of Lupinus mutabilis in healthy volunteers and subjects with dysglycemia. Nutr. Hosp. 2012, 27, 425–433. [Google Scholar] [CrossRef]
- Hall, R.S.; Thomas, S.J.; Johnson, S.K. Australian sweet lupin flour addition reduces the glycaemic index of a white bread breakfast without affecting palatability in healthy human volunteers. Asia Pac. J. Clin. Nutr. 2005, 14, 91–97. [Google Scholar]
- Tapadia, M.; Johnson, S.; Utikar, R.; Newsholme, P.; Carlessi, R. Antidiabetic Effects and Mechanisms of Action of γ-Conglutin from Lupin Seeds. J. Funct. Foods 2021, 87, 104786. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Black Bean Peptides Inhibit Glucose Uptake in Caco-2 Adenocarcinoma Cells by Blocking the Expression and Translocation Pathway of Glucose Transporters. Toxicol. Rep. 2018, 5, 552–560. [Google Scholar] [CrossRef]
- Li, Y.; Song, Z.; Kerr, K.A.; Moeser, A.J. Chronic Social Stress in Pigs Impairs Intestinal Barrier and Nutrient Transporter Function and Alters Neuro-Immune Mediator and Receptor Expression. PLoS ONE 2017, 12, e0171617. [Google Scholar] [CrossRef]
- Blum, H.; Beier, H.; Gross, H.J. Improved Silver Staining of Plant Proteins, RNA and DNA in Polyacrylamide Gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Ziraldo, R.; Shoura, M.J.; Fire, A.Z.; Levene, S.D. Deconvolution of Nucleic-Acid Length Distributions: A Gel Electrophoresis Analysis Tool and Applications. Nucleic Acids Res. 2019, 47, e92. [Google Scholar] [CrossRef] [Green Version]
- Awati, A.; Rutherfurd, S.M.; Plugge, W.; Reynolds, G.W.; Marrant, H.; Kies, A.K.; Moughan, P.J. Ussing Chamber Results for Amino Acid Absorption of Protein Hydrolysates in Porcine Jejunum Must Be Corrected for Endogenous Protein: Determining Amino Acid Absorption Using Ussing Chambers. J. Sci. Food Agric. 2009, 89, 1857–1861. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In Vitro Human Digestion Models for Food Applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Ozorio, L.; Mellinger-Silva, C.; Cabral, L.M.C.; Jardin, J.; Boudry, G.; Dupont, D. The Influence of Peptidases in Intestinal Brush Border Membranes on the Absorption of Oligopeptides from Whey Protein Hydrolysate: An Ex Vivo Study Using an Ussing Chamber. Foods 2020, 9, 1415. [Google Scholar] [CrossRef]
- Capraro, J.; Magni, C.; Faoro, F.; Maffi, D.; Scarafoni, A.; Tedeschi, G.; Maffioli, E.; Parolari, A.; Manzoni, C.; Lovati, M.R.; et al. Internalisation and Multiple Phosphorylation of γ-Conglutin, the Lupin Seed Glycaemia-Lowering Protein, in HepG2 Cells. Biochem. Biophys. Res. Commun. 2013, 437, 648–652. [Google Scholar] [CrossRef]
- Baker, D.H. Animal Models in Nutrition Research. J. Nutr. 2008, 138, 391–396. [Google Scholar] [CrossRef]
- Hilgendorf, C.; Spahn-Langguth, H.; Regårdh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX Co-cultured Cell Lines: Permeabilities Via Diffusion, Inside- and Outside-Directed Carrier-Mediated Transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- Ghiselli, F.; Rossi, B.; Piva, A.; Grilli, E. Assessing Intestinal Health. In Vitro and Ex Vivo Gut Barrier Models of Farm Animals: Benefits and Limitations. Front. Vet. Sci. 2021, 8, 723387. [Google Scholar] [CrossRef]
- Herrmann, J.; Schröder, B.; Klinger, S.; Thorenz, A.; Werner, A.-C.; Abel, H.; Breves, G. Segmental Diversity of Electrogenic Glucose Transport Characteristics in the Small Intestines of Weaned Pigs. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, 161–169. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Duranti, M.; Magni, C.; Morandi, S.; D’Agostina, A.; Arnoldi, A. Proteins of White Lupin Seed, a Naturally Isoflavone-Poor Legume, Reduce Cholesterolemia in Rats and Increase LDL Receptor Activity in HepG2 Cells. J. Nutr. 2004, 134, 18–23. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Peptides Derived from Soy and Lupin Protein as Dipeptidyl-Peptidase IV Inhibitors: In Vitro Biochemical Screening and in Silico Molecular Modeling Study. J. Agric. Food Chem. 2016, 64, 9601–9606. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowicz, T.; Czubiński, J.; Billert, M.; Półciennik, A.; Nowak, K.W.; Skrzypski, M. Suppressive Effects of γ-Conglutin on Differentiation of 3T3-L1 Preadipocytes. Int. J. Food Sci. Technol. 2018, 53, 2624–2630. [Google Scholar] [CrossRef]
- Muñoz, E.B.; Luna-Vital, D.A.; Fornasini, M.; Baldeón, M.E.; Gonzalez de Mejia, E. Gamma-Conglutin Peptides from Andean Lupin Legume (Lupinus mutabilis Sweet) Enhanced Glucose Uptake and Reduced Gluconeogenesis in vitro. J. Funct. Foods 2018, 45, 339–347. [Google Scholar] [CrossRef]
- Barbiroli, A.; Capraro, J.; Marulo, S.; Gamba, M.; Scarafoni, A. Effects on the Caco-2 Cells of a Hypoglycemic Protein from Lupin Seeds in a Solution and Adsorbed on Polystyrene Nanoparticles to Mimic a Complex Food Matrix. Biomolecules 2019, 9, 606. [Google Scholar] [CrossRef]
- Lima-Cabello, E.; Alché, J.D.; Morales-Santana, S.; Clemente, A.; Jimenez-Lopez, J.C. Narrow-Leafed Lupin (Lupinus Angustifolius L.) Seeds Gamma-Conglutin Is an Anti-Inflammatory Protein Promoting Insulin Resistance Improvement and Oxidative Stress Amelioration in PANC-1 Pancreatic Cell-Line. Antioxidants 2019, 9, 12. [Google Scholar] [CrossRef]
- Guzmán, T.J.; Düfer, M.; Wiedemann, M.; Olguín-Alor, R.; Soldevila, G.; Gurrola-Díaz, C.M. Lupin γ-Conglutin Protects against Cell Death Induced by Oxidative Stress and Lipotoxicity, but Transiently Inhibits in Vitro Insulin Secretion by Increasing KATP Channel Currents. Int. J. Biol. Macromol. 2021, 187, 76–90. [Google Scholar] [CrossRef]
- Grácio, M.; Rocha, J.; Pinto, R.; Boavida Ferreira, R.; Solas, J.; Eduardo-Figueira, M.; Sepodes, B.; Ribeiro, A.C. A Proposed Lectin-mediated Mechanism to Explain the in Vivo Antihyperglycemic Activity of Γ-conglutin from Lupinus Albus Seeds. Food Sci. Nutr. 2021, 9, 5980–5996. [Google Scholar] [CrossRef]
- Gętek, M.; Czech, N.; Muc-Wierzgoń, M.; Grochowska-Niedworok, E.; Kokot, T.; Nowakowska-Zajdel, E. The Active Role of Leguminous Plant Components in Type 2 Diabetes. Evid.-Based Complement. Altern. Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A. Soybean- and Lupin-Derived Peptides Inhibit DPP-IV Activity on In Situ Human Intestinal Caco-2 Cells and Ex Vivo Human Serum. Nutrients 2018, 10, 1082. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A. Three Peptides from Soy Glycinin Modulate Glucose Metabolism in Human Hepatic HepG2 Cells. Int. J. Mol. Sci. 2015, 16, 27362–27370. [Google Scholar] [CrossRef]
- Johnson, S.K.; McQuillan, P.L.; Sin, J.H.; Ball, M.J. Sensory Acceptability of White Bread with Added Australian Sweet Lupin (Lupinus angustifolius) Kernel Fibre and Its Glycaemic and Insulinaemic Responses When Eaten as a Breakfast. J. Sci. Food Agric. 2003, 83, 1366–1372. [Google Scholar] [CrossRef]
- Lee, Y.P.; Mori, T.A.; Sipsas, S.; Barden, A.; Puddey, I.B.; Burke, V.; Hall, R.S.; Hodgson, J.M. Lupin-Enriched Bread Increases Satiety and Reduces Energy Intake Acutely. Am. J. Clin. Nutr. 2006, 84, 975–980. [Google Scholar] [CrossRef]
- Skalkos, S.; Moschonis, G.; Thomas, C.J.; McMillan, J.; Kouris-Blazos, A. Effect of Lupin-Enriched Biscuits as Substitute Mid-Meal Snacks on Post-Prandial Interstitial Glucose Excursions in Post-Surgical Hospital Patients with Type 2 Diabetes. Nutrients 2020, 12, 1239. [Google Scholar] [CrossRef]
- Ward, N.C.; Mori, T.A.; Beilin, L.J.; Johnson, S.; Williams, C.; Gan, S.K.; Puddey, I.B.; Woodman, R.; Phillips, M.; Connolly, E.; et al. The Effect of Regular Consumption of Lupin-Containing Foods on Glycaemic Control and Blood Pressure in People with Type 2 Diabetes Mellitus. Food Funct. 2020, 11, 741–747. [Google Scholar] [CrossRef]
- Pavanello, C.; Lammi, C.; Ruscica, M.; Bosisio, R.; Mombelli, G.; Zanoni, C.; Calabresi, L.; Sirtori, C.R.; Magni, P.; Arnoldi, A. Effects of a Lupin Protein Concentrate on Lipids, Blood Pressure and Insulin Resistance in Moderately Dyslipidaemic Patients: A Randomised Controlled Trial. J. Funct. Foods 2017, 37, 8–15. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Kiehntopf, M.; Jahreis, G. Consuming a Mixed Diet Enriched with Lupin Protein Beneficially Affects Plasma Lipids in Hypercholesterolemic Subjects: A Randomized Controlled Trial. Clin. Nutr. 2015, 34, 7–14. [Google Scholar] [CrossRef]
- Radtke, J.; Schutkowski, A.; Brandsch, C.; Hirche, F.; Hasenkopf, K.; Stangl, G.I. Isolated Conglutin γ from Lupin, but Not Phytate, Lowers Serum Cholesterol Without Influencing Vascular Lesion Development in the ApoE-Deficient Mouse Model. Plant Foods Hum. Nutr. 2015, 70, 113–118. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Sánchez, C.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Cantarero, S.; Arrebola, F.; Fernández-Segura, E.; et al. Health Promoting Effects of Lupin (Lupinus Albus Var. Multolupa) Protein Hydrolyzate and Insoluble Fiber in a Diet-Induced Animal Experimental Model of Hypercholesterolemia. Food Res. Int. 2013, 54, 1471–1481. [Google Scholar] [CrossRef]
- Brandsch, C.; Kappis, D.; Weiße, K.; Stangl, G.I. Effects of Untreated and Thermally Treated Lupin Protein on Plasma and Liver Lipids of Rats Fed a Hypercholesterolemic High Fat or High Carbohydrate Diet. Plant Foods Hum. Nutr. 2010, 65, 410–416. [Google Scholar] [CrossRef]
- del Carmen Millán-Linares, M.; del Mar Yust, M.; Alcaide-Hidalgo, J.M.; Millán, F.; Pedroche, J. Lupine Protein Hydrolysates Inhibit Enzymes Involved in the Inflammatory Pathway. Food Chem. 2014, 151, 141–147. [Google Scholar] [CrossRef]
- del Carmen Millán-Linares, M.; Millán, F.; Pedroche, J.; del Mar Yust, M. GPETAFLR: A New Anti-Inflammatory Peptide from Lupinus angustifolius L. Protein Hydrolysate. J. Funct. Foods 2015, 18, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Millan-Linares, M.C.; Toscano, R.; Lemus-Conejo, A.; Martin, M.E.; Pedroche, J.; Millan, F.; Montserrat-de la Paz, S. GPETAFLR, a Biopeptide from Lupinus angustifolius L., Protects against Oxidative and Inflammatory Damage in Retinal Pigment Epithelium Cells. J. Food Biochem. 2019, 43, e12995. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; del Carmen Millán-Linares, M.; del Mar Yust, M.; Pedroche, J.; Millán, F.; Lardone, P.J.; Carrera-Sánchez, C.; Guerrero, J.M.; Carrillo-Vico, A. Lupine Protein Hydrolysates Decrease the Inflammatory Response and Improve the Oxidative Status in Human Peripheral Lymphocytes. Food Res. Int. 2019, 126, 108585. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Álvarez-Ríos, A.I.; Santos-Sánchez, G.; Pedroche, J.; Millán, F.; Carrera Sánchez, C.; Fernández-Pachón, M.S.; Millán-Linares, M.C.; Martínez-López, A.; et al. Safety and Efficacy of a Beverage Containing Lupine Protein Hydrolysates on the Immune, Oxidative and Lipid Status in Healthy Subjects: An Intervention Study (the Lupine-1 Trial). Mol. Nutr. Food Res. 2021, 65, 2100139. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.-E.; Jayasena, V. Phytochemical Composition and Bioactivities of Lupin: A Review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Bryant, L.; Rangan, A.; Grafenauer, S. Lupins and Health Outcomes: A Systematic Literature Review. Nutrients 2022, 14, 327. [Google Scholar] [CrossRef]


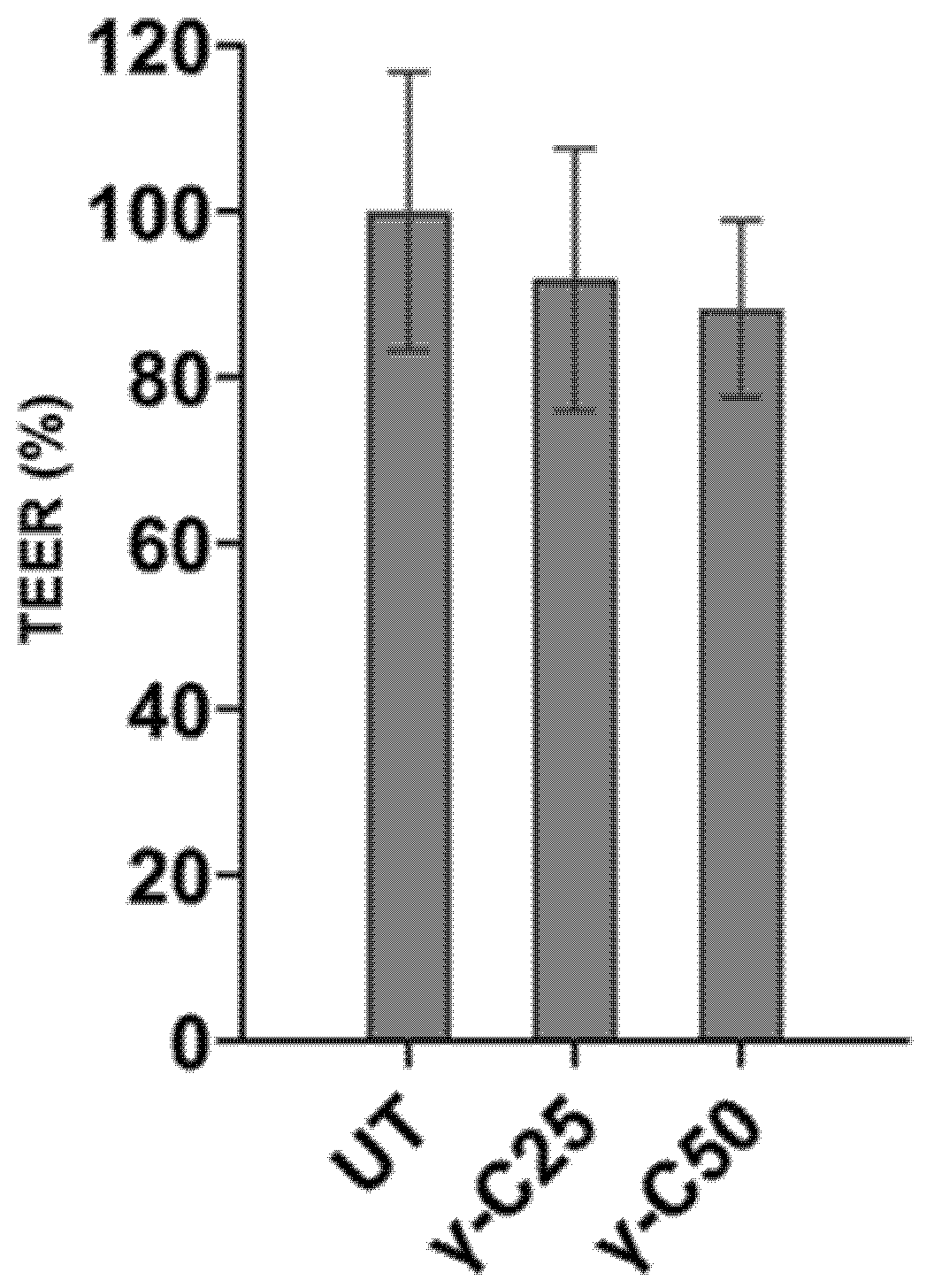
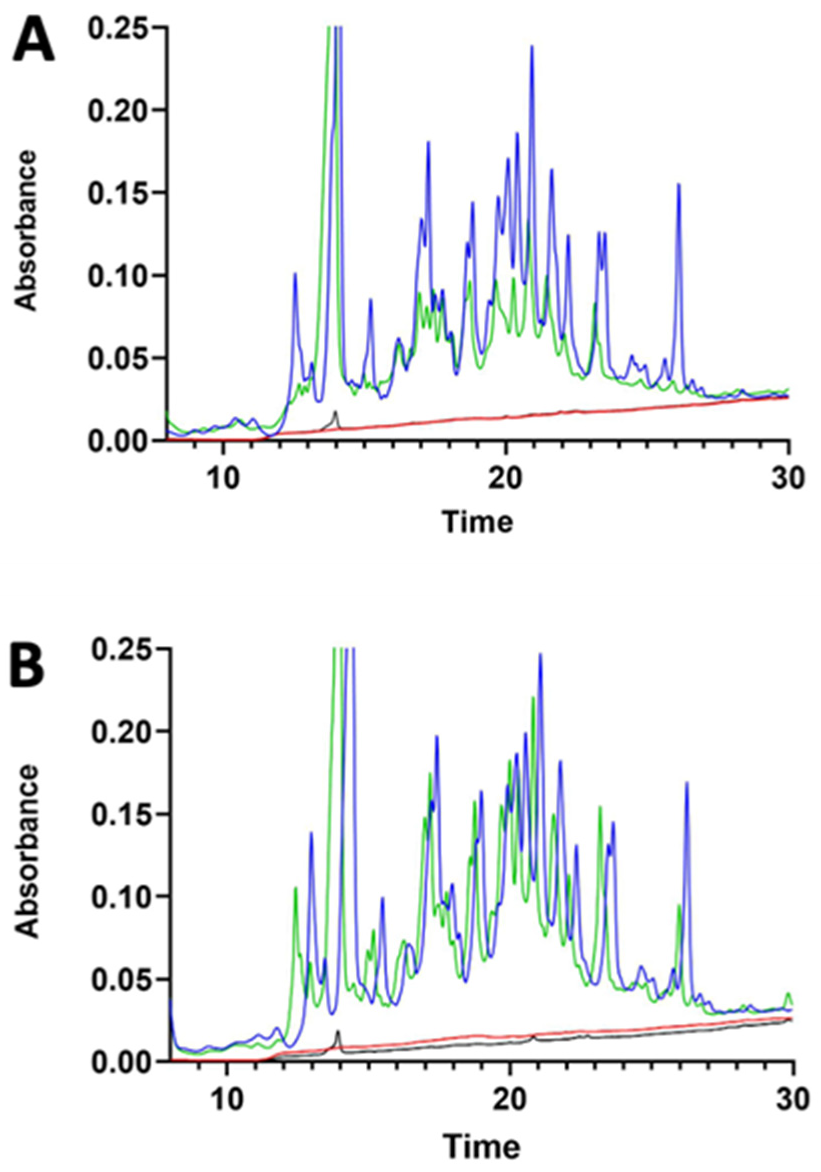

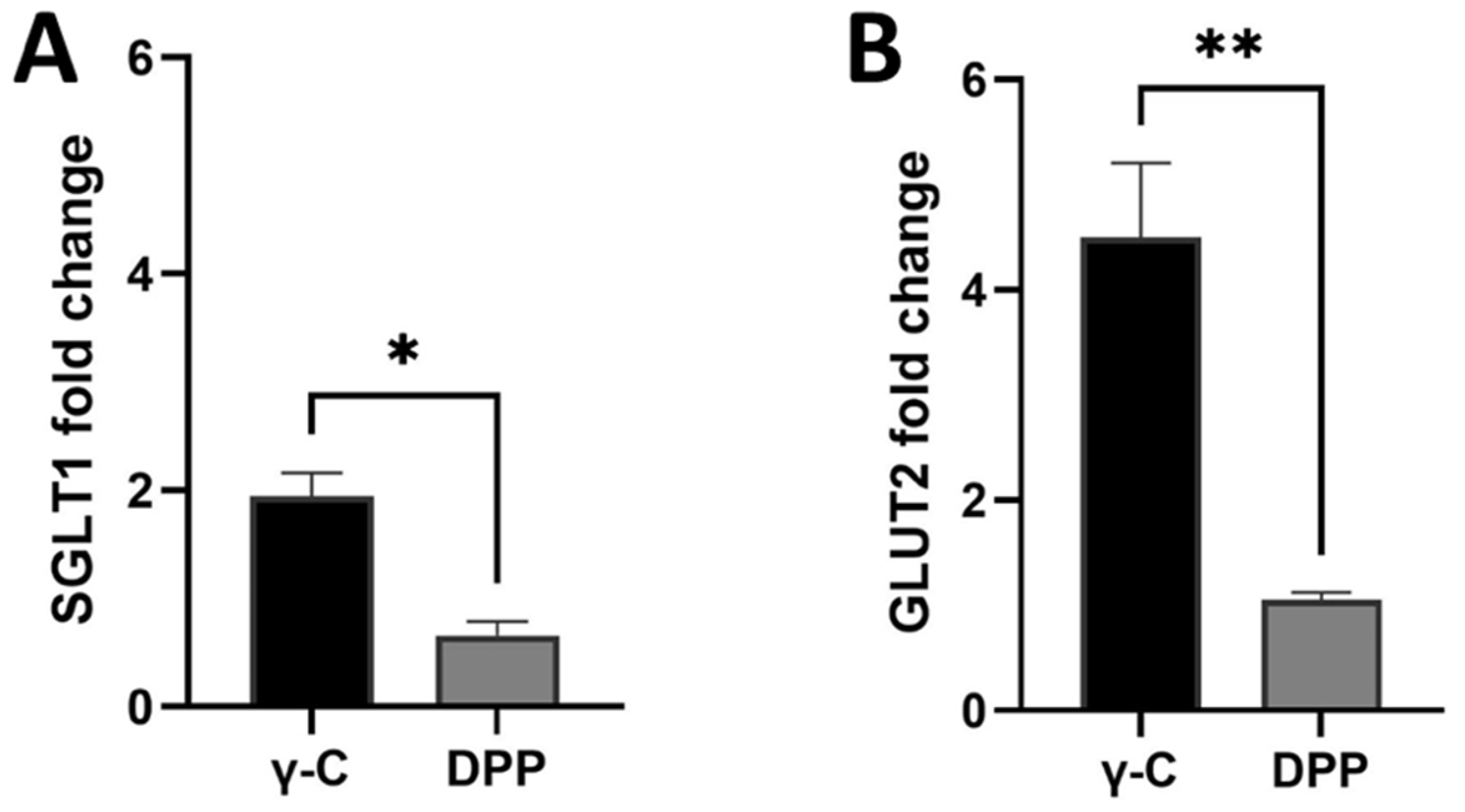

| UT | γ-C25 | γ-C50 | SEM | p-Values | |
|---|---|---|---|---|---|
| ΔIsc-Glucose (µA) | 0.18 | 0.08 | 0.06 | 0.02 | 0.02 |
| Δlsc-Forsk (µA) | 1.07 | 0.98 | 0.95 | 0.05 | 0.64 |
| TEER (Ω) | 100 | 91.8 | 88.2 | 3.35 | 0.36 |
| UT | γ-C | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|
| Jejunum | Ileum | Jejunum | Ileum | SEM | T | S | TxS | |
| Δ-Isc (µA) | 6.77 | 9.18 | 3.57 | 7.89 | 1.07 | 0.02 | 0.27 | 0.64 |
| TEER (Ω) | 38.3 | 58.9 | 42.7 | 78.4 | 7.62 | 0.11 | 0.08 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinzl, G.C.; Tretola, M.; De Benedetti, S.; Silacci, P.; Scarafoni, A. Lupinus albus γ-Conglutin: New Findings about Its Action at the Intestinal Barrier and a Critical Analysis of the State of the Art on Its Postprandial Glycaemic Regulating Activity. Nutrients 2022, 14, 3666. https://doi.org/10.3390/nu14173666
Heinzl GC, Tretola M, De Benedetti S, Silacci P, Scarafoni A. Lupinus albus γ-Conglutin: New Findings about Its Action at the Intestinal Barrier and a Critical Analysis of the State of the Art on Its Postprandial Glycaemic Regulating Activity. Nutrients. 2022; 14(17):3666. https://doi.org/10.3390/nu14173666
Chicago/Turabian StyleHeinzl, Giuditta C., Marco Tretola, Stefano De Benedetti, Paolo Silacci, and Alessio Scarafoni. 2022. "Lupinus albus γ-Conglutin: New Findings about Its Action at the Intestinal Barrier and a Critical Analysis of the State of the Art on Its Postprandial Glycaemic Regulating Activity" Nutrients 14, no. 17: 3666. https://doi.org/10.3390/nu14173666
APA StyleHeinzl, G. C., Tretola, M., De Benedetti, S., Silacci, P., & Scarafoni, A. (2022). Lupinus albus γ-Conglutin: New Findings about Its Action at the Intestinal Barrier and a Critical Analysis of the State of the Art on Its Postprandial Glycaemic Regulating Activity. Nutrients, 14(17), 3666. https://doi.org/10.3390/nu14173666